1. Introduction
Ardusi (Adhatoda vasica Nees syn. Justicia adhatoda L.), a shrub with an unpleasant smell, is popularly known as Malabar nut or Vasaka (in Sanskrit) [1]. It is an important member of the Acanthaceae family. In Unani and Ayurveda, this shrub is highly treasured owing to its healing properties against asthma, cold, cough and tuberculosis [2]. It acts as antispasmodic and expectorant as well [3]. A. vasica leaf, shoot and root prevalently possess quinazoline alkaloids like vasicine and vasicinone [4], and a non-crystalline steroid (vasakin), along with several essential oils, fatty acids, glycosides, sterols, and other phenolic components [5]. Due to an immoderate exploitation of plant parts for the purpose of constant phytochemical extraction by pharmaceutical industries, the natural population of A. vasica is under threat. As a consequence, the ever-increasing demand for its plant-part-based secondary metabolites cannot be fulfilled. Seed germination rate of A. vasica is quite poor and clonal propagation is occasional as well [6], [7]. Owing to these drawbacks, tissue culture techniques i.e. direct and indirect organogenesis has been preferred [7], [8], [9], [10].
2. Distribution and description
A. vasica is widely spread over India (up to an altitude of 1300 m), few parts of Sri Lanka, Bhutan, Pakistan, Afghanistan, and is progressively introduced to other countries like China, Hong Kong, Taiwan, Cyprus, Ethiopia etc. It is also found throughout the tropical regions of Southeast Asia [11] and some parts of Germany and Sweden [12], as well. A. vasica is a typically evergreen shrub, perennial, and grows at a height of about 1.2–2.5 m; leaves are characteristically perfect, elliptic-lanceolate, borne on short petioles and leathery to touch. The leaves carry an unpleasant smell and have a bitter taste. Chloral hydratepreparations of leaves showed oval stomata encircled by two crescent-shaped cells at right angles to the ostiole [13]. The branching habit is opposite and ascending with white, purple or pink flowers. But when the flowers become dry, they turn dull brownish in color. White with yellow or red barred throats with large bracts are seen in the flowers. Fruit-capsules and seeds are globular in nature [1].
3. Pharmaceutical/therapeutic importance
Ardusi contains numerous bioactive compounds, for instance, vasicinol, 5-hydroxy vasicine, vasicine, vasicine glycoside, deoxyvasicine, vasicinone, adhavasicinone, vasicolinone, adhatodine, anisotine and vasnetine [14], [15], [16], [17]. Vasicine shows bronchodilatory activity under in vitro and in vivocondition, whilst, vasicinone exhibited its effectiveness towards bronchoconstriction in vivo. Simultaneous effect of these two alkaloids was preferably administered for bronchodilatory activity both under in vitro and in vivo. A combination of vasicine and vasicinone also showed a significant reduction in cardiac depressant effects. Vasicinone produced from the roots, prevents shrinkage of intestine and cardiac depression in guinea pigs, and transient hypotension in cats, thus displaying decent anticholinesterase activity[18]. Vasicine produces ambroxol and bromhexine that have a pH-dependent growth inhibitory influence on Mycobacterium tuberculosis, which suggests that it may play a significant part in the primary treatment of tuberculosis [19]. Both vasicine and vasicinone have sucrose inhibitory activity, signifying that they can be explored as natural antidiabetic agents [20]. It has been reported that vasicine and its derivatives are excreted through urine [21]. By way of intramuscular and intravenous administration, for the first 18 and 22 h, 55% of the excreted product was vasicine, whilst, on oral administration, it was 18% during the first 24 h. The leaves of A. vasica possess anti-ulcer activity, which was tested in rats. The ardusi leaves have the highest degree of anti-ulcer activity (80%) as detected in the ethanol induced ulceration model when compared to that of the actions of pylorus and aspirin [22]. The syrup made from A. vasica leaves improved symptoms of dyspepsia as well [23]. A. vasicaextracts exhibited antimutagenic activity when cadmium-intoxicated mice was treated with the same, wherein, it showed marked decline in inhibition of lipid peroxidation and xanthine oxidase activity [24]. Swiss albino mice when exposed to Cobalt-60 radiation, was affected with radiation-induced ailment, displaying noticeable effects in histology of testis. This effect was significantly reduced when A. vasica plant extract was applied. This suggests that the ardusi plant extracts have radioprotective effects on testis [25].
4. In vitro regeneration
Conventionally, A. vasica is propagated through seed or nodal cuttings. Nevertheless, the frequency of propagation is limited since the seed setting is insufficient; seed germination is poor and clonal propagation via stem cuttings is exclusively season-dependent [7], [8]. As an alternative to the conventional methods, in vitro propagation through plant cell, tissue and organ culture becomes a proficient technique for accelerated production of propagules in large-scale, exploring the variability among the propagules, and, to induce new attributes of commercial importance, as well as to develop novel variant via genetic transformation [26], [27]. There are several in vitro techniques that have been applied for direct and indirect regeneration in A. vasica till date. It is now quite essential to compare the reported in vitro techniques and classify them based on their efficacy, in order to select the suitable need-based protocol [28], [29], [30]. Accordingly, in this review, we’ve compared the reported methods of micropropagation in A. vasica, for instance direct organogenesis via multiple shoot culture and indirect organogenesis mediated by callus culture, along with some improved technologies like artificial seed development and in vitroproduction of secondary metabolites.
4.1. Explant selection
Appropriate selection and collection of explants is the first and foremost step for a successful in vitro regeneration study. Even though A. vasica is a perennial shrub and collection of explants can be done round the year; the most active growth stage was considered to retain the regeneration ability of collected explants. Preferable time of explant collection for in vitro regeneration is considered to be between November and March [8], [31], based on certain aspects like ontogenetic or physiological age and position (certain part of the plant, from where explants are collected) or size of explants. A number of explants, such as whole leaf, leaf disc, petiole, shoot tip, nodal segment, axillary meristems and root have been utilized for initiation of in vitro direct or indirect regeneration of A. vasica that has been summarized in Table 1. Among these explants, the sole use of nodal segments from field-grown plants was the most prevalent in majority of the reports [8], [9], [10], [32], [33], [34]. Additionally, when the regeneration efficiency of nodal segments was compared with other explants like shoot tip [35], [36], [37], the nodal segment explants displayed better response based on multiple shoot initiation and subsequent proliferation. Similar trend was also observed in case of indirect regeneration, where the nodal segment explants induced higher frequency of callogenesis in comparison to shoot tip, petioles, and leaf disc explants [38]. To induce cell culture and to obtain maximum cell biomass, Singh et al. [39], unconventionally used root segment explants and attained significant results. On the other hand, Madhukar et al. [40] used leaf explants to develop cell suspension culture via friable callus induction from leaf explants. Even after considering the superior morphogenetic competence of nodal segment explants, leaf explants was preferred for induction and subsequent regeneration of callus [7], [31], [41], [42], [43], [44]. In couple of the instances, the specific age of the explant source (mother plant) was mentioned either as 2–3 years old plant [35] or 6–7 year old flowering plant [8]; however in majority of the reports the age and stage of mother plants were not mentioned, which is considered to be a major factor during explant selection.
Table 1. Factors involved and their influence on micropropagation of Adhatoda vasica (arranged in chronological order).
| Explant | Surface sterilization | Culture medium composition | Culture condition | Regeneration response | Acclimatization | Reference |
|---|---|---|---|---|---|---|
| Leaf (in vitro) | 0.1% HgCl2 for 5 min → 4 interim rinse with sterile water | MS + 1.0 mg/1 BA + 0.1 mg/1 NAA | 25 ± 1 °C temp, 60% RH, 16 h photoperiod, with an irradiance of 3000 lux | Maximum number of shoots with optimum callus growth | Regenerated plantlets were acclimatized in soil | [49] |
| PGR-free MS | Rooting | |||||
| Nodal segment | 1% Savlon 10 min → 80% ethanol for 30 sec → 0.1% HgCl2 for 7–10 min → 4–5 interim rinse with sterile water | MS + 0.5 mg/l BA + 0.1 mg/l NAA | 26 ± 1 °C temp, 60% RH, 16 h photoperiod, with an irradiance of 2000–3000 lux | Maximum 10.5 shoots of 4 cm and 2.8 of leaves/ shoot | 80% survival on garden soil, sand and compost (2:1:1) | [32] |
| PGR-free MS | 100% rooting, 3.5 roots/shoot with 4 cm length in 15 days | |||||
| Leaf | Not mentioned | B5 + 1 mg/l 2,4-D | Not mentioned | Callus induction | Wasn’t carried out | [41] |
| B5 + 0.1 mg/l Kn | Shoot regeneration | |||||
| Shoot tip | 2–3 drops teepol for 5–10 min → 0.01% HgCl2 for 7–18 min → thorough rinse with sterile water | MS + 0.5 mg/l BA + 15% CM | 25 ± 1 °C temp, unspecified RH, 16 h photoperiod, with an irradiance of 3000 lux | 4.3 shoots per explant with 5 leaves 3.2 nodes/shoot | 85% plants were acclimatized in sterilized sand and soil mixture (3:1) | [47] |
| PGR-free MS | 9.33 roots per shoot with 0.6 cm length | |||||
| Leaf | 5 drops Tween 80 for 5 min → 0.1% HgCl2for 5 min → 3 rinse with sterile water | MS + 21.5 μM NAA + 19.7 μM IBA + 9.3 μM Kn | 25 ± 2 °C temp, unspecified RH, 16 h photoperiod, with an irradiance of 30 μE/m2/s PPFD | 76% callus with precocious roots | Wasn’t carried out | [42] |
| Petiole | MS + 4.5 μM 2,4-D + 2.3 μM Kn | 62% callus with precocious somatic embryos | ||||
| Nodal segment | 0.1% HgCl2 for 5 min → 4–5 rinse with sterile water; or, 0.1% HgCl2 for 5 min → Geneticin treatment → 4–5 interim rinse with sterile water | MS + 10 mg/l BA | 25 ± 2 °C temp, 80% RH, 16 h photoperiod, with an irradiance of 100 μmol/m2/s PPFD | 7.75 shoots/explant in 4 weeks | Successfully acclimatized for 3 weeks in soilrite with liquid ½ MS nutrient spraying | [8] |
| MS + 1 mg/l BA + 1 mg/l Kn | 30 shoots/explant in 6 weeks | |||||
| MS + 0.1 mg/l IBA | 90% rooting | |||||
| Shoot tip, Nodal segment | 1% Savlon (w/v) and 2 drops Tween 80 for 20 min → unspecified HgCl2 for 5 min | MS + 2 mg/l BA + 0.2 mg/l NAA | 25 ± 2 °C temp, unspecified RH, 16 h photoperiod, with an irradiance of 3000 lux | 90% of explants produced 7 shoots/explant with 4.9 cm length in 28 days | 80% plantlets were acclimatized in garden soil + cow dung + sand (1:1:1) | [35] |
| MS + 1 mg/l IBA | 80% rooting with 3–4 roots/shoot of 3 cm length were recorded in 28 days | |||||
| Leaf, Petiole, Nodal segment | Unspecified | MS + 10.7 µM NAA + 2.2 µM BA | Unspecified | 90% repeatability to induce callus with 7 day callus induction | Wasn’t carried out | [53] |
| Leaf | 0.1% HgCl2 for 5 min → 3 rinse with sterile water | MS + 1.5 ppm 2,4-D + 1.5 ppm IAA + 1.5 ppm Kn + 1.5 ppm BA | 24 ± 2 °C temp, unspecified RH, 16 h photoperiod, with an irradiance of 3000 lux | 75% callus induction and proliferation with 18.16 g fresh weight | Wasn’t carried out | [43] |
| Nodal segment, Shoot tip, Petioles, Leaf disc | 0.1% HgCl2 for 5 min → thorough rinse with sterile water | MS + 2.0 mg/l BA + 0.5 mg/l NAA | Unspecified | Maximum callus induction from nodal segments | Rooted plantlets were acclimatized in a mixture of sandy soil and FYM (1:1) | [38] |
| MS + 2.0 mg/l 2,4-D, 0.5 mg/l Kn and 0.5 mg/l GA3 | Embryogenic callus proliferation | |||||
| ½ MS + 0.5 mg/l IBA + 0.2 g/l AC | Rooting | |||||
| Shoot tip | Unspecified | MS + 22.20 µm BA | Unspecified | High frequency and maximum number of multiple shoots | The rooted plantlets were hardened and established at 50–60% | [50] |
| PGR-free MS | High frequency of rooting | |||||
| Axillary meristems, Leaf, Nodal segment | 0.1% HgCl2 for 2 min → thorough rinse with sterile water | MS + 1 mg/l BA + 1 mg/l GA3 | 25 ± 2 °C temp, unspecified RH, 10 h photoperiod, with unspecified irradiance | High frequency shoot multiplication | Wasn’t carried out | [36] |
| MS + 1 mg/l Kn + 2 mg/l 2,4-D 1 mg/l BA + 0.1 mg/l Pic | Callus initiation within 5 days, both friable and green calli | |||||
| Nodal segment | 0.2% HgCl2 for unspecified duration → 4–5 interim rinse with sterile water | MS + 15% (v/v) CW + 5 mg/l BA | 25 ± 2 °C temp, unspecified RH, 16 h photoperiod, with unspecified irradiance | 14 shoots with 3 cm long in 8 weeks | 80% plants were acclimatized under laboratory conditions → transferred to pots filled with sterilized soil: sand mixture (1:3) | [33] |
| MS + 1 mg/l IBA | Rooting (unspecified) | |||||
| Nodal segment | Unspecified HgCl2 + Tween-20 for 5 min → thorough rinse with sterile water | MS + 1.0 mg/l BA + 0.05 mg/l NAA | 24 ± 2 °C temp, unspecified RH, 16 h photoperiod, with an irradiance of 2000–2500 lux | 10 shoots/explant within 4 weeks with maximum elongation | Wasn’t carried out | [9] |
| MS + 0.5 IBA | Rooting with root length 3.5–4 cm after 3 weeks | |||||
| MS + 0.05 mg/l NAA + 0.1 mg/l BA + 0.1 mg/l Kn | Dark green, compact and hard callus | |||||
| Leaf | Unspecified | MS + 1 mg/l 2,4-D + 0.5 mg/l Kn | 27 ± 2 °C temp, unspecified RH, 16 h photoperiod, with unspecified irradiance | 70% callusing from leaf explants after 4 weeks | Wasn’t carried out | [7] |
| Petiole | MS + 1 mg/l 2,4-D + 1 mg/l Kn | 45% callusing from petiole explants after 4 weeks | ||||
| Friable calli | MS (liquid) + 1 mg/l 2,4-D + 0.5 mg/l Kn | Rotary shaker at 120 ± 5 rpm | Cell suspension culture | |||
| Leaf | Few drops Tween 80 for 15–20 min → 0.1% HgCl2 for 3–4 min | MS + 6 mg/l IAA + 6 mg/l Kn | 25 ± 2 °C temp, 55–6-% RH, 16 h photoperiod, with an irradiance of 2000 lux | Induction and proliferation of friable calli | Wasn’t carried out | [44] |
| Nodal segment | MS + 3 mg/l IBA + 3 mg/l BA | Induction and proliferation of friable calli | ||||
| Root | MS + 3 mg/l IBA + 6 mg/l BA | Induction and proliferation of friable calli | ||||
| Friable calli | PGR-free MS (liquid) | Rotary shaker at 120 rpm | Cell suspension culture | |||
| Shoot tip, Nodal segment | 1% Dettol for 10 min → 0.1% HgCl21–4 min → thorough rinse with sterile water | MS + 2 mg/l BA + 0.5 mg/l NAA + 0.5 mg/l TDZ | 25 ± 2 °C temp, unspecified RH, 16 h photoperiod, with an irradiance of 1000 lux | 100% of explants produced 23.3 shoots/explant in 28 days | 98.2% plantlets were acclimatized in garden soil + sand + vermicompost (1:1:1) in 28 days | [37] |
| MS + 0.1 IBA | 5.8 roots/shoot with 2.5 cm root length in 17 days | |||||
| Petiole | 0.1% HgCl2 for 5 min → 5 interim rinse for 10 min with sterile water | MS + 0.25 mg/l TDZ + 0.25 mg/l NAA | 25 °C temp, unspecified RH, 16 h photoperiod, with an irradiance of 35 μmol/m2/s PPFD | 100% callus induction, 90.6% regeneration with 8.10 shoots per callus | 90% were acclimatized in garden soil within 12 weeks | [31] |
| SH + 0.5 mg/l IBA | 75% rooting with 9–10 roots/ shoot | |||||
| Root | 0.1% HgCl2 for 1–1.5 min → 3 rinse with sterile water | MS + 3.5 mg/l NAA + 1.25 mg/l BA | 25 ± 1 °C temp, unspecified RH, 16 h photoperiod, with an irradiance of 3000 lux | Induction and proliferation of friable calli | Wasn’t carried out | [54] |
| Leaf | 1% Bavistine® solution for 10 min → 1% Savlon 10 min → 70% ethanol for 30 sec → 0.1% HgCl2 for 3 min → 5–7 interim rinse with sterile water | MS + 1 ppm 2,4‑D + 1 ppm BA + 1 ppm IAA | 25 ± 1 °C temp, 70% RH, 16 h photoperiod, with an irradiance of 2000–3000 lux | Profuse growth of soft creamy colored calli | Wasn’t carried out | [40] |
| MS (liquid) + KNO3 + NaCl | Rotary shaker at 120 rpm | Cell suspension culture | ||||
| Nodal segment | 70% alcohol for 1 min → 0.1% HgCl2(w/v) for 5 min → 4–5 interim rinse with sterile water | MS + 10.0 mg/l BA | 25 ± 2 °C temp, unspecified RH, 12 h photoperiod, with unspecified irradiance | 93.33% explants produced 10.6 shoots with 5.2 cm length | 80% plantlets were acclimatized in garden soil and compost (2:1) | [34] |
| MS + 0.05 mg/l IAA + 0.05 mg/l NAA + 1.0 mg/l BA | 100% induction of light green callus in 14 days | |||||
| MS + 10 mg/l BA | Callus mediated shoot regeneration | |||||
| MS + 1 mg/l IBA | Rooting | |||||
| Nodal segment | 3% (v/v) H2O2 for 2 min → 95% (v/v) ethanol for 1 min → 3 interim rinse with sterile water | MS + 1.1 mg/l BA | 25 ± 1 °C temp, a 16 h photoperiod, with an irradiance of 60 μmol/m2/s PPFD | Shoot initiation in 6 days, 7.4 shoots of 7.2 cm length, 2.8 of leaves/ shoot | Initially in soil and sand (1:1; v/ v) for 4 weeks recording a survival rate of 95%. Finally, plantlets were established in sand, soil and farmyard manure (1:1:1; v/v) for another 4 weeks | [10] |
| MS + 1 mg/l IBA + 0.25 mg/l NAA | 94% rooting, 8.4 roots/shoot with 5.6 cm length | |||||
| MS + 1 mg/l 2,4-D | 46% callus induction that subsequently induced 60 roots per callus, devoid of adventitious shoots | |||||
| Root segment | 0.1% HgCl2 (w/v) for 5 min → 3 interim rinse with sterile water | MS + 1 mg/l 2,4-D + 4 mg/l BA | 25 ± 2 °C temp, 60% RH, unspecified photoperiod, 8 days on rotary shaker (120 rpm) | Cell culture, maximum cell biomass (47.43 g/flask) was achieved | Wasn’t carried out | [39] |
2,4-D 2,4-dichlorophenoxyacetic acid; AC activated charcoal; B5 B5 medium, or Gamborg’s medium [52]; BA N6-benzyladenine; CM coconut milk; CW coconut water; GA3 gibberellin A3; IAA indole-3-acetic acid; IBA indole-3-butyric acid; Kn kinetin or 6-furfurylaminopurine; MS Murashige and Skoog medium [48]; NAA α-napthalene acetic acid; PGR plant growth regulator; Pic piclorum; SH Schenk and Hildebrandt [55]; TDZ thidiazuron.
4.2. Surface sterilization
The most crucial step for establishment of any in vitro culture is sterilization of explants that are to be inoculated in the media, since there persists a high chance of microbial contamination in the plant materials, collected from fields [45]. There are three key parameters of surface sterilization: the category of disinfectant, their levels and duration of exposure. These parameters should be standardized in such a way that the sterilization would eradicate the contaminants without disturbing the regeneration ability of the explants. In majority of the instances, these three parameters depend upon the nature of explant tissue; softer or juvenile tissue requires an exposure of lower levels of disinfectants for a briefer time span in comparison to mature and hard tissues [29], [46]. As noted in the published literatures (Table 1), the surface sterilization of A. vasica was done by the way of exposing the explants to 1% (v/v) Savlon for 10 min, 80% (v/v) ethanol for 30 sec and 0.1% (w/v) HgCl2 for 7–10 min with 3–5 interim rinse with sterile water (Table 1). However, in many of the reports it was found that prior to ethanol or HgCl2 exposure, the explants were usually treated with 2–3 drops Teepol for 5–10 min [47] or 2 drops Tween-80 for 15–20 min [35], [44] or Tween-20 for 5 min [9] or 1% (v/v) Dettol for 10 min [37] as an alternative to Savlon solution. There are few other reports that used several other alternative surface sterilants. For example, Madhukar et al. [40] used 1% (w/v) Bavistine® solution for 10 min prior to the treatment with Savlon, ethanol and HgCl2. Use of 3% (v/v) H2O2 treatment for 2 min, before HgCl2 exposure was reported by Panigrahi et al. [10]. In a unique approach, Abhyankar and Reddy [8] used Geneticin solution after treating with HgCl2 to make the explants free from any contamination.
4.3. Multiple shoot formation
Following the collection, surface sterilization and preparation, the explants undergo processing for optimization of in vitro regeneration protocol via standardization of type and formulation of basal media, vitamins, carbohydrates, levels of solidifying agent, pH and plant growth regulators (PGRs). Influence of these factors on micropropagation of A. vasica has been summarized in Table 1. For multiple shoot initiation and subsequent proliferation (Fig. 1a and b), full strength Murashige and Skoog [48] (MS) medium was the only choice as found in all the published reports on A. vasica. Supplementation of PGRs in MS medium significantly varied, as displayed by the reports on shoot multiplication. In several reports, combination of cytokinin and auxin were preferred. For instance, 0.5–2 mg/l N6-benzyladenine (BA) was used as cytokinin in combination with 0.05–0.2 mg/l α-napthalene acetic acid (NAA), which was used as auxin [9], [32], [35], [49]. As an additional cytokinin source, 1 mg/l 6-furfurylaminopurine (Kinetin or Kn) was used along with equal concentration of BA for shoot multiplication [8]. Similarly, Roja et al. [36] used 1 mg/l supplementation of gibberellin A3 (GA3) with 1 mg/l BA to enhance the shoot multiplication frequency of A. vasica. Later on, Lone et al. [37] added 0.5 mg/l NAA and 0.5 mg/l thidiazuron (TDZ) with 2 mg/l BA to improve the regeneration efficiency of BA; wherein, 100% of explants produced the maximum (23.3) shoots/explant in 28 days. However, in contrast, there are several reports on the sole use of BA for initiation of high frequency multiple shoots, wherein very high concentrations of 10 mg/l [8], [34] or 22.20 µm [50]BA were used. As an outcome, 93.33% explants produced ∼11 shoots/explant, each 5.2 cm in length. Exceptionally, a very low level (1.1 mg/l) of BA alone induced multiple shoots in 6 days of culture and eventually produced 7.4 shoots of 7.2 cm length with 2.8 of leaves/shoot [10].

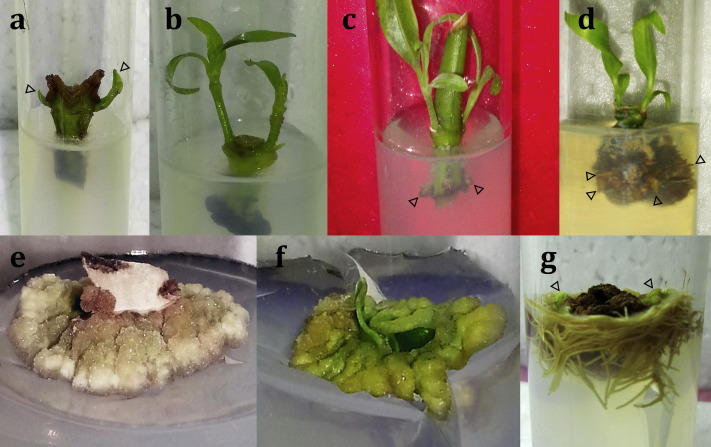
Fig. 1. Micropropagation of Adhatoda vasica: (a) Direct shoot initiation (arrows) from nodal segment explant after one week of inoculation in MS medium with 1.1 mg/l BA, (b) elongation and proliferation of initiated shoots at 10 days of culture, (c) multiple shoot proliferation and root initiation (arrows) in MS medium with 1 mg/l IBA and 0.25 mg/l NAA, (d) in vitro rooting in clump after 14 days of inoculation (arrows), (e) induction of friable calli in MS medium with 3 mg/l 2,4-D, (f) induction of organogenic calli in MS medium with 0.5 mg/l 2,4-D, (g) indirect rooting and shoot bud (arrows) initiation after 21 days. [Photographs are not in scale].
Source: Original and unpublished photographs from the experiments, carried out by the authors.