1. Introduction
Over the past several decades, a growing number of multifunctional nanomaterials such as liposomes, dendrimers, carbon nanotubes, gold and iron oxide nanoparticles, titanium dioxide, and MNS, have been designed and synthesized for their potential biomedical applications [[1], [2], [3], [4], [5]]. Among these nano-systems, MNS, a versatile material comprised of a honeycomb-like porous structure with hundreds of mesopores, has gained increasing attention due to its enormous advantages, such as high surface area (>1000 m2 g−1), large pore volume (0.5–2.5 cm3 g−1), tunable pore diameter (1.3–30 nm), high chemical and thermal stability as well as good biocompatibility and biodegradability [[6], [7], [8]].
Discovered in 1992, ordered mesoporous molecular sieves (MCM-41) were prepared by a “liquid-crystal templating” mechanism at the Mobil Research and Development Corporation [9]. This achievement has been regarded as a crucial breakthrough in material science that could lead to a wide variety of applications ranging from food processing through pharmaceutical technology [10, 11]. In 2001, Vallet-Regi and co-workers introduced a novel promising application of MCM-41, its capacity for significant encapsulation and controlled delivery of therapeutic compounds [12]. In this regard, they encapsulated ibuprofen (IB), a commonly used analgesic and anti-inflammatory drug, into two MCM-41 nanomaterials with different pore sizes, 2.5 and 1.8 nm, and thus the in vitro drug release test was performed under simulated body fluid. Results of this study indicated that approximately 30% of IB could be loaded into the MCM-41 and the prepared MCM-41 showed a slow and sustained release up to 3 days.
Compared to the conventional silica-based materials, MNS's unique structure allows them to effectively encapsulate drugs or bioactive agents and protect them from enzymatic degradation that induced by pH and temperature changes of the surrounding media. However, the loaded cargos would burst release and poorly dispersible from the unmodified MNS, caused by weak non-covalent interactions of hydrogen bonding. Moreover, Bharti, et al. also reported that the silanol groups of MNS could interact with the phospholipid layers of the red blood cell membranes, leading to hemolysis. Thereby, modification of MNS with appropriate functional groups, presenting attractive interactions with biological agents, may provide an effective control on bio-molecules amount and sustained release rate [13].
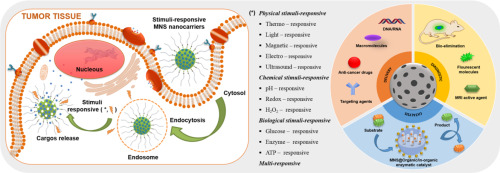
In this review, we intend to discuss the combination of different intelligent materials, leading to the development of multifunctionalized MNS that respond to the demand of physical stimuli (thermo, light, magnetic field, ultrasound, and electricity), chemical stimuli (pH, redox, H2O2), and biological stimuli (enzyme, glucose, and ATP), individual or in combination. Moreover, recent applications of multifunctionalized MNS, primarily focusing on drug and other therapeutic agents delivery, diagnostic imaging, and catalysis were also reviewed.
2. Multifunctionalized MNSs: from concept demonstration to multifunctionalization
The development of MNSs has experienced three generations, from the first-ever MNSs generation (i.e., MCM-41, SBA-15, etc.) which is the concept demonstration of MNS, to the second generation with nanosized pores and tunable composition that provide the standard for in vitro and in vivo evaluations, and finally is multifunctionalized MNSs generation [14, 15]. Unlike previous generations, interior pores and the exterior surface of multifunctionalized MNSs enable the conjugation of various functional groups and materials such as ligands, functional polymers or materials that possess unique physicochemical properties, etc. owning to the abundant presence of silanol groups (Si-OH) on the outer and inner surface. Therefore, multifunctionalized MNSs not only prevent the premature release of encapsulated cargo but also promote the targeted and controlled transport under specific stimulation [16, 17]. Also, a combination of MNSs with magnetic nanoparticles, fluorescent agents, quantum dots, nanocrystals, etc. would further broaden the versatility of multifunctionalized MNSs, satisfying multiple requirements of theranostic and other applications [17, 18].
Recently, many efforts have been made for the development of multifunctionalized MNSs, among those, MNSs that are modified for the demand response to specific stimuli, obtaining targeted and controlled MNS-based delivery system are intensively researched (Fig. 1). In the following sections, multifunctionality of modified MNSs that selectively respond to physical stimuli (thermo, light, magnetic field, ultrasound and electricity), chemical stimuli (pH, redox, H2O2), biological stimuli (enzyme, glucose, and ATP), and multi-stimuli (i.e. pH-redox, pH-thermo, light-pH, magnetic-thermo, etc.) will be discussed in more detail.
 Fig. 1. Advantages of the functionalized MNS-based delivery system over traditional drug administration. Stimuli-responsibility allows MNS carriers to unload their cargo in a site-specific manner, preferably intracellularly.
Fig. 1. Advantages of the functionalized MNS-based delivery system over traditional drug administration. Stimuli-responsibility allows MNS carriers to unload their cargo in a site-specific manner, preferably intracellularly.2.1. Physical stimuli-responsive multifunctionalized MNSs
2.1.1. Thermo-responsive multifunctionalized MNSs
Thermo-responsive, or temperature-sensitive, delivery systems are among the most highly investigated types of physical stimuli-responsive systems, thanks to their practical applications. In fact, thermo-responsive systems can be manipulated to function in physiological temperature and be sensitive to a tumor-targeted temperature range which involves hyperthermia (42 °C). Therefore, it is a substantially potential strategy in drug release. Since MNS itself is not essentially responsive to temperature changes, it is usually compositely coated or hybridized with other materials which manifest this characteristic [[19], [20], [21], [22], [23], [24], [25], [26]]. The coating materials provide the nanovalves to the pores of MNSs, switching to trap or release the loads accordingly to surrounding temperature. The most well-known polymer that has been extensively studied in composition with MNSs is poly N-isopropylacrylamide (PNIPAAm) [27]. In 2011, Chen et al. proposed a method of synthesizing thermos-responsive modified nanosilica combined with PNIPAAm using reversible addition-fragmentation chain transfer (RAFT) and Click chemistry. Silica nanoparticles with their surface modified with azide groups were Click reacted with alkyne-terminated PNIPAAm polymerized by RAFT procedure. The lower critical solution temperature (LCST) of PNIPAAm was established to be 32 °C, below which hydrogen bonds form and give the polymer its water solubility and surface activity, above which the hydrogen bonds break and cause a coil-to-globule transition of the polymer. These temperature-induced changes of the PNIPAAm while conjugated to the surface of MNSs could help control the drug release, as drug molecules could be blocked in MNSs pores when PNIPAAm becomes hydrophobic above LCST, then be released when PNIPAAm chains extend below LCST [28]. Also used PNIPAAm as the thermo-responsive component, Zou's research reported another method of grafting with direct surface-initiated photopolymerization. The method was described to be simple and facile, without the need of photo-initiator immobilization and additional initiator. Briefly, the process was carried out in two steps: thiol-functionalized silica particles synthesis, and NIPAAm photopolymerization at the particles' surface. The resulted nanocomposite particles showed well-defined thermo-sensitivity. At 22 °C, the PNIPAAm chains were in a random coil state, ideal for releasing cargo, while higher temperature (up to 38 °C), the chains collapsed and provided a suitable trap for drug loading. The behaviors of PNIPAAm were also found to be reversible when cycling the temperature between 22 and 38 °C [29].
Recently, another way of coating MNSs has been reported, which produces a kind of inorganic-organic hybrid nanoparticles with lipid bilayer, or liposomes. In a 2017 article, Zhang and co-workers produced a thermo-responsive mesoporous silica/lipid bilayer hybrid and tested for its drug release behavior with doxorubicin (DOX). The DOX-loaded MNSs was first prepared, then assembled with the support lipid bilayer (SLB) by sonication. The results confirmed MNSs were enveloped with SLB and produced spherical particles in nanoscale. Release test showed a 30–40% increase at 47 °C compared to 37 °C. The temperature-responsive manner was reasoned to result from the phase transition temperature (Tm) of SLB, beyond which the lipid bilayers are more permeable [30].
In general, thermo stimuli is the most exploited strategy among the physical stimuli. However, the concerns in fabricating novel thermo-responsive nanomaterials lie in the safety and sensitivity for the materials to be functional in a physiological environment.
2.1.2. Light-responsive multifunctionalized MNSs
Light has been recognized as attractive stimuli to trigger controlled drug release, thanks to its ability in remote stimulation with extremely high precision spatially and temporally, and its ease of control, with a broad range of parameters (wavelength, light intensity, exposure duration and size of the beam). Hence, the development of light-responsive systems has received high attention recently. Most photo-responsive systems make use of light-sensitive chromophores such as azobenzene (AB), spiropyran, or nitrobenzyl [[19], [20], [21], [22], 24, 32, 33]. ABs have been utilized for many photosensitive molecular nanomaterials, based on their reversible photoisomerization from trans to cis forms and vice versa by irradiation at 365 nm and 450 nm, respectively. The trans-isomer with rod-like and apolar shape can bind to both α- and β-cyclodextrin (CD) with high affinity, while the cis-isomer does not fit in either CD [34]. Ferris et al. exploited such characteristic in a 2014 paper when they prepared pseudorotaxane design from α-CD, and two new Abs originated from P-nitrobenzoic acid, which acted as stalks and caps when attached with MNSs. The nanoparticles were loaded with ethanoic ARS dye solution; then cargo was sealed by washing with water. Treating with UV light at 377 nm reversed the sealing by dissociating the AB and the β-CD, but the removal of light allowed recomplexation after cis-AB converted back to trans-AB and resealed the pores [35].
Same principle yet with another compound as the gatekeepers, He's research group (2012) reported a reversible MNS-based light-responsive system with thymine derivatives [36]. Gate closing/opening mechanism in this study was dependent on a photodimerization-cleavage cycle of thymine due to a different wavelength. At 365 nm UV light, cyclobutane dimer was formed and thus locked the pores and trapped the cargo inside. To release the guest molecules, UV light irradiation at a different wavelength of 240 nm was introduced, resulting in the photocleavage of cyclobutane dymer and subsequently opened the pores. Ru(bipy)32+ was used as a model guest molecules to test the efficiency of this system. The results suggested an excellent loading amount (53 μmol·g−1 MNS), controlled release pattern, and a good reversibility in light-responsive loading and release (Fig. 3).
Another approach in synthesizing light-responsive delivery system is to rely on light-induced cleavage of bonds. A 2008 report by Wu et al. proposed a MNS-based system with a photoactive o-nitrobenzyl bromide linkage. The authors synthesized silica nanoparticles functionalized with amino and methyl phosphonate groups, then covalently conjugated the photoactive o-nitrobenzyl bromide molecules with the amino groups. The TEM and absorption spectroscopy results showed an average diameter of 70 nm and confirmed the successful conjugation. To assess the light-responsive behavior of the particles, 5(6)-carboxytetramethylrhodamine (CTMR) was incorporated. No detectable release of CTMR was recorded in dark conditions, while the longer duration of UV light exposure resulted in more fluorescence intensity of the released CTMR [37].
Another strategy is to make use of photoresponsive linkers, which are able to change physicochemically under light irradiation. Various linkers have been established at different wavelengths, for example, 7-amino-coumarin derivative (at visible or NIR), S-coordinated Ru(bpy)2(PPh3)-moieties (at visible), and thioundecyl-tetraethylene-glycol-ester-o-nitrobenzylehtyl dimethyl ammonium bromide (TUNA) (at UV region). In 2010, Knezevic et al. reported a MNSs-based assembly with [Ru(bpy)2(PPh3)Cl]Cl molecules acted as caps for the nanopores. [Ru(bpy)2(PPh3)Cl]Cl can form coordination bonds with mercaptopropyl moieties on the surface of the MNSs and seal the cargo. Nitrogen adsorption/desorption measurements confirmed the successful capping of the mesopores with the [Ru] complex. Irradiation by visible light at 455 nm cleaved the bonds and successfully released the entrapped sulforhodamine 101 dye molecules and the capping [Ru] in the form of hydroxo-substituted complex ion [Ru]OH. An observed hypsochromic shift of Sr101 UV–Vis bands also confirmed that the dye molecules were entrapped inside the pores rather than adsorbed on MNSs surface [38].
Despite the common use of UV/visible light in drug delivery, its clinical application is hindered by the fact that regions with wavelength < 700 nm show low penetration depth (~10 mm), which means its application is limited to skin or external layers of organs. Moreover, UV light has been associated with risks of DNA damage [24]. Such drawback can be effectively countered by developing system responsive to longer wavelength NIR laser (700–1000 nm). NIR laser displays some desirable characteristics, such as lower scattering properties, deeper penetration, and fewer risks for clinical application. NIR-absorbing materials, like gold [39] and CuS single-walled carbon nanotubes (SWNTs), can convert photon energy received to thermal energy and even induce hyperthermia, which is greatly beneficial in cancer treatment since hyperthermia therapy has been recognized as effective in killing cancer cells. Yang et al. reported a novel NIR-stimulus system based on gold nanocages as photothermal cores (Au-nanocage) and thermo-responsive PNIPAAm-gated MNSs as supporters to improve the loading capacity for anticancer drugs (MNSs@PNIPAAm), Au-nanocage@MNSs@PNIAAm. Internal heating by the gold nanocages upon treating with NIR light triggered the collapse of the PNIPAAm shell and thereby released the DOX drug entrapped. The system was evaluated by its in vitro drug loading and release and its cytotoxicity assay. The results showed a 23.5 wt% drug loading capacity, and an NIR-triggered burst release at both pH 7.4 and 5.0. The release rate was observed to slow down again after stopping the stimulus. Cytotoxic assay with HeLa cells revealed lower cell viability for Carrier + DOX. With NIR irradiation, the cell killing efficacy Carrier + DOX was remarkably higher (80.1%) than without NIR irradiation (14.5%), or with photothermal therapy alone (19.4%) [39].
2.1.3. Magnetic-responsive multifunctionalized MNSs
Magnetically guided and responsive strategies in delivery show great potential in improving therapeutic profile of drugs and biologically active molecules since they can help distribute the carriers toward target site and then trigger release, as well as hamper off-target interactions [[19], [20], [21]]. Aside from usual concerns about biocompatibility and biodistribution, magnetic delivery system must be magnetized with ferrimagnetic or magnetite [40, 41]. In a paper published in 2005, Giri and co-workers reported the synthesis of a superparamagnetic iron oxide nanoparticle-capped, MCM-41 type mesoporous silica nanorod-based controlled-released delivery system (Magnet-MNS) and assessed the magnetic sensitivity by using fluorescein as encapsulated molecules. The MNS was functionalized by 3-(propyldisulfanyl)propionic acid to later be covalently bonded with the 3-aminopropylsiloxy-derivatized superparamagnetic iron oxide (APTS-Fe3O4) nanoparticles, after loading the 3 mm mesopores with fluorescein molecules. Various spectroscopic tests results proved the successful incorporation of APTS-Fe3O4 to the MNS matrix, and the magnetically responsive behavior of the system was also shown. However, the magnetic field only worked as a directional guide to this system, which means the release of entrapped cargo was not triggered magnetically, but rather chemically [42]. In 2010, Chen et al. reported a novel nanocarrier (MNS@Fe3O4), constructed by chemically capping MNSs with monodispersed Fe3O4nanoparticles (Fig. 4). The superparamagnetic Fe3O4 nanoparticles were functionalized with meso-2,3-dimercaptosuccinic acid (DMSA-Fe3O4nanoparticles), then chemically bound by amidation with amine-MNSs. Without stimulation with a magnetic field, only few drugs could be released from the MNS@Fe3O4, which was negligible. Under magnetic stimulus, the breaking of chemical bonds occurred, Fe3O4 nanoparticles were freed from the MNSs surface, resulting in burst release of drugs, which could be well-controlled by changing the strength and exposure duration [43].
Hyperthermia induced by the magnetic field is also an important mechanism in triggering drug release. In fact, magnetic MNSs have been extensively studied as a multifunctional platform for both drug delivery and hyperthermia therapy [44, 45]. The magnetic particles incorporated in the MNSs are able to generate thermal energy under alternative magnetic field [46]. Thomas et al. incorporated zinc-doped iron oxide nanocrystals (ZnNCs) into a surface-modified mesoporous silica framework to produce a non-invasive remote-controlled delivery system. The authors first prepared magnetic-core silica nanoparticles by forming mesostructured nanoparticles around the stabilized ZnNCs, then finished with assembling the cucurbit[6]uril nanovalves and cargo loading. Under the application of an alternative magnetic field, ZnNCs locally heated up and triggered the valve opening, releasing the entrapped molecules [47].
Ruiz-Hernández and his group developed a controlled delivery system with iron oxide superparamagnetic nanocrystals loaded MNSs gated by double-stranded DNA (Fig. 2). The report showed that after both DNA strands were hybridized, the pores of the magnetic silica particles were sufficiently capped. Thermal energy generated by magnetic particles under an alternating magnetic field of 24 kA m−1 and 100 kHz melted the double-stranded DNA, uncapping the pores and releasing the cargo. The assembly and disassembly of the DNA strands were reversible, providing an ‘on-and-off’ release mechanism [31].
 Fig. 2. Representation of another thermo-sensitive MNSs utilizing double-stranded DNA (15 bp, Tm = 47.3 °C) and magnetic nanoparticles. As temperature rises above Tm due to an applied magnetic field, the DNA double strands melted, opening the gate for cargo release [31].
Fig. 2. Representation of another thermo-sensitive MNSs utilizing double-stranded DNA (15 bp, Tm = 47.3 °C) and magnetic nanoparticles. As temperature rises above Tm due to an applied magnetic field, the DNA double strands melted, opening the gate for cargo release [31]. Fig. 3. Representation of the light-responsive reversible MNS system. The light-responsive release mechanism of the system is based on the photodimerization and photocleavage of thymine modified on MNS [36].
Fig. 3. Representation of the light-responsive reversible MNS system. The light-responsive release mechanism of the system is based on the photodimerization and photocleavage of thymine modified on MNS [36]. Fig. 4. Schematic structure and drug release under magnetic stimulus of MNS@Fe3O4 nanoparticles as caps [43].
Fig. 4. Schematic structure and drug release under magnetic stimulus of MNS@Fe3O4 nanoparticles as caps [43]. Fig. 5. Schematic release of MNS based on thermal effects of ultrasound. Copolymer (red) of thermo-sensitive and ultrasound-sensitive moieties create an ultrasound triggered release strategy [49]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5. Schematic release of MNS based on thermal effects of ultrasound. Copolymer (red) of thermo-sensitive and ultrasound-sensitive moieties create an ultrasound triggered release strategy [49]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.) Fig. 6. Sol-gel method for synthesis of MNSs.
Fig. 6. Sol-gel method for synthesis of MNSs. Fig. 7. Illustration of pH-triggered release of DOX from MNS-GPTMS-Hydrazine [56].
Fig. 7. Illustration of pH-triggered release of DOX from MNS-GPTMS-Hydrazine [56]. Fig. 8. Schematic demonstration of the redox-responsive DOX/MNS-SS-ADA@CD-HEP-mPEG nanoparticles formation steps and redox-triggered release of model drug DOX by breaking the disulfide bonds [6].
Fig. 8. Schematic demonstration of the redox-responsive DOX/MNS-SS-ADA@CD-HEP-mPEG nanoparticles formation steps and redox-triggered release of model drug DOX by breaking the disulfide bonds [6]. Fig. 9. Illustration of a MNS-based H2O2 responsive system for controlled release of metal chelators. Arylboronicacid functionalized MNS were capped with human IgG via boronate ester bond. The presence of H2O2 oxidized the erylboronic esters, releasing IgG cap and guest molecules clioquinol, which helped chelate Cu2+ and inhibit H2O2 production by Aβ plaques [66].
Fig. 9. Illustration of a MNS-based H2O2 responsive system for controlled release of metal chelators. Arylboronicacid functionalized MNS were capped with human IgG via boronate ester bond. The presence of H2O2 oxidized the erylboronic esters, releasing IgG cap and guest molecules clioquinol, which helped chelate Cu2+ and inhibit H2O2 production by Aβ plaques [66]. Fig. 10. Schematic illustration of the functionalization MNS-based platform and its enzyme-responsive release [74].
Fig. 10. Schematic illustration of the functionalization MNS-based platform and its enzyme-responsive release [74]. Fig. 11. Schematic controlled release from glucose-responsive MNS-based system with cyclic AMP loaded in-pore and gluconic acid-modified insulin functioning as both caps and delivered bioactive molecules. Glucose introduced to the system would compete for boronic acid on MNS surface, resulting in release of G-Ins and cAMP [77].
Fig. 11. Schematic controlled release from glucose-responsive MNS-based system with cyclic AMP loaded in-pore and gluconic acid-modified insulin functioning as both caps and delivered bioactive molecules. Glucose introduced to the system would compete for boronic acid on MNS surface, resulting in release of G-Ins and cAMP [77].