1. Introduction
Aquaculture has rapidly grown as a source of seafood worldwide [1,2]. In response to the decline in wild fisheries, aquaculture has grown further than other meat industries, such as livestock and poultry, which produce similar nutrients (e.g., protein, omega-3 fatty acids, iron, zinc, niacin, and B vitamins) [3]. Global aquaculture production has achieved a remarkable US$232 billion market value as of 2016 [2], and the aquafeed market will continue to grow exponentially to meet the increasing demand for seafood and changing lifestyles [1]. Fish oil and fishmeal are principal ingredients in aquafeeds because of their high content of long-chain polyunsaturated fatty acid, such as eicosapentaenoic acid, and high-quality protein [3]. The population of wild-harvested fish could decline when they are used to produce fishmeal [3].
Our reliance on fish-derived diets for aquaculture threatens marine biodiversity and food safety and security [4]. Owing to the increasing consumption of fish products worldwide, the proportion of fish processed into fishmeal is not likely to grow [3]. Given the considerable increase in global fish farming, the price of fishmeal has risen geometrically [3]. Oily fish are often used for direct human consumption. Hence, the amount of fish available for fish oil production is reduced and not likely to change in the near future [5].
Alternatives to fish-based feed are necessary because its excessive use cannot be sustained [6]. Fish grow well on diet consisting of plant-based ingredients, but this feed has several disadvantages, such as low feed quality, low digestibility, alteration in nutritional qualities, and insufficient vital elements such as lysine, threonine, and tryptophan [7,8]. A variety of plant-based lipidsare available, including vegetable, camelina, cottonseed, and soybean oils [4]. However, they do not provide enough polyunsaturated fatty acids (PUFAs) (omega 3-PUFA) to support high-quality fish production and growth [9,10]. Furthermore, many of these alternative sources lack adequate nutrients and contain antinutrients, which may alter the structure of the beneficial bacteria in the GIT of the host and negatively affect the metabolism [7].
Compared with fishmeal and fish oil, microalgae provide a more sustainable source of aquafeed [11], [12], [13], [14]. Fish diets can be produced using microalgal species in broth or whole dried samples of algal cells [15]. A variety of microalgae groups, including green algae, diatoms, and cyanobacteria, are used in aquaculture [3]. A mixture of these algae groups must be employed to provide a balanced dietary component [3]. The rich carotenoid pigments in microalgae give some fish species such as salmonids and shellfish a desirable color, and the inclusion of these pigments in fishmeal- or grain-based feeds increases the nutritional value [3,16]. Furthermore, algae-based feed improves fish health and provides nutritional benefits [3]. Microalgal species produce antimicrobial compounds that may be useful against a wide range of pathogens [17]. New technologies enable the engineering of algal species to produce designer chemicals, such as vaccines, growth hormones, or antibacterial agents, thus expanding the potential application of algae in feed production [18], [19], [20].
To play a role in sustainable aquaculture, microalgae strains must have many beneficial characteristics, such as simple cultivation, no toxic substances or biotoxins, quality nutrient composition, and food-grade cell walls for rapid nutrient absorption [21], [22], [23]. These characteristics are modulated by several factors, such as illumination (quality and quantity), nutrient availability/limitation, temperature, and salinity, which influences metabolite profile.
In this review, we describe the biochemical and nutritional attributes of economic microalgae used in aquaculture by focusing on high-value compounds.
2. Microalgal biomass as a source of functional aquafeeds
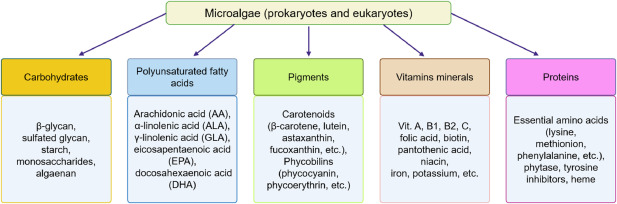
Microalgal biomass has health benefits beyond enriching nutrition. As a component of functional foods, it contribute toward optimal health and reduced health risks or disease prevention [24]. Many proteins, oils, carbohydrates, vitamins, carotenoids, and other nutrients are found in various species of microalgae [25], [26], [27] (Fig. 1). These bioactive ingredients offer a variety of health benefits to terrestrial and aquatic organisms. For instance, they protect against diseases, prevent nutrient deficiencies, and promote proper growth and development among aquaculture species [3,25,28].
 Fig. 1. Components of microalgal biomass rich in ingredients for aquaculture meal.
Fig. 1. Components of microalgal biomass rich in ingredients for aquaculture meal.3. Microalgal protein constituents
Compared with other protein sources, microalgal proteins have good quality and amino acid profiles, and thus, they could be a viable alternative to fishmeal protein [3,29]. An analysis of selected red algal groups (Porphyra dioica, Porphyra umbilicalis, and Gracilaria vermiculophylla) used in aquaculture reported their significant content of essential amino acids, mainly leucine (9.01–16.63 mg/g dry sample), valine (6.84–12.79 mg/g dry sample), and threonine (6.21–12.27 mg/g dry sample) [30]. Other studies evaluated the composition of microalgal biomass in terms of its chemical and elemental components [31], [32], [33] and indicated that microalgal protein can be incorporated into feeds. Existing evidence supports the use of biomass from Spirulina species, Chlorellaspecies, Scenedesmus species, Nanofrustulum species, and Tetraselmis suecica as fishmeal protein replacement [34] (Table 1).
Table 1. Analysis of the feeding of aquaculture with various microalgal cultures.
| Algal culture | Fish type | Percentage replacement of fish meal or fish oil or level of diet inclusion | Influence of microalgal- derived nutrition | References |
|---|---|---|---|---|
| Schizochytriumsp. | Shrimp | <5% addition in diet | Reasonable increase in specific growth rate but no impact on survival, digestive enzyme activities, or oil composition was observed | [40] |
| Schizochytriumsp. | Tilapia | 100% substitution of fish oil | Enhanced weight gain, feed conversion ratio, and protein efficiency ratio and no substantial survival rate improvement | [6] |
| Arthrospira platensis | Rainbow trout | 10% addition in diet | Considerable rise of red and white blood level, hemoglobin, total protein and albumin concentrations of fish and can be used as an immune booster in nutrition | [41] |
| Chlorella vulgaris | Freshwater prawn | 6%–8% substitution of fish meal | Improved growth rate, boosted immune response, and disease resistance | [42] |
| Arthrospira platensis | Red tilapia | Supplemented with >25% carotenoid diet | Enhanced fish color | [3] |
| Arthrospira sp. | Golden barb | 20% substitution of fish meal | Substantially improved fish growth | [43] |
| Arthrospira platensis | Ornamental carp | Supplemented with 7.5% carotenoid feed | Enhanced fish color | [44] |
| Dunaliella salina | Shrimp | 5%–10% addition in diet | Enhanced the immune system and antioxidant activities and boosted the survival level | [45] |
| Isochrysis sp. | European sea bass | ≤36% substitution of fish oil | Growth or feed intake relative to control is not negatively affected | [46] |
|
Nannochloropsissp. and Schizochytriumsp. |
Olive flounder | 100% substitution of fish oil | No adverse impact on growth, feed efficiency, or ingredient value | [47] |
| Schizochytriumsp. | Atlantic salmon | ≤5% substitution of fish oil |
Absence of toxicity, stress, inflammation, or any adverse impact of dietary inclusion; food-grade level |
[48] |
| Spirulina sp. | Rainbow trout | 7.5% substitution of fish meal | Noticeable strong weight gain | [49] |
|
Nannochloropsis gaditana, Tetraselmis chuii, and Phaeodactylum tricornutum |
Gilthead sea bream | 0.5%–1% addition in feed | Boosted immune system | [50] |
| Haematococcus pluvialis | Pacific white shrimp | 12.5% substitution of fish meal | Absence of adverse impact on shrimp function and develop shrimp coloration | [35] |
|
Nannochloropsissp. Pavlova viridis |
European sea bass | 100% substitution of fish oil | Absence of adverse impact on fish growth function and nutrient utilization | [51] |
| Phaeodactylum tricornutum | Atlantic salmon | 6% substitution of fish meal | Absence of adverse impact on growth, feed conversion of protein, lipid, energy, ash | [52] |
| Dunaliella sp. | Shrimp | Approximately 2% addition of microalgal carotenoid | Shrimps' survival rate rose | [53] |
| Scenedesmus almeriensis | Gilthead sea bream | 38% substitution of fish meal | Feed intake was unaltered | [54] |
|
Nannochloropsis salina Navicula sp. |
Juvenile red drum | Approximately 10% substitution of fish meal | Absence of observable negative impact on growth function | [36] |
| Arthrospira platensis | Nile tilapia | 0.5%–2% addition of microalgal biomass | Significantly enhanced the health status of fish and boosted antioxidant function | [55] |
| Arthrospira sp. | Tilapia | Up to 43% substation of fish meal | Absence of adverse effect on growth or feed intake; preferred FCR to corn–gluten diet standard | [56] |
|
Nanofrustulumsp. Tetraselmis sp. |
Atlantic salmon, common carp Pacific white shrimp |
Up to 10% substitution of fish meal | No significant difference in growth function/feed utilization compared fish meal diet | [57] |
| Nannochloropsissp. and Isochrysis sp. | Juvenile Atlantic cod | 15% substitution of fish meal | Enhance diet intake and growth in fish | [58] |
In addition to being rich in essential amino acids, microalgae without oil content serve as a potential supplement or feed for aquaculture [35]. For example, shrimp fed with diet containing 12.5% fishmeal protein replacement showed a higher growth rate and a lower feed conversion ratio than shrimp fed with control diet after a 2-month feeding test [36]. In another study, marine juvenile red drum fish (Sciaenops ocellatus) showed no significant growth reduction even when fed with lipid-extracted algae (Navicula sp. and Nannochloropsis salina) as a replacement for crude protein from fishmeal or soy protein concentrate [36] (Table 1). Similarly, fishmeal was replaced with microalgal biomass in a diet for juvenile Pacific white shrimp (Litopenaeus vannamei) [37]. Related data were collected for red cherry shrimp (Neocaridina davidi) fed with Spirulina platensis (8%–10%) diet [38]. As a source of alternative protein, microalgae are a viable option for fishmeal due to its nutritional composition and relative digestibility as long as feed intake is maintained. In one study, intensely compressed microalgal biomass improved the digestibility of Atlantic salmon [39]. The amount of microalgae meal included in aquafeed varies depending on the microalgae and aquaculture species (Table 1). The production of microalgal protein is generally beneficial for aquaculture development because it provides essential nutrients for growth, immune system, and overall health.
4. Microalgal essential oil
Microalgae contain PUFAs, including docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), α-linolenic acid (ALA), and arachidonic acid (AA), that are essential to fish development [11,23,59]. An analysis of several strains of microalgae underlined the importance of these essential PUFAs in the growth and productivity of aquaculture [60,61] (Table 2). The amount of EPA in most microalgal species ranges from 7% to 34% [61,62]. A relatively high amount of DHA (≥11%) can be found in prymnesiophytes (Pavlova sp., Tisochrysis sp., and cryptomonads), and <5% AA can be found in eustigmatophytes (Nannochloropsisspp.) and diatoms. C20 and C22 PUFAs are lacking in chlorophytes (Dunaliellaand Chlorella spp.), although some species contain EPA in modest amounts (<3.5%). PUFA deficiencies render chlorophytes nutritionally inferior and unsuitable as sole food sources [63]. Compared with commercial oils, PUFAs enrich zooplankton with DHA [64] and produce a DHA-to-EPA ratio of 1:2, which is suitable for feeding fish larvae [63]. When EPA and AA uptake is balanced, eicosanoid dysfunctions can be prevented and many metabolic disorders can be treated [65]. Bacillariophyceae (diatoms), Chlorophyceae, Chrysophyceae, Cryptophyceae, Eustigmatophyceae, and Prasinophyceae all contain EPA [15]. EPA derived from Nannochloropsis sp. is widely used in aquaculture nutrition [66]. Porphyridium purpureum also contains high levels of EPA [67].
Table 2. Nutrient-rich microalgal polyunsaturated fatty acids [60,69].
| Polyunsaturated fatty acids | Microalgal groups | Uses | Recommended daily allowance (kg) |
|---|---|---|---|
|
α-linolenic acid (ALA) |
Chlorella vulgaris |
Dietary supplement (single-cell oil) |
1–2 |
|
Eicosapentaenoic acid (EPA) |
Nannochloropsis oculata, Phaeodactylum tricornutum, Monodus subterraneus, Isochrysis galbana |
Dietary supplement, therapeutic medicine, brain development in children, heart disease |
0.25–0.5 |
|
Docosahexaenoic acid (DHA) |
Schizochytrium limacinum, Crypthecodinium cohnii, Pavlova lutheri |
Dietary supplement, essential for brain and eye development in fetus and children, crucial for healthy heart, adult dietary supplements in food |
0.25–0.5 |
|
γ-linolenic acid (GLA) |
Arthrospira platensis |
Dietary supplements, anti-inflammatory, autoimmune diseases |
0.5–0.75 |
|
Arachidonic acid (AA) |
Porphyridium cruentum, Mortierella alpina, Parietochloris incisa |
Food supplements, pain reliever, muscle formulations that have anabolic properties (for sports lover) |
0.05–0.25 |
Researchers fed fish and shrimp with diets rich in these fatty acids and found that dietary DHA is essential for marine fish and shrimp [30]. Phosphoglyceride-rich biological membranes have a bilayer thickness and phase structure that remain relatively constant despite changes in environmental variables, such as pressure, temperature, and salinity. In natural PUFAs, methylene double bonds occur in the cis position and additional double bonds are found in the cis faction [23,64]. Shrimp require dietary docosahexae DHA as a part of the vitellogenic process and as precursors for enzymatic and hormonal reactions [68]. A highly mobile and flexible energy source, such as the one provided by PUFA, is essential for the production of ecdysone for egg production, growth, and molting [68]. A dietary DHA:dietary EPA ratio of approximately 2:1 is generally found in fish phosphoglycerides [68]. Dinoflagellates contain a large amount of dietary DHA, and diatoms contain a large amount of dietary EPA but a negligible amount of dietary DHA [68]. The vast majority of hatcheries do not culture microalgae species with high PUFA levels as a source of dietary DHA and an alternative source of PUFAs for shrimp (and other marine eukaryotes) diets [11]. In addition to being energy reservoirs, microalgal oils (such as traditional lipids) play an important role in membrane synthesis and cell membrane structural component by acting as an information-sharing center [30].
5. Microalgal polysaccharides
Carbohydrates with nutritional and pharmaceutical values are significant components of microalgae [25,70]. The soluble fiber beta-1–3-glucan found in Chlorella sp. is an antioxidant that helps in reducing blood cholesterol levels[15,26,71]. Porphyridium cruentum, a red unicellular alga, can produce sulfated galactan exopolysaccharide, a possible alternative for carrageenan in food and dairy industries [72]. A number of species-dependent monosaccharides, including xylose, mannose, glucose, galactose, and rhamnose, can be obtained from microalgal polysaccharides [73]. Glucose dominates the carbohydrates of several green algae species, comprising 47%–85% of their total carbohydrates [73] (Table 3).
Table 3. Diversity of microalgae species in terms of biochemical composition [74].
| Microalgae | Protein (%) | Lipids (%) | Carbohydrates (%) |
|---|---|---|---|
| Anabaena cylindrical | 43–56 | 4–7 | 25–30 |
| Aphanizomenonflos-aquae | 62 | 3 | 23 |
| Chaetoceros calcitrans | 36 | 15 | 27 |
| Chlamydomonas reinhardtii | 48 | 21 | 17 |
| Chlorella vulgaris | 51–58 | 14–22 | 12–17 |
| Chlorella pyrenoidosa | 57 | 2 | 26 |
| Diacronema vlkianum | 57 | 6 | 32 |
| Dunaliella salina | 57 | 6 | 32 |
| Dunaliella bioculata | 49 | 8 | 4 |
| Euglena gracilis | 39–61 | 22–38 | 14–18 |
| Haematococcus pluvialis | 48 | 15 | 27 |
| Isochrysis galbana | 50–56 | 12–14 | 10–17 |
| Porphyridium cruentum | 28–39 | 9–14 | 40–57 |
| Prymnesium parvum | 28–45 | 22–38 | 25–33 |
| Scenedesmus obliquus | 50–56 | 12–14 | 10–17 |
| Scenedesmus dimorphus | 8–18 | 16–40 | 21–52 |
| Scenedesmus quadricauda | 47 | 1.9 | 21–52 |
| Spirogyra sp. | 6–20 | 11–21 | 33–64 |
| Spirulina maxima | 60–71 | 6–7 | 13–16 |
| Spirulina platensis | 46–63 | 4–9 | 8–14 |
| Synechococcus sp. | 63 | 11 | 15 |
| Tetraselmis maculata | 52 | 3 | 15 |
6. Microalgal carotenoid pigments
Microalgae species produce carotenoid pigments in different quantities and compositions, and their production ability is affected by their culture parameters [75], [76], [77], [78]. Fish, such as crustaceans, salmonids, and other farmed fish, consume these compounds through diets to enhance their coloration [35]. Carotenoids are also metabolized for normal bodily functions [79]. As antioxidants, they can neutralize free radicals and reactive oxygen species in humans upon dietary consumption [79], thus reducing the risk of inflammation, heart disease, type 2 diabetes, and cancer; enhancing vision; and preventing neuron damage [79]. As a result, carotenoids have many applications and are commercially relevant. In addition, the utmost importance placed on consumer health explains why these pigments are produced at such large scales.
6.1. Lutein
Lutein is one of the carotenoid molecules naturally found in microalgae. Human serum and food are rich in this pigment [80]. In addition to its use for pigmenting animal tissues and products, lutein serves as a natural colorant for food, pharmaceuticals, and beauty products. It slows down chronic diseases, stimulates the immune system, prevents cataracts and early atherosclerosis, and impedes blindness or loss of vision caused by age-related macular degeneration [81], [82], [83]. In 2004, lutein accounted for US$139 million in global sales and was considered the fastest growing carotenoid [84]. Currently, the most widely used source for lutein production is the French marigold(Tagetes patula) [85]. Aztec marigold and Tagetes, both containing lutein, have been commercialized in the USA. Marjoram (Tagetes patula L.) produces lutein in three forms (marjoram orange, marjoram yellow, and marjoram red). Although marigold plantations occupy a large portion of land, the climate and seasons can easily affect their performance [83]. Dietary carotenoids have recently attracted considerable attention because of their antioxidant properties and potential role in the prevention of some chronic diseases caused by free radicals [86] owing to their ability to quench singlet oxygen damage. Hence, algae-derived carotenoids could be a useful natural resource for the development of functional ingredients [86] in aquaculture to enhance the immune system. Chlorella, Scenedesmus, and Muriellopsis species are also important sources of lutein from chlorophycean microalgae [83,84]. Heterotrophically grown Chlorella sp. can produce significant amounts of lutein [87].
6.2. Astaxanthin
The natural pigment astaxanthin has long been added as a source of color in cultured salmon, sea bream, and ornamental fish [88]. Natural astaxanthin from microalgae can be considered a safe pigment for aquaculture because its usage up to the maximum allowed dietary level for salmon and trout poses no threat to the safety of consumers [88,89]. Fish culture relies on pigmentation for the growth of ornamental fish and salmon. The sensory panel and consumers prefer salmon meat with pink colorings that contain a high amount of astaxanthin [90,91]. Moreover, the market value of ornamental fish is partly determined by the color of their skin or muscle. Fish cannot synthesize pigments on their own; instead, they absorb pigments from diets. Microalgae containing astaxanthin are widely used as feed supplements to enhance fish pigmentation, particularly in salmon, trout, sea bream, and ornamental species [92,93]. Salmon, trout, and sea bream are excellent sources of nutrition because they are rich in protein, lipids, natural pigments, and minerals [88,90] and are marketed according to the pigmentation of their skin and muscles [88].
Salmon pigmentation depends on the quantity and source of astaxanthin [90]. When the astaxanthin concentration in the diet of rainbow trout was increased up to 27.6 mg/L, the total carotenoids in muscle and chroma values also increased [94].
The pigmentation of ornamental fish, such as koi carps, goldfish, and cherry barbs, can be enhanced using astaxanthin [95], [96], [97]. When the astaxanthin contents were 0 and 100 mg/kg in goldfish diet, the pigmentation scores were 3.40 and 6.67, respectively, indicating that astaxanthin improves the pigmentation of ornamental fish [97]. Goldfish fed with diet containing Chlorella vulgaris and Haematococcus lacustris had more red texture and yellow coloration than the control fish [96]. In addition, microalgal biomass outperforms synthetic astaxanthin in pigmenting ornamental fish [96].
Adding natural astaxanthin to ornamental fish diets can influence pigmentation and color-based behavior. One study found that astaxanthin added to fish diets effectively reduces mirror-image aggression and changes the behavior of cherry barbs when choosing mates [80]. Thus, astaxanthin from microalgae can be used in the pigmentation and behavioral regulation of ornamental fish.
Aquatic animals could easily gain weight when they consume certain amounts of astaxanthin. One report stated an improvement in the specific growth rate of rainbow trout from 4.27% to 5.34% when the amount of dietary astaxanthin was increased from 0 to 33.3 mg/kg [93]. When the amount of astaxanthin added to a discus fish culture diet was increased from 0 to 50 mg/kg, a slight increase in body weight was observed [98]. Consequently, astaxanthin-rich microalgae can be added to fish diets to promote fish growth in aquaculture. Some microalgae contain astaxanthin, essential amino acids, unsaturated fatty acids, and polysaccharides [99], [100], [101]. Along with astaxanthin, these value-added compositions should contribute to successful fish rearing.
Excessive amounts of H. lacustris in the diet of fish may have adverse effects on their growth. When over 25% of defatted Haematococcus was added to the diet of juvenile yellow perch, the fish grew slower than expected [102]. Therefore, the astaxanthin content in fish diet should be limited to a reasonable range and optimized on a case-by-case basis to avoid adverse effects on fish growth [103]. Using natural astaxanthin from microalgae is a better choice than synthetic astaxanthin to promote the accumulation of astaxanthin in fish skin and muscle in aquaculture.
6.3. β-carotene
Approximately 35% of total revenues (US$432.2 million) in 2015 were generated by algal beta-carotene [104]. A number of companies have introduced synthetically produced carotenoids to meet the limited supply of natural sources and the high demand of the global market. Beta-carotene (provitamin A) is one of the few carotenoid compounds that have the potential to transform into vitamin A (retinol) [105]. Several strains of microalgae genus Dunaliella can produce and accumulate large amounts of beta-carotene within their cells [106,107]. Dunaliella sp. is commonly found in oceans, brine lakes, salt marshes, salt lagoons, and salt water ditches near the sea with high salinity levels and high magnesium concentration. Among eukaryotes, Dunaliella sp. is the most salt tolerant and can withstand salt concentrations as low as 0.1 M and as high as 4 M [108]. An all-trans and a 9-cis form of beta-carotene was found in unicellular halotolerant alga Dunaliella bardawil [105]. Beta-carotene contains a chemical analogue of vitamin A that can be applied topically to treat skin cancer [109]. Daily doses of Dunaliella sp. beta-carotene can protect against exercise-induced asthma because of its antioxidative properties [110].
Natural beta-carotene, a provitamin A compound, is used in animal feeds for poultry and shrimp (aquaculture) [59,108].
6.4. Zeaxanthin
Xanthophyll pigment zeaxanthin appears in the eyes and skin of humans [111]. In conjunction with lutein, zeaxanthin accumulates as a macular pigment in the cornea to shield the retina from blue light and enhance the sight [112]. Zeaxanthin is also effective as an anti-inflammatory, antioxidant, and neuroprotector [113]. Despite their relatively low zeaxanthin contents of 16.3, 16.7, and 24.9 mg/g, raw and cooked scallions, orange peppers, and corn, respectively, are still good choices for the dietary intake of this pigment [114,115].
The zeaxanthin content in various kinds of marigold petals varies between 10 and 300 g/g [116]. This pigment is in high demand due to the growing number of age-related macular degeneration sufferers worldwide [113]. Zeaxanthin production by Chlorella ellipsoidea is ninefold that by red pepper (4.26 mg/g DW) [117]. Moreover, microalgae-derived zeaxanthin partly exists in free form as opposed to the mono- or diesters found in floral and fruit compounds. As a result, microalgae are productive and bioavailable sources of zeaxanthin.
In combination with N-starvation, C. zofingiensis bkt1 accumulates 7.00 ± 0.82 mg/g DW when exposed to high light. However, high light is not always preferred for zeaxanthin accumulation by microalgae. Under constant low light (100 mol photons m−2 s−1), D. salina zea1 accumulates 5.9 mg/g DW of zeaxanthin, which is higher than its 4.18 mg/g DW accumulation under high light [118].
6.5. Fucoxanthin
Fucoxanthin accounts for a relatively large portion of total carotenoid production [119]. Its global production amounted to approximately 500 tons in 2016, and it is expected to annually grow by 5.3% [120]. This pigment has a unique molecular structure with an allenic bond, a carbonyl group conjugated to a monoepoxide molecule, and an acetyl group. The antioxidant fucoxanthin and its derivatives have anti-cancer, anti-inflammatory, and anti-obesity properties [121,122].
More than 20,000 species of algae are rich in fucoxanthin, especially the members of family Synurophyceae (up to 26.6 mg/g DW), diatom class (up to 21.67 mg/g DW), and Prymnesiophyceae (up to 18.23 mg/g DW) [123]. Isochrysiszhanjiangensis can produce 23.29 mg/g DW of fucoxanthin under low light and can convert carbon skeletons into carotenoids, especially fucoxanthin [124]. In a nitrogen-replete medium under low light, Odontella aurita can accumulate 18.47 mg/g DW fucoxanthin [125]. Furthermore, the purified fucoxanthin from O. aurita is (all-E)-fucoxanthin, which has a strong antioxidant ability and high bioavailability. Tisochrysis lutea exhibits the highest fucoxanthin productivity (9.81 mg/L/day) [126].
7. Dietary minerals and vitamins
Microalgal biomass shows potential in improving aquaculture diets and supplying essential vitamins [127]. The well-being of an organism depends on various vitamins, including vitamin A (a retinoid antioxidant); B vitamins such thiamine (B1), niacin (B2), nicotinate (B3), pantothenic acid (B5), pyridoxepin (B6), biotin (B7), folic acid (B9), and cobalamin (B12); and micronutrients (sodium, potassium, calcium, magnesium, and iron) [128], [129], [130]. Microalgae naturally accumulate vitamins and minerals, which make them easier to digest compared with artificial foods [131]. Vitamin synthesis is dependent on several factors, such as strain, amount of light, culture medium composition, and growth phase. High amounts of thiamine, pyridoxine, nicotinic acid, and pantothenic acid were found in Tetraselmis sp. culture, and elevated levels of riboflavin, cobalamin, and β-carotene were observed in Dunaliella under culture conditions. Selenium (Se) reduces free radical production, improves immunity, and protects against disease in humans and fish [132]. This element can be found in microalga Nannochloropsis oculata, which received particular interest in aquaculture nutrition [132]. Zinc is another essential element that can be obtained from the protein-rich microalga Spirulina [133]. In addition to being an antioxidant, zinc contributes to nutrient digestion and enzyme activity, improves plasma membranes, and plays a role in transcriptional elements that facilitate nucleic acid metabolism and cell division [133]. In the fight against reactive oxygen species, iodide is one of the powerful and ancient compounds [134]. Among its sources is Tisochrysis lutea (microalga). This antioxidant provides electrons to halo-peroxidase enzymes to rapidly and nonenzymatically stop the H2O2 reaction. Most algae contain iodomethane (CH3I) or its derivatives as their source of iodine [134]. These minerals serve as a key component of enzymes, vitamins, hormones, bioactive compounds, and enzyme activators and a cofactor that promotes metabolism in animals, including Penaeus monodon [135]. Gills and body surfaces of shrimp can directly absorb minerals from the surrounding water.
8. β−1,3-glucan
In response to pathogen surface molecules, β−1,3-glucan initiates host defense reactions [59]. Apart from oil, beta-1,3-glucan is the most important component of Chlorella sp. This species generates more than US$38 billion in global sales annually [136]. In 1970, many studies on β−1,3-glucan were conducted in Japan. β−1,3-glucan is essential to our health. In one study, feeding immune-stimulating β−1,3-glucan to healthy fish increased their nonspecific and specific immunity levels and protection against bacterial infection compared with the controls [137]. As a prophylactic treatment, β−1,3-glucan effectively reduced anthrax infection in mice and showed potential to inhibit the growth of cancer cells in vivo by stimulating three cytokines in the body [138]. Furthermore, β−1,3-glucans have positive effects in patients who underwent cardiopulmonary bypass, inhibit antiviral activity in HIV-infected patients, and are routinely used in patients undergoing immunotherapy [139]. Lipopolysaccharide and β−1,3-glucan binding protein interact with phenoloxidase to enhance its activity in shrimp [140].
9. Improving immunity and intestinal functions of fish
Several studies on fish and some farm animals fed with microalgae produced new information regarding growth, performance, gut function, and microbiome[3,11,129,141]. Microalgae-based diets can enhance immunity and can act as a powerful antioxidant. Diets containing Euglena viridis powder (up to 2%) intensified various immune responses, such as superoxide anion production and serum bactericidal activity, in Rohu fish (Labeo rohita) [142]. Euglena viridisshowed an increased resistance to Aeromonas hydrophila, a bacterial pathogen. Spirulina platensis-rich feedings enhanced the phagocytic and lysozymeactivation and A. hydrophila resistance of Nile tilapia (Oreochromis niloticus) [143]. A significant improvement in immune parameters, including total hemocyte count, phenoloxidase activity, and superoxide dismutase (SOD) activity, was observed in Pacific white shrimp raised on diets with thraustochytrid meals rich in DHA and AA [144]. Compared with that in the control group, survival was increased in shrimp fed with thraustochytrid-meal-supplemented diets containing Vibrio harveyi. The immune response of freshwater prawns (Macrobrachium rosenbergii) raised on larval diets containing Chlorella vulgaris (2%–8%) was assessed by introducing the bacterial pathogen A. hydrophila. An increase in prophenol oxidase activity and total hemocyte counts was observed in the prawns fed with C. vulgaris diets. Meanwhile, the prawns exposed to A. hydrophila and fed with C. vulgaris diets had a higher survival rate than their controls [45]. A separate experiment on gilthead sea bream (Sparus aurata) and Pacific red snapper (Lutjanus peru) revealed significant improvements in their antioxidant capabilities and immune response after the inclusion of Navicula sp. with Lactobacillus sakei. However, whether Navicula sp. alone is responsible for the improvement remains unclear [145,146]. A dietary inclusion of Schizochytrium sp. (3%) improved the growth performance and nonspecific immunity of golden pompano, but it did not affect its antioxidant capacity at either the transcriptional level or enzymatic activity [147]. These results indicated that the microalgae-mediated immune response varies based on the recipient species because the benefits are not consistent across species [147]. The addition of two microalgae species to a diet of European sea bass(Dicentrarchus labrax) enhanced the nonspecific immune responses and had no adverse effects on gut digestion and absorption [148]. In addition to the antibacterial effects, microalgae supplementation of live cells and pellet feed can inhibit white spot syndrome virus (WSSV), a pathogen that represents a significant economic burden in shrimp farming. Microalga D. salina (0.5%–2%), an important source of antioxidant β-carotene, acted as a possible prophylactic agent against WSSV in the infected shrimp group. The D. salina-fed shrimp had strong antioxidant activity and induction of immunological markers, such as prophenoloxidase (ProPO), SOD, and catalase (CAT), compared with the control group. However, the mechanism underlying the antiviral activity of D. salinaremains unclear. Madhumathi et al. [48] investigated the effects of the consumption of Nannochloropsis gaditana, Tetraselmis chuii, and Phaeodactylum tricornutum and its interaction with the immune-associated genes (EF-1α, IgMH, TCR-β, MHCIα, MHCIIα, CSF-1R, and β-defensin) and immune characteristics of gilthead sea bream. The treatment with N. gaditana and T. chuii increased hemolytic complement activity, phagocytosis, and expression of genes related to immunity, such as MHCIIα, CSF-1R, and β-defensin. However, a diet containing P. tricornutum did not exert much influence on gene expression but exhibited immunostimulating effects. A study on the effects of dietary supplementation with Haematococcus pluvialis (1–10 g/kg) on antioxidant activity and specific enzyme markers on rainbow trout found that 0.3% and 1% H. pluvialis administration increased the antioxidant system and aspartate aminotransferase activity, respectively, suggesting liver damage [149]. The effect of Spirulina platensis (0%–10%) on the hematopathological and serum biochemical parameters of rainbow trout was examined [41]. S. platensis-supplemented fish groups showed an increase in the levels of red blood cells, white blood cells, hemoglobin, and protein but not in hematocrit, cholesterol, triglyceride, or lactate levels. A similar experiment was conducted to investigate the effect of S. platensis meal (0%–10%) on the expression of antioxidant genes, SOD, CAT, and total antioxidant capacity of rainbow trout [150]. The fish groups administered with S. platensis showed significant increases in the expression of SOD and CAT genes. In addition, the antioxidant capacity increased significantly with the levels of S. platensis inclusion.
10. Opportunities for new microalgae products
As a part of the effort to promote the sustainable development of the aquaculture industry, modern feed ingredients that primarily come from the bottom of the food chain are essential [21], [22], [23]. As one of the pioneer organisms of the universe, photosynthetic microalgae can convert 1% of light energy received into chemical energy. As flexible organisms, microalgae grow in closed and open systems and require only basic nutrients and light to thrive. Aquaculture nutrition derived from microalgae may offer more competitive advantages compared with other sources in terms of cost, environmental impact, and potential resource reclamation and emission reduction. Several microalgae strains have recently been analyzed for bioenergy production potential. Aquaculture nutrition researchers have expressed a great deal of interest in the biochemical composition of microalgae. Their supply of essential amino acids, essential oil, vitamins, minerals, carotenoids, and other high-value molecules has been proven suitable for fish diets. A few countries such as Japan, Australia, China, and the United States can produce large quantities of microalgae, but they rely heavily on only a few species. The most commonly used microalga species are Spirulina spp., Chlorella spp., Haematococcus spp., diatom, and Dunaliella spp. (Table 4). The average cost of their dry biomass is estimated at US$150,000 per ton. Currently, the vast majority of this biomass is produced for human health food. Nevertheless, many producers expressed interest in expanding into the aquafeed market when production levels are increased and the price is lowered.