1. Introduction
Cancer is the major life threatening therapeutic consequence in the modern world as evidenced by estimates of World Health Organization in 2011 [1,2]. Mortality rate in global population due to cancer has crossed the number of deaths caused by morbid cardiovascular complications [1]. Adaptation of modern sedentary unhealthy life style, toxicants exposure untreated or ignored infections and use of immune-suppressant drugs etc. are assumed to be few of the underlying causes of this dangerous disease [3]. Surgery and/or radiation followed by compulsory use of chemotherapeutic agents are the basic/core treatments for healing cancer [4,5]. However, severe toxicities of the chemotherapeutic agents have brought challenge to the formulation scientists to deliver desired chemotherapeutics to the site of action in a therapeutic concentration with reduced toxicities towards healthy tissues.
PTX is a semisynthetic plant alkaloid, which is isolated from the bark of North American pacific yew tree, Taxus bravifolia and has been approved as chemotherapeutic agent by Food and Drug Administration. It blocks the mitotic pathway of cell division through stabilization of microtubule and triggers apoptosis/cell death [6,7]. It is an important chemotherapeutic agent in breast, ovarian, head and neck carcinoma, non-small cell lung carcinomas, and AIDS-related Kaposi's sarcoma etc. Despite of its clinical popularity in the treatment of life threatening cancer clutches, application of this chemotherapeutic agent is grossly restricted by poor water solubility (~0.4 μg/mL), development of resistance by the cancerous cells and quick elimination. Thus the commercial formulation is a concentrated solution of PTX in an organic mixture of dehydrated alcohol and polyoxyethylated castor oil (Cremophor-EL) at a ratio of 1:1 (v/v) [[7], [8], [9]]. However, severe toxicities of the marketed formulation of PTX, Taxol® is attributed by the presence of Cremophor-EL, viz. hypersensitivity, neurotoxicity and nephrotoxicity reactions [7,8]. Furthermore, Cremophor-EL disrupts the pharmacokinetic profile of the drug in vivo, leading to establishing unpredictable nonlinear pharmacokinetics [7]. Alternatively development of multi-drug-resistance (MDR) confines towards effective chemotherapy, where it can be developed by activation of detoxifying systems, decreasing drug uptake by means of increasing drug efflux, evasion of drug-induced apoptosis, DNA repair mechanisms etc. [[10], [11], [12]]. Therefore, stimulation of specific ATP-dependent transporter P-glycoprotein [P-gp] efflux or up-regulating the efflux protein expression [10]. Various approaches have been made to overcome the cellular efflux, potential by incorporating various P-gp inhibitors (e.g., PSC 833, verapamil, etc.) along the PTX formulation, although unsatisfactory outcomes with altered pharmacokinetics/bio-distribution and potential toxicity limits its progress [13,14]. Finally, metabolism by cytochrome P450 enzymes eliminates the drug rapidly from the circulation [15]. Thus, parenteral formulations requires increased dose of the formulation to maintain the therapeutic drug level for a significant period of time which ultimately results in severe toxicities [16]. Beside these noticeable issues with PTX, the marketed formulation is associated with pronounced toxicities including life threatening responses in 1.5–3.0% of the patients [17,18].
Nanoparticulate colloidal delivery systems constitute a developing approach with improved drug delivery options along with tumor targeting which resolves most of the limitations in conventional chemotherapy. Incorporation of biodegradable and biocompatible polymers, such methoxy-poly(ethylene-glycol)-poly(ε-caprolactone) (mPEG-PCL), poly(lactic-co-glycolic acid) (PLGA), poly(lactide) (PLA), etc., in consort with options to fabricate surface functionalization provides distinctive prospects to target and interact with the tumor microenvironment [[19], [20], [21]]. Such interactions of the nanoparticulate carriers with the cancer cell lead transportation of the chemotherapeutic agents to interact with intracellular organelles to produce favourable cytotoxic effect without producing non-specific toxicity to the normal cells, however they have some limitation like acid degradation, autocatalytic effect, slow degradation rate and rapid clearance via liver [22].
Vitamin E TPGS-the water-soluble derivative of vitamin E is a well-known surfactant in nanocarrier science. It contains a hydrophilic polar head and the lipophilic alkyl tail, which has shown to have beneficial effect in solubilisation of PTX, inhibition of P-gp mediated MDR and enhancement of permeation in the cancer cell. Being amphiphilic in nature and existence of several advantages of TPGS, viz.: PEG molecule of TPGS act as polar head whereas tocopherol succinate group work as lipophilic tail, large surface area, bulky shape, miscibility with water and solubility in oil establish TPGS as an important surface active agent to the formulation scientists for different oil in water immiscible systems. TPGS is pharmaceutically advantageous to improve oral bioavailability of P-gp substrates by hindering P-gp efflux on intestinal brass borders alongside assist in permeability of the drug. Additionally, TPGS has proved its important role in chemotherapy through inducing arrest of cell cycle and promoting apoptosis [5,23]. The aforesaid limitations of hydrophobic polymers could perfectly be overcome through the incorporation of TPGS into the hydrophobic polymer backbone. Various roles of TPGS in the advancement of chemotherapy have been discussed in our previous article [5]. Thus, it can be inferred incorporation of TPGS and specific ligand in nanocarriers facilitates active and passive transport of chemotherapeutics into the cancer microenvironment to achieve effective therapy (Fig. 1). Although, process and formulation variable have significant effect on physiochemical properties of NP which governs the drug loading, drug release, cellular uptake and targeted delivery of NPs. For instance, selection criteria for surfactant plays critical role in order to obtain stable particles in nano-range. Sharma and team evaluated the effect of homogenization speed, aqueous and organic phase ratio, surfactant concentration, polymer type and concentration, on physicochemical properties of PTX-loaded biodegradable polymeric NPs, where high homogenization speed (15,000 rpm) resulted smaller particle size (438 nm) in comparison to NPs (899 nm) prepared at lower homogenization speed (10,000 rpm). This difference in the particle size due to reduction in emulsion globules allows the formation of smaller NPs. Similarly, encapsulation efficiency has been increased from 65.3% to 77.5% as the speed increased, however this increment was not in linear pattern. Furthermore, larger particles exhibited slower rate of release of the drug, due to decreased surface area as well as longer pathways for diffusion of drug to travel to reach dissolution medium. Additionally, ratio of aqueous and organic phase directly affects in the viscosity of the emulsion, thus effect on particle size and also on cumulative drug release. The cumulative drug release also decreased with increase in surfactant concentration due to larger particle size. Inclusion of other surfactant including TPGS had achieved decreased particles size and fast release of drug. The results of this investigation elucidates that the formulation and process variables could be effectively altered to achieve the desired characteristics [24]. In the report by Mu and Feng combined TPGS and biodegradable polymers as matrix material for fabrication of NPs with excellent encapsulation efficiency of almost 100% in conjunction with controlled release profile [25]. It is important to mention that the cracks and/or caves existed on the particle surfaces with rough and smooth surface reinforced the hypothesis that both diffusion and matrix erosion mechanism may involve in release of drug from the developed formulation. Thus the authors commented that combination of TPGS and hydrophobic polymer can develop a controlled release nanocarrier of lipophilic chemotherapeutics including gene delivery [25]. In another approach of using TPGS decorated reduced bovine serum albumin NP of PTX, where it showed a sustained release of the drug at specified pH to exhibit enhanced cytotoxicity via inducing apoptosis in even MDR cells, which may be attributed by significant inhibition of reduced ATPlevel and P-gp activity due to the presence of TPGS [26]. In this current manuscript, we focussed on TPGS based nanoparticulate approaches to overcome the limitations associated with PTX chemotherapy. With the emerging field of nanotechnology, research outcomes of this chemotherapeutic agent have been improved by various means, which has been briefed in the connecting section.
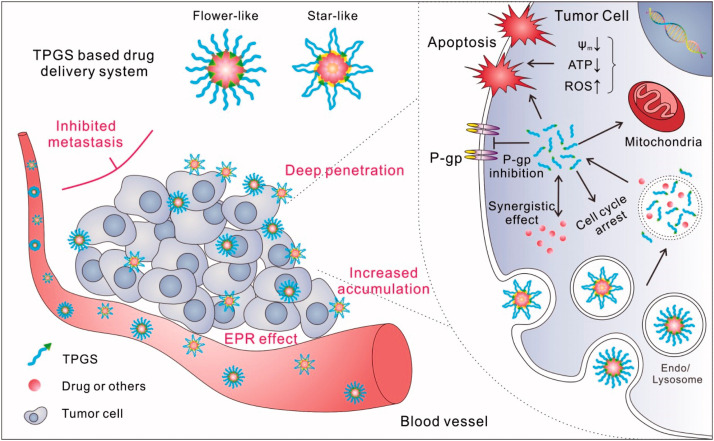
 Fig. 1. Schematic representation of potential bio-function of TPGS-based delivery nanosystem for the treatment of cancer [23].
Fig. 1. Schematic representation of potential bio-function of TPGS-based delivery nanosystem for the treatment of cancer [23].2. Nanotechnology based strategies in PTX delivery: pharmaceutical attributes and clinical outcomes
Recent advancement of nanotechnology has gained attention towards diagnosis, prevention, and treatment of cancer patients where nanocarrier tool deals with the challenges in the delivery of chemicals to the diseased site [[27], [28], [29], [30], [31], [32]]. Hence, a unique structure of modern nanocarrier is considered to be the leading platform for PTX delivery by overcoming aforementioned limitations:
-
•
Solubility could be increased greatly when entrapped into the lipid based nanocarrier, or else conjugated with water soluble polymers
-
•
EPR effect due to their nanometric size range (10–200 nm) where these carriers can escape recognition by the endogenous RES
-
•
Improved efficacy due to delivery of drugs to the target site
-
•
Modification of the pharmacokinetic profile of the entrapped drug
-
•
Increased tumor uptake through attachment of specific functionalized ligand for active targeting to the specific cancerous cells, simultaneously, decreases systemic cytotoxicities and increases maximum tolerated dose (MTD) (Fig. 2) [[33], [34], [35]].
 Fig. 2. TPGS-based nanoparticle-mediated active and passive targeting in the management of cancer [35].
Fig. 2. TPGS-based nanoparticle-mediated active and passive targeting in the management of cancer [35].
Therefore, nanocarriers are being investigated around the world with diverse composition and biological properties to deliver therapeutic components to the target site, however several underline factors are to be considered while formulating and delivering nanocarriers, including release pattern of drug(s), stability of entrapped drug, biological interactions and targeting behaviour and molecular mechanisms of cell signalling with safer delivery [5,29,36]. In due course, different characterisation methods are incorporated to analyse the optimization of the developed formulation and reproducibility of the nanocarrier; such characterisation process incorporate utilization of analytical instruments such as Fourier transform infrared (FTIR), high performance liquid chromatography (HPLC), scanning electron microscopy (SEM), dynamic light scattering (DLS), atomic-force microscopy (AFM), transmission electron microscopy (TEM) and nuclear magnetic resonance (NMR) [32,37]. In the subsequent section of the article we have summarised diverse TPGS based nanoparticulate strategies adopted to improve the efficacy and safety of the deliverable PTX formulation.
3. PTX containing TPGS nanoparticles for treatment of various cancers
As discussed earlier, being a good chemotherapeutic agent, PTX incurs low therapeutic index and limited aqueous solubility which limits clinical application of PTX. Moreover, Taxol® and Abraxane®, the available commercial product of PTX also exhibit severe side effects such as neuro- and nephrotoxicity, hypersensitivity reaction, lower blood cell count etc. [18,38,39]. To setback such constrain, many research has been done to enhance the drug accumulation at tumor site by using nanocarriers. In construction of nanocarrier, TPGS functionalized polymeric nanoparticles is well accepted in which TPGS acts as stabilizer, solubilizer, penetration enhancer and emulsifier [5]. The most fascinate use of TPGS in cancer therapy is P-gp inhibition by reducing P-gp ATPase activity which leads in reduction of efflux of drug from cell. TPGS won't influence the P-gp expression on cancer cell surface, however it exert its effect by inhibiting the bio-function of receptor. P-gp inhibiting ability of TPGS improves drug accumulation and treatment efficacy in various types of cancer [5,23]. Accumulation of drug to the cancer environment also enhances by interaction of vitamin-E moiety of TPGS with additional surface receptor which induces receptor-mediated endocytosis and improves the absorption [40].
This section of the article highlighted with the proof-of-concept to the readers on recent TPGS-based PTX nanoparticulate drug delivery approach for the treatment of cancers with different strategies to improve pharmacological profile, where Table 1 summarises the outcomes of different NP approaches.
Table 1. Nanoparticulate approaches of paclitaxel in the improvement of chemotherapeutic potential for effective control of cancer.
| Type of nanoparticle | Method used for preparation | Cancer type | Targeting ligand | Cell line | Outcome | Source |
|---|---|---|---|---|---|---|
| PCL-TPGS NPs | Emulsion-solvent evaporation, homogenized with an ultra sonicator | Estrogen-dependent and independent breast cancer | – | MCF-7 and MDA-MB-231 |
Smallest NPs exhibited a slow and prolonged release of PTX. PTX-loaded PCL–TPGS NPs showed superior anti-cancer efficacy than the commercial formulation Abraxane® and PTX solution. IC50value for PTX-loaded PCL–TPGS NPs against MDA-MB-231 breast cancer cells was 7.8 times lower than IC50 value of Abraxane®. Finally, in vivostudies demonstrated that the developed NPs exhibited slower plasma elimination rate than the commercial formulations. |
[52] |
| PLA-TPGS NPs | Emulsification/solvent evaporation method | Colorectal tumor | Folate | HeLa cell line | The spherical NPs were around 50 nm in size with low PDI. PTX loaded nanoparticles exhibited great benefits compared to free PTX and subsequently, folate decoration significantly lead towards effective targeting of drug to cancer cell in both in vitro and in vivo. | [66] |
| Pluronic P85-PEI/TPGS/PTX/shSur complex NPs (PTPNs) | Thin-film hydration method | PTX resistance lung cancer | Survivin shRNA | A549/T and A549 cell line | PTX-induced apoptosis and cell arrest in G2/M phase resulted enhanced efficacy of PTPNs against lung cancer cells. PTPN mediated down-regulation of survivin protein, could lower the apoptosis threshold of drug resistant cells and render more effective chemotherapeutic effect. Moreover, P85 mediated inhibition of Glutathione S-transferase activity, was found to increase PTX accumulation in A549/T cells. The in vivo antitumor efficacy further proved its improved efficacy over Taxol®. | [46] |
| TPGS emulsified PLGA NPs | Solvent extraction/evaporation technique | Breast cancer | – | Achieved sustained release profile in in vitrorelease study due to presence of combination of TPGS and hydrophobic polymer, PLGA in developed nanocarrier where encapsulation efficiency can be attained close to 100%. | [25] | |
| PLA-TPGS polymeric nanoparticle | Solvent extraction/evaporation method | Breast cancer and brain tumor | Folate | MCF-7 and C6-glioma cell line | Developed folate decorated PLA-TPGS NPs might improve therapeutic effect and subside the side effects of chemotherapy compared to pristine drugdut to the targeting effect and nanosize of NPs. | [69] |
| (Curcumin + paclitaxel) co-loaded PLA-TPGS nanoparticles | Emulsification solvent evaporation method | Breast cancer | – | MCF7 |
Developed NPs in diagnostic and therapeutic function showed to have more cellular uptake due to its smaller size. MCF7 spheroids study indicated that the activity of the developed NP was comparable with commercially used paclitaxel-oregon for biodistribution study. |
[57] |
| TPGS based NPs | Facile self-assembly | Breast cancer | MCF7/ADR nonresistant cells (HeLa) | The particle size of TPGS/PTX and TPGS/PTX/TQR NPs was found to be below 150 nm. The tumor cell inhibition efficiency was found to be significantly more in dual drug loaded NPs due to inhibition of P-gp efflux by TQR. | [63] | |
| Paclitaxel loaded PLA–TPGS NPs | Modified emulsification/solvent evaporation method | Colorectal cancer | Folate | HeLa cells |
In vitro targeting study revealed that Fol/PTX/PLA–TPGS NPs showed obvious differences in morphology of cancer cells than free drug and PTX/PLA–TPGS NPs. Results of in vivotargeting on tumor bearing nude mice describe effective inhibitory effect of Fol/PTX/PLA–TPGS NPs on tumor volume. |
[66] |
| Paclitaxel loaded PLGA NPs | Modified solvent evaporation technique | NA | – | NA | Particle size, encapsulation efficiency and drug release of nanoparticles can be altered by changing homogenization speed, aqueous and organic phase ratio, surfactant and co surfactant ratio etc. | [24] |
| PLA-TPGS NPs loaded with curcumin, PTX and doxorubicin | Emulsification and solvent evaporation method | Breast cancer, colorectal cancer | Folate receptor | MCF7, Hep-G2, HeLa and HT29 |
PLA-TPGS nanoparticles showed high loading capacity with remarkable increase in solubility of paclitaxel (500 fold) and curcumin (350 fold). Cellular uptake of Fol/DOX/PLA-TPGS NPs was remarkable in comparison to DOX/PLA-TPGS NPs due to folate receptor mediated endocytosis. |
[65] |
| PTX loaded M-PLA-TPGS NPs | Nanoprecipitation method | Prostate cancer | PC-3 cells | M-PLA-TPGS NPs has higher entrapment efficiency than linear PLA TPGS NPs and the PLGA NPs. Cellular uptake efficiency of M-PLA-TPGS NPs was found to be higher than that of the PLA TPGS NPs. PTX loaded M-PLA-TPGS NPs inhibited tumor growth efficiently. | [72] | |
| TPGS-SS-PLA NPs copolymer with a disulfide linkage | Nanoprecipitation method | – | Synthetic peptides containing the arginine–glycine–aspartate (RGD) | B16F10, A2780 and A2780/T cell lines | Results suggested that TPGS-SS-PLA NPs and iT-P NPs showed negligible level of hemolysis. PTX loaded nanoparticles (iT-P/PTX NPs and T-P/PTX NPs) showed significant cytotoxicity due to higher cellular uptake against these three cell lines. Further, iT-P/PTX NPs showed 3.0- and 1.2-fold higher accumulation than Taxol® in A2780/T or B16F10 cells, respectively. Additionally, in the iT-P/PTX NPs treated group significant increased in G2/M phase arrest was observed for B16F10 cells compared to Taxol® treated group. Finally, T-P/PTX NPs and iT-P/PTX NPs significantly inhibit tumor growth in tumor bearing Kunming mice. | [82] |
| PLGA-TPGS-NPs | Modified single emulsion solvent extraction/evaporation technique | – | – | – | PLGA-TPGS NPs resulted in high entrapment efficiency for PTX along with controlled release profile over a prolonged period of time for better stay in the targeting site of cancer patient. | [25] |
| TPGS-b-PCL and mPEG-b-PCL NPs | Ring opening polymerization of ∈ − caprolactone using microwaved radiation followed by solvent evaporation technique | Breast cancer |
MCF-7 MB-231 |
TPGS-b-PCL NPs resulted better in vitro anticancer performance in breast cancer cell lines compared to mPEG-b-PCL NPs. | [53] | |
| Methoxy PLGA-block-(lactic-co-glycolic acid) copolymers (PLGA-mPEG) NPs | Nanoprecipitation method with minor modifications | Human ovarian cancer cell line | IGROV1, human ovarian cancer cell line | The developed NPs resulted in sustained release of loaded PTX over 4 d within the therapeutic window and reduced side effects. In vivostudies in athymic mice established that the PTX NP significantly enhance the median lethal dose of paclitaxel by 10-fold without altering pharmacodynamic outcome. | [74] | |
| Wheat germ agglutinin (WGA)-conjugated-PTX-loaded solid lipid NPs | Emulsification and evaporation method | Lung cancer cells | Lectin receptors | A549 |
Developed WGA conjugated PTX NPs showed higher anticancer activity on A549 cell line compared to free PTX. Conjugation of WGA to the developed NP focused on improved oral bioavailability and targeting towards lung because of bioadhesive nature of nano-carrier. |
[42] |
| Monomethoxy-poly(ethylene glycol)-bpoly(lactide)-PTX (MPEG-PLA-PTX) and TPGS-folate (TPGS-FOL) NPs | Solvent extraction/evaporation method | Human cervical carcinoma and brain tumor | Folate receptor | HeLa and glioma C6 cells | TPGS-FOL NPs showed to be a potential carrier due to the cellular uptake through folate receptor-mediated endocytosis because of folate-decorated hybrid polymeric structure, thereby resulted in efficient absorption by cancerous cells | [70] |
3.1. PTX containing TPGS nanoparticles for treatment of lung cancer
Lung cancer is one of the leading causes of death triggered by cancer among adults in the USA. Control of lung cancer is presently targeted through screening of lung cancer patients and awareness of cessation of dangerous effects of smoking [41]. Research on nanotechnology has already crossed the laboratory dimension, studied in clinical patients and many products have been marketed [32]. Despite in the advancement of the NP based IV chemotherapy for PTX with Abraxane® (PTX-albumin NP conjugate) and Opaxia® (PTX-polyglutamate conjugate), Pooja and team recently focuses on most convenient oral route of drug administration for PTX, freed from alcohol and chremophor-EL. Therefore, a natural plant derived lectin, wheat germ agglutinin was conjugated with the NP to prolong gastric residence. Increase stay through lectin binding to abundantly present N-acetyl-D-glucosamine receptors on gastric surface resulted in complete absorption to target increased oral bioavailability. Researchers in this group have introduced succinoyl-TPGS instead of TPGS in the NPs for the purpose of inhibiting P-gp efflux from the intestinal brass border as well as from the MDR cancer cells. This lectin conjugated NP's produced significantly lower IC50 values in human lung adenocarcinoma cell lines (A549) when compared to suspension and unconjugated NP's of PTX, because the unconjugated form of NP's enters into the cell through clathrin-mediated endocytosis, whereas the lectin conjugation promotes lectin-receptor mediated endocytosis (Fig. 3). Contrarily, cytotoxicity of the conjugated NP's was found to decrease significantly in the presence of N-acetyl-D-glucosamine, competitive ligand for lectin receptors. Finally, improved oral bioavailability was reported by the pharmacokinetics interpretation in healthy Wistar rats, consequently, concentration of the drug was found to be accumulated in lungs due to conjugation of lectin into it [42]. On the other hand, copolymerization of biodegradable aliphatic polyesters PCL, and polyglycolide (PGA) has shown to improve physical characteristics of the PTX NP, subsequently adornation with TPGS was found to decrease recognition by RES system. PTX-loaded NPs of [PGA-co-PCL]-b-TPGS2k showed superior internalization in in vitro lung cancer cells and better efficacy in reducing the tumor growth in tumor bearing mice due to faster release and higher cytotoxicity compared to commercial formulation [43]. In another report by Wang and team on PTX loaded TPGS-functionalized PLGA NPs revealed similar mechanism for its superior control of cancer cell growth in the experimental nude mice with A549 lung cancer xenografted, i.e., enhanced intracellular uptake of the NP by the cancerous cells. Accumulation potential of the fluorescence labelled (DiR) NPs at 8 h post injection has been depicted in Fig. 4, however the authors represented that the major distribution of the NP was observed in cancer tissues followed by other internal organs [44]. Recent research on cancer delivery emphasises on blocking the specific P-gp efflux or down-regulating the efflux protein expression in the cancer cell to reverse drug resistance [10]. In order to achieve the desired goal against MDR lung cancer, Gao et al. investigated PTX nano-suspension coated with TPGS for reversal of drug resistance in P-gp overexpressed human lung cancer cells (H460). The results showed significantly improved cytotoxicity activity and 5 time increment in tumor growth inhibition with PTX nanoemulsion compared to PTX solution in reverse drug resistance of H460/RT cells [45]. Similarly, Shen et al. developed a new co-delivery system of pluronic-P85-polyethyleneimine (P85-PEI)/TPGS-NP complex for delivery of PTX and survivin shRNA (shsur) (PTPNs). When mass ratio of polymer and shRNA was >2, PTPNs could condense shRNA firmly as evidenced by reduced particle size. Fastest release of PTX from PTPNs was observed in acetic buffer solution whereas slow and prolonged release in RPMI 1640 medium represents higher stability of NP at extracellular environment. Due to sturdy selective effects to the drug resistance cells exerted by pluronics, cellular uptake of RNA was more in A549/T cells than A549 cells and downregulate shSur to lower the apoptotic threshold, particularly in drug resistant cells. In vitro cytotoxicity results revealed a 360-fold lower IC50 of PTPNs against A549/T cells compared to free PTX which might be due to synergistic effects exhibited by co-delivery of chemotherapeutic agent, PTX and shSur. Additionally, pluronic P-85 resulted inhibition of glutathione activity and further assist in accumulation of PTX in A549/T cells. Simultaneously, PTX induced apoptosis to sensitive A549/T cells, along with cell block in G2/M phase, down-regulation of survivin protein and reduce glutathione activity by PTPNs might paved an alternative and effective chemotherapy. Finally, the in vivo antitumor efficacy in A549/T cells bearing nude mice were also represented superiority of PTPNs compared to Taxol®. Thus, PTPNs could be a powerful weapon to get an effective chemotherapy against PTX resistant human lung cancer [46]. On other hand, Hou et al. synthesized PTX loaded micelleswith TPGS and Plasdone®S-630 Copovidone to improve absorption, anticancer activity as well as to enhance solubility of PTX. The results revealed significant reduction in efflux ration in Caco-2 cells along increased cytotoxicity, it also showed 4 time increment in oral bioavailability and higher antitumor activity in C57BL/6 mice compared with PTX in Lewis based. These findings demonstrate the potential of these PTX loaded micelles for effective cancer therapy [47].