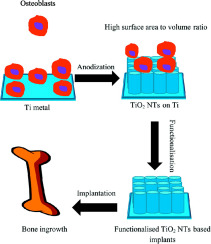
1. Introduction
Medical implants are important structures that function to support, replace or augment a diseased or injured biological tissue. Implants are used throughout the body as artificial hearts, prosthetic blood vessels, vascular stents, bone implants and dental structures [1], [2], [3]. The essential requirements of a medical implant are that it be bio-inert; not provoking an inflammatory response, be biocompatible; promoting a good interaction between the implant and the surrounding tissue, and be non-toxic; not leaching toxic substances into the body [4], [5], [6]. Additional requirements are specific to the site of implantation, such as shape and mechanical properties, for example, with sufficient structural integrity under compressive load for bone implants. Implant surface chemistry and topography are also important considerations, as these affect the implant biocompatibility and integration with the surrounding tissues [3], and should be tailored to the particular tissue being replaced.
Implants are predominantly manufactured from three types of materials: metals, ceramics and polymers. When considering bone implants, such as for total knee and hip replacements (Fig. 1) [7], metal implants are superior to other materials, with superior strength, stiffness and toughness, suitable for load-bearing applications [8], [9]. Conversely, ceramics (including bioglass) have lower fracture toughness and higher elastic modulus than bone, so do not mechanically perform as needed in vivo [8], [9]. However, the mechanical properties of ceramics may be tailored to some extent by manipulating the processing conditions; temperature, length of thermal step and choice of powder [10]. Lastly, although polymers permit easy fixation to the surrounding tissue, polymer implants may evoke inflammation of the tissue if monomers leach out of the implant [11].
 Fig. 1. Implants for complete reconstruction of hip (left) and knee (right) joints (reprinted from Ref. [7]).
Fig. 1. Implants for complete reconstruction of hip (left) and knee (right) joints (reprinted from Ref. [7]).Bones have a hierarchical structure that comprises of components with length ranging from the macroscale to the nanoscale (Fig. 2) [12]. This hierarchical structure lends itself to high toughness and high strength, with high resistance to fracture.
 Fig. 2. Schematic of the hierarchical structure of bone: (a) hydroxy-apatite crystal (b) mineralized collagen fibrils (c) lamellar bone (d) trabecula and osten (e) macroscopic view of bone (reprinted from Ref. [12]).
Fig. 2. Schematic of the hierarchical structure of bone: (a) hydroxy-apatite crystal (b) mineralized collagen fibrils (c) lamellar bone (d) trabecula and osten (e) macroscopic view of bone (reprinted from Ref. [12]).Various metals have been used for bone implants such as stainless steel (SS316L), cobalt-chromium (Co-Cr) alloys, titanium (Ti) and Ti-based alloys. Of these, Ti and Ti-based alloys, such as nitinol (TiNi) have proved to be most suitable as bone implants, owing to their superior mechanical properties and biocompatibility [7]. For example, the elastic modulus of nitinol is 40 GPa, compared to 30 GPa for bone [7]. However, surface modification of Ti and Ti-based implants is still required to promote osteogenesis (bone tissue formation), with osteoconduction (bone growth on the implant) and osseointegration (integration of the implant with the surrounding bone tissue).
Efforts to surface modify Ti based implants have been reported to include mechanical surface modification [13], physical surface modification [14], and chemical surface modification [15]. Recently, considerable attention has been devoted to the generation of titanium dioxide (TiO2) through electrochemical anodization. In particular, TiO2 nanotubes (NTs) with a diameter in the range of 30–100 nm have resulted in enhanced cell attachment [16] and osseointegration [17], [18]. Recent review articles discuss the fabrication of TiO2NT on Ti and Ti-based alloys [19], [20], [21], [22].
This review summarizes the research progress in the etching of TiO2 NTs on the surface of Ti implants. The processing conditions required for the surface modification of Ti-based implants on Ti-based implant surfaces are discussed in this review. Furthermore, this review investigates methods of improving the functionality of these TiO2 NTs, using antibacterial agents to reduce post-operative infection, and hydroxy-apatite (HA) to improve osseointegration. Additionally, methods of improving cell attachment using chemical modification of the implant surface with carbon nanotubes (CNTs), polymers, and proteins is also discussed.
2. Electrochemical anodization: deposition of TiO2 NTs on Ti surfaces
Electrochemical anodization is a method that produces nanostructures on the surface of metal-based implants such as Ti, Ti-based alloys, Tantalum (Ta) and Zirconium (Zr). Fig. 3a illustrated the apparatus for the electrochemical anodization. The electrochemical cell comprises of an anode, which can be either; Ti or a Ti-based alloy and a cathode; platinum (Pt), and an electrolyte solution; typically containing HF or NH4F. Three consecutive steps occur during the electrochemical anodization process of Ti in the fluoride-based electrolyte; see Fig. 3b. Firstly, the oxidation of Ti to TiO2 takes place, forming a compact oxide thin film on the surface of the Ti. Following this, fluoride anions (F−) adsorbed on the surface of the Ti react with the oxide layer, resulting in a porous thin film. Finally, the formed soluble titanium fluoride complex [(TiF6)− 2] gradually dissolves from the Ti surface into the bulk of the solution, which results in the porous TiO2 NT structures.
 Fig. 3. Schematic of the electrochemical anodization: (a) experiment setup, (b) mechanism of TiO2 NTs deposition.
Fig. 3. Schematic of the electrochemical anodization: (a) experiment setup, (b) mechanism of TiO2 NTs deposition.
A number of factors affect the geometry, crystallography and topography of the fabricated TiO2 NTs, including applied voltage, time of anodization, type of electrolyte, solvents, annealing conditions, temperature and state of electrolyte solution during anodization. Fabrication of NTs from an aqueous electrolyte reduces both the voltage and processing time, compared to that of an organic electrolyte, in which wider NTs may result. For example, TiO2 NTs of diameters up to 100 nm and lengths up to 4 μm have been fabricated at 20 V and 3.5 h from an aqueous electrolyte containing 1 M (NH4)2 SO4 and NH4F (Fig. 4), while high voltage (60 V) and longer time (18 h) were required for fabricating TiO2NTs of similar diameters, but 45 μm length, from an organic electrolyte solution containing NH4F, H2O and ethylene glycol [23], [24].
 Fig. 4. SEM images of TiO2 NTs (a and c) top and (b and d) cross-sectional views fabricated in 1 M (NH4)2SO4 + NH4F at 20 V (a and b) (reprinted from Ref. [23]) and in NH4F + H2O + ethylene glycol at 60 V (c and d) (reprinted from Ref. [24]).
Fig. 4. SEM images of TiO2 NTs (a and c) top and (b and d) cross-sectional views fabricated in 1 M (NH4)2SO4 + NH4F at 20 V (a and b) (reprinted from Ref. [23]) and in NH4F + H2O + ethylene glycol at 60 V (c and d) (reprinted from Ref. [24]).Following anodization the NTs on the metal substrate are often subjected to an annealing treatment at high temperatures (400 to 600 °C) to transform the TiO2from an amorphous phase to a crystalline phase [25]. Crystalline phase NTs possess a stronger adhesion to the Ti substrate [26], better electrical properties, and high corrosion resistance in Hank's solution than the amorphous TiO2 NTs [25], [26].
3. Biocompatibility of TiO2 NTs modified Ti and Ti-based alloys
The presence of hollow nanotubes on flat Ti surfaces alters the structural properties of the base Ti substrate, rendering it more attractive in tissue engineering applications because the higher porosity and increased surface area improve cell attachment and tissue ingrowth, and therefore osseointegration. Fig. 5 shows human osteoblast cell attachment on blank Ti and TiO2 modified Ti after 3 days culture [15].
 Fig. 5. Human osteoblast cell attachment and proliferation on blank Ti (a) and TiO2 NTs (b) after 3 days of culture (reprinted from Ref. [15]).
Fig. 5. Human osteoblast cell attachment and proliferation on blank Ti (a) and TiO2 NTs (b) after 3 days of culture (reprinted from Ref. [15]).During anodization, OH- and F- species from the anodizing solution are adsorbed on to the TiO2 NT surface, resulting in the now negatively charged NTs attracting the positively charged proteins in the medium, which facilitates cell attachment [27].
NT annealing alters the crystalline characteristics, which increased NT hydrophilicity [28]. As a result, cell-NT interactions are improved [28], [29]. Additionally, NT annealing at high temperatures decreases the amount of fluoride remaining in the NTs, which might be toxic to the cells [28], [29].
Table 1 lists the NT modified Ti and Ti alloy metallic biomaterials reported in the recent literature and their cell response. It was indicated that although the length of the NT did influence its biocompatibility [30], and nanotube diameter had a critical impact on cell proliferation, TiO2 NTs with a diameter of 15 nm were found to be the maximum permissible diameter prior to cell apoptosis for mesenchymal stem cells [31], [32]. In contrast, other studies showed cell proliferation to be highest on TiO2 NTs with diameters exceeding 100 nm for osteoblast cells [15]. These conflicting results suggest that the cell-substrate interaction mechanism is not yet clearly understood. In summary, the processing conditions of NT fabrication affect the NT physicochemical properties, biocompatibility and cell response.
Table 1. Recent studies on NT formation on Ti and Ti alloy based metallic implants and corresponding cell response.
| Metallic biomaterials | Synthesis conditions of NTs | NT dimensions | Cell response | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Electrolyte | Applied voltage | Anodization time | Other treatments | Diameter | Wall thickness | Length | |||
| Ti | HF | 20 V | 60 min | NT annealing | 70 nm | 15 nm | 250 nm | Osteoblasts showed adhesion on TiO2 NTs. | [33] |
| Ti | H3PO4 + HF | 1–20 V | 10–15 nm | Excellent biocompatibility for mesenchymal stem cells on 15 nm TiO2 NTs, compared to cell apoptosis on 100 nm NTs. | [31] | ||||
| Ti | H2SO4 + NaF + Citric acid | 20 V | 4 h | NT annealing | 51 nm | 51 nm | 600 nm | Osteoblastic precursor cells showed significant cell division within 5 days of seeding. | [34] |
| Ti | H3PO4 + HF | 20 V | 1 h | Stirring during anodization | > 100 nm | Human osteoblasts showed good cell adhesion, with viability up to 7 days culture. | [15] | ||
| Ti | H3PO4 + HF | 1–20 V | 1 h | 15–100 nm | Osteoblast and osteoclast apoptosis when cultured on TiO2NTs of diameter > 15 nm. | [32] | |||
| Ti | Acetic acid/HF | 5–20 V | NT annealing | 30–100 nm | Improved osteoblast elongation when cultured on TiO2 NTs of 100 nm diameter, compared to 30 nm NTs. | [35] | |||
| Ti | Acetic acid/HF | 5–20 V | 30 min | NT annealing | 30–100 nm | Human mesenchymal stem cell adhesion on NTs of both 30 and 100 nm diameter, with less cell differentiation on 30 nm NTs. | [36] | ||
| Ti coated Zr | NH4F + Ethylene glycol | 20 V | 15 min | NT annealing | 50 nm | 200 nm | Mouse osteoblasts exhibited higher adhesion on TiO2 NTs coated Zr, compared to uncoated Zr. | [37] | |
| Ti | NH4F + Ethylene glycol | 30 V | 3 h | NT annealing | 100 nm | 4 μm length | Osteoblast-like cells demonstrated high viability on NTs. | [38] | |
| Ti | H3PO4 + HF | 20 V | 60 min | 100 nm | 1 μm | Osteoblasts showed higher bone formation on Ti coated with TiO2 NTs compared to uncoated Ti. | [16] | ||
| Ta coated Ti | Acetic acid + HF | 20 V | 30 min | NT annealing and coating with Ta (20 nm thickness) via sputter technique | 100 nm | 10 nm | 300 nm | Human osteoblasts demonstrated better cell viability and faster rate of cellular matrix of mineralization (30%) on TiO2NT coated Ta, compared to uncoated Ti and uncoated TiO2NTs. | [39] |
| Ti coated Ti6Al4V | HF | 30 nm | 10 nm | Human mesenchymal stem cells demonstrated better cell response, protein adsorption and gene expression when cultured on TiO2 NTs, compared to uncoated Ti6Al4V. | [40] | ||||
| Ti | NH4F + Ethylene glycol | 70 nm | 500 nm | The hierarchical NT surface promoted greater bone formation and protein adsorption, compared to blank Ti and micro porous surface. | [17] | ||||
| Zr |
First step: NH4F + (NH4)2 SO4 |
20 V |
First step: 30 min |
NT annealing | 40 nm | 10 μm | ZrO2 NTs possessed a biocompatible topography for osteoblasts. Furthermore, a large amount of Ca and P was deposited by the cells on ZrO2 NTs, almost covering the NTs. | [41] | |
|
Second step: NH4F + (NH4)2 SO4, |
Second step: 15 min |
||||||||
| Ta | HF + H2SO4 + DMSO | 2.3–11.1 μm | TaO2 NTs were more biocompatible than blank Ta, in terms of osteoblast adhesion, proliferation and differentiation. | [42] | |||||
4. Functionalization of TiO2 NTs
Although TiO2 NTs enhanced biocompatibility, osteoconduction, and osseointegration. They still face some challenges [32], [33]. For example, TiO2NTs have very limited antibacterial properties [43]. To reduce post-operative infection, antibacterial agents such as metallic nanoparticles or drugs have been incorporated into TiO2 NTs during NT fabrication. Hydroxy-apatite has been deposited onto TiO2 NTs either by wet chemistry, through immersion of the NTs in simulated body fluid containing calcium and phosphorous, or by physical or chemical or electrochemical deposition. The presence of HA on TiO2 NTs was found to increase the integration of the implants to the bone tissue. In addition, TiO2 NTs were also modified using CNTs, polymers, and proteins to enhance bone ingrowth. A combination of these strategies has also been reported to improve the bacterial resistance, osteogenesis, and rate of osseointegration.
4.1. Antibacterial functionalization of TiO2 NTs
4.1.1. Metallic nanoparticles
Nanoparticles (NPs) from silver, gold, zinc, and copper nanoparticles have been investigated to enhance the antibacterial properties of TiO2 NTs [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54]. The influence of TiO2 NT phase structure (mineral form) and morphology have also been investigated [43]. The antibacterial action of Ag NPs is due to the interaction between the positive Ag ions and the negatively charged bacterial cell wall, with the Ag NPs increasing bacterial cell wall permeability and therefore inhibiting bacterial growth [44], [45], [46].The attachment of Ag NPs to TiO2 NTs was found to enhance the antibacterial properties of TiO2 NTs [43], with the antibacterial effect increased from 20% (for NTs alone) to 100% (for AgNP-TiO2 NTs). Fig. 6a shows the FESM of TiO2 NTs incorporated with Ag NPs and Fig. 6b shows the antibacterial rate of TiO2 NTs alone, Ag NPs modified blank Ti and Ag NPs modified TiO2 NTs.
 Fig. 6. (a) FESEM of Ag NPs deposited TiO2 NTs and (b) Effect of Ag NPs deposition on TiO2 NTs bacterial activity (reprinted from Ref. [43]).
Fig. 6. (a) FESEM of Ag NPs deposited TiO2 NTs and (b) Effect of Ag NPs deposition on TiO2 NTs bacterial activity (reprinted from Ref. [43]).TiO2 NTs modified with Ag NPs, ranging in size from 10 to 20 nm, were exposed to Staphylococcus aureus in an antibacterial study [47]. The Ag NP modified TiO2NT surface was found to exhibit a more pronounced antibacterial effect than that of the blank Ti surface alone (Fig. 7). This antibacterial effect increased with increasing amount of Ag NPs [47]. At a low Ag NP concentration (0.5 M), the antibacterial efficiency was found to significantly decrease with incubation time; reaching 30% efficiency after 30 days of incubation, whereas at high Ag NPs concentration (2 M), the TiO2 NTs showed antibacterial efficiency of 50% after 30 days of incubation [47]. However, the Ag NPs/TiO2 NT surface became cytotoxic at higher Ag concentration due to the released Ag+ ions at a high content causing osteoblast cells [47], [48], [49].
 Fig. 7. Fluorescence photos of S. aureus cells on (a) Ti, (b) TiO2 NT and (c) Ag NP- TiO2 NT surfaces after 7 days of incubation. Green colour represents live bacteria cells whilst orange colour shows dead bacteria cells (reprinted from Ref. [47]).
Fig. 7. Fluorescence photos of S. aureus cells on (a) Ti, (b) TiO2 NT and (c) Ag NP- TiO2 NT surfaces after 7 days of incubation. Green colour represents live bacteria cells whilst orange colour shows dead bacteria cells (reprinted from Ref. [47]).The antibacterial effect of Ag NP-TiO2 NTs have also been evaluated using four strains of bacteria, including; Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa [50]. It was found that the Ag NP-TiO2 NTs influenced the shape of the bacteria cells by interacting and damaging the bacteria cell membrane. As shown in Fig. 8a,b [50]. Fig. 8a shows the S. epidemidis bacteria cells cultured on blank Ti and Fig. 8b shows S. epidemidisbacteria cells cultured on Ag NP modified TiO2 NTs.
 Fig. 8. FESEM micrographs of S. epidemidis cells cultured onto (a) Ti and (b) Ag NP-TiO2 NTs (reprinted from Ref. [50]).
Fig. 8. FESEM micrographs of S. epidemidis cells cultured onto (a) Ti and (b) Ag NP-TiO2 NTs (reprinted from Ref. [50]).Silver oxide nanoparticles (Ag2O NPs) have also been used to modify TiO2 NTs by sputtering a TiAg thin film on a Ti substrate and then electrochemically anodizing in a fluoride solution [51]. In this case, the Ag2O NPs were mainly embedded inside the NTs. The Ag2O content varied from 1.27 to 27.23%, the Ag2O NP-TiO2 NTs exhibited a good Ag+ ion release profile and good antibacterial properties against both Escherichia coli and Staphylococcus aureus. After 28 days of incubation, the scaffold still maintained up to 97% antibacterial efficiency [51].
Zinc oxide nanoparticles (ZnO NPs) were also incorporated into TiO2 NTs using a wet chemistry method to improve the antibacterial ability [52].The efficiency of the ZnO NP-TiO2 NTs against Staphylococcus aureus showed dose dependency, with the highest efficacy observed at a concentration of 1.5 mM of ZnO NPs [52]. ZnO NPs tended to have higher efficiency with the bacterial membrane [52]. Furthermore, the critical ZnO NP concentration for a cytotoxic effect to mesenchymal stem cells and osteosarcoma cells was found to be very low, just 750 μM [53].
ZnO NPs and Ag NPs have been co-incorporated into TiO2 NTs using two consecutive strategies, starting with Ag sputter coating and then electrodeposition of ZnO NPs onto the TiO2 NT surface [53]. The ZnO NPs and Ag NPs were evenly distributed on the surfaces of TiO2 NTs and showed good antibacterial efficacy [54]. Apart from Ag and ZnO NPs, CuO nanoparticle (CuO NP) were also deposited on TiO2 NTs. The CuO NP TiO2 NTs can be synthesized by anodization of Ti-10Cu alloy or Ti-10 Cu-0.45Al alloy in a fluoride containing solution. The presence of CuO NPs enhanced antibacterial activity up to 99% compared to blank alloys [54].
4.1.2. Drug delivery (loading of drug)
Drugs have been loaded into TiO2 NTs to enable the implant to directly deliver the drug to the site of implantation [55]. The NT processing conditions show an effect on the release kinetics of the loaded drug. TiO2 NTs fabricated from a fluoride-based aqueous electrolyte, and a fluoride-based organic electrolyte, were loaded with vancomycin and silver nitrate via an impregnation technique. The release of the vancomycin from NTs fabricated from the organic electrolyte was slower (415 μg after 300 days) than from those fabricated from the aqueous electrolyte (320 μg after 140 days) [56]. Similarly, when gentamicin was loaded into TiO2 NTs fabricated at different voltages (20, 40 and 60 V), the lowest release time (up to 4 days) was obtained for the NT fabricated at the lowest voltage [57]. This may have been due to differences in nanotube dimensions, as a result of the different processing conditions.
Multi-drug delivery has been attained with the use of polymer micelles with either lipophilic or hydrophilic cores, loaded into TiO2 nanotubes. Controlled drug release from these NTs was maintained by the addition of some empty polymer micelles, with the ratio of the loaded drug polymer micelles to empty micelles adjusted to delay the release of the drug [58]. Additionally, stimuli response drug release from TiO2 NT-based implants can be done by several ways, such as ultrasound, UV light and laser radiation [59], [60].
Finally, antibacterial agents can be combined, to increase the antibacterial properties of the substrate; TiO2 NTs loaded with either Ag NPs or an antibiotic, such as cephalothin, minocycline, or amoxicillin, or Ag NPs with attached antibiotic, have been evaluated against a range of bacteria strains [61]. The combination of two antibacterial materials was found to be more effective on the bacterial strains up to 90% efficiency after 60 min incubation, compared to using just one approach [61].
The release kinetics of a drug from the NT takes the form of an initial burst due of the drug from the open end of the NT, with a steady drug release following this. This has advantages in some applications, such as the prevention of bone infection, while it is not beneficial in the application of long term treatment. Therefore, research has been devoted to controlling the release kinetics of drugs from NTs. One approach is to cap the drug loaded into the TiO2 NTs. Plasma polymerization was reported to steady the drug release for 6 to 8 weeks [62], [63], [64], [65], [66], [67], [68]. The thickness of the polymer end may be varied by altering the plasma deposition time which in turn alters the release rate of the drug [66], [67], [68], [69]. This method has been used in the delivery of water-insoluble drugs, such as non-steroidal anti-inflammatory drug (indomethacin), antibiotics (gentamicin, vancomycin, levofloxacin), and proteins [69].
Coating biodegradable polymers is another method to control the drug release kinetics from TiO2 NTs. By coating the entire nanotube using a simple and inexpensive technique, such as dip [70] or spin coating [70], drug release rate can be reduced by 30% - 60% [70]. Using this method, TiO2 NTs loaded with ibuprofen and a polymer coating layer were prepared, the molecular weight of the polymer used affected the ibuprofen release profile [70]. The NTs coated with a high molecular weight polymer was slower (at 9 days) than that with a low molecular weight polymer (at 5 days) [70].
The drug release kinetics have been shown to be dependent on NT length, for NTs exceeding 100 nm in diameter [71]. When filled with gentamicin, wider diameter NTs (160 and 200 nm) produced a stronger antibacterial effect than narrower NTs (80 and 120 nm) [72], because of a sustained drug release from the wider nanotubes.
4.2. Deposition of hydroxy-apatite on TiO2 NTs
4.2.1. Hydroxy-apatite grown in simulated body fluid
The mechanism of hydroxy-apatite (HA) growth on TiO2 NTs from simulated body fluid (SBF) is due to the dipolar nature of the TiO2/HA interface. Hydroxyl groups (OH−) are adsorbed from SBF to form Ti-OH groups. When the pH is approximately 7.4, the Ti-OH groups are negatively charged, due to the presence of deprotonated acidic hydroxides. Calcium ions (Ca2 +) are then adsorb from the SBF to the surface of the NTs, as a result of the negatively charged surface, so that HOPO42 − or H2PO4− can easily react with the as-adsorbed Ca2 + to finally produce calcium phosphate [73].
Repetitive dipping and drying of TiO2 NTs in the SBF enhances the growth of the hydroxy apatite, as a consequence of filling the inner and outer surfaces of the NTs. Fig. 9a,b shows a dense hydroxy apatite layer fabricated after repetitive dipping (eight dips), which was more obvious compared to fewer dips (two dips) [73].
 Fig. 9. FESEM micrographs of TiO2 NTs dipped in SBF (a) twice and (b) eight times (reprinted from Ref. [73]).
Fig. 9. FESEM micrographs of TiO2 NTs dipped in SBF (a) twice and (b) eight times (reprinted from Ref. [73]).TiO2 NTs chemically treated with sodium hydroxide (NaOH) solution have been used as a substrate for HA growth through immersion in SBF [74]. The process involved first making sodium titanate (Na2Ti5O11 or Na2Ti6O13) by immersion of the NTs in NaOH solution. Hydroxy-apatite was then deposited on the NT surface upon soaking in SBF. It was found that the longer the length of the TiO2NT, the higher the amount of HA formed on its surface, with 2 μm long NTs promoting increased HA growth, compared to lengths of 200 nm [74]. Furthermore, the annealing of TiO2 NTs prior to immersion in SBF may shorten the growth time of the HA for shorter nanotubes [75]. Additionally, annealed TiO2 NTs, with an anatase structure, of length 1.66 μm and diameter 100 nm, exhibited higher growth rate of Ca (from 0.067 to 52.77 mmol/L) and P (from 0.058 to 36.27 mmol/L) upon extending the exposure time from 0.5 to 5 h, compared to non-annealed NTs, with an amorphous structure [76]. However, although non-annealed TiO2 NTs have showed higher HA growth rate than the blank Ti substrate, the non-annealed NTs possessed a non-uniform HA layer [76].
Increasing the ion concentration of SBF by three-fold (3SBF) has been found to increase HA growth on TiO2 NTs [73], 9 μm thickness of HA layer could deposit on TiO2 NTs after 3 days of immersion in 3 SBF. When mechanically tested, it was found that the HA-TiO2 NTs bond strength was on average 15.3 MPa [73]which is greater than the ISO requirement for apatite coating on surgical implants (15 MPa) [73].
Other methods include immersion of a NT surface in boiling solutions, Wang et al. [78] reported that immersion of TiO2 NTs in a boiling Ca (OH)2 solution, followed by immersion in SBF for 4 days led to the formation of a HA layer on the NTs. When the immersion period was extended to 12 days, a dense HA film was formed on the substrate surface, whilst the untreated TiO2 NTs (without immersion in Ca (OH) 2 solution) only formed an insignificant HA layer after 12 days immersion in the SBF [77].
Ag NPs were sputtered into TiO2 nanotubes which in turn led to the fabrication of Ag NPs/TiO2 NTs [78]. When soaked in SBF, a hydroxy-apatite layer grew on Ag NPs/TiO2 NTs. The resulting ternary combination of Ca-P/Ag/TiO2 NTs was expected to lead to improvements in terms of biocompatibility and bacteriocytotoxicity, due to the low Ag+ ion release, as well as the molar concentration of the formed CaP, which was close to stoichiometric hydroxy-apatite [78].
4.2.2. Electrochemically deposition
An hydroxy-apatite layer can be electrochemically deposited onto TiO2 NTs at 80 °C, with subsequent annealing of the HA/TiO2 NTs improving the bond strength between the HA and TiO2 NTs up to 40 MPa, compared to non-annealed HA/TiO2 NTs (25 MPa) [79]. Using the same strategy, calcium hydrogen phosphate was electrochemically deposited from an electrolyte containing calcium and phosphorus and then converted to HA upon treatment with NaOH, with a resulting bond strength of approximately 7.41 MPa [80].
A recent investigation on the electrodeposition of hydroxy-apatite on TiO2 NTs using Ca (NO3)2 and NH4H2PO4 found that static magnetic field assisted electrodeposition was able to enhance both the uniformity and the bond strength of the deposited HA [81]. The generated magnetic field, as a consequence of the magnetohydrodynamic effect, significantly facilitated the mass transport of the calcium and phosphorus ions. As a consequence of applying perpendicular and parallel magnetic fields to the direction of applied current, the shape of the formed HA crystals changed from plate-like for the control sample without magnetic field applied, to needle-like and spherical, for the perpendicular and parallel magnetic field, respectively [81]. The incremental increase in the magnetic field intensity, from 0.25 T to 1 T, led to an improvement in adhesive strength of the fabricated HA crystals from 20.1 MPa and 16.7 MPa to almost 26.5 MPa and 24.7 MPa for perpendicular and parallel magnetic field conditions, respectively [81]. TiO2 NTs incorporated with calcium phosphate nanoparticles (CaP NPs) through electrodeposition provided better hydrophilicity than bare TiO2 NTs, with these CaP NP/TiO2 NTs having a higher rate of HA crystals formations than TiO2 NTs alone, when immersed in SBF for 2 days [82]. Substitution of Ca2 + ions by Mg2 + during electrodeposition of hydroxy-apatite on TiO2 NTs was found to yield MgHA instead of CaHA coated TiO2 NTs, with the resulting morphology of the HA crystals changed from needle-like for the CaHA to plate-like crystals for the MgHA (Fig. 12a,b). It was found that the MgHA deposited TiO2 NTs provided a higher corrosion resistance(Ecorr = − 0.228 V) than the bare TiO2 NTs (Ecorr = − 0.354 V) and HA coated TiO2NTs (Ecorr = − 0.315 V), (Fig. 12c) [83]. Additionally, MgHA deposited on TiO2 NTs slightly increased the bond strength from 17.3 MPa, in the case of HA deposited on TiO2 NTs, to 18.1 MPa [83].
4.2.3. Physical vapour deposition
Tooth ash has been used as a HA source to deposit on TiO2 NTs using physical vapour deposition technique [85]. The HA coated TiO2 NTs had improved corrosion resistivity, where the surface current density of bare Ti-30Ta-15Zr NTs (1.122 × 10–5 A/cm2) was higher than that of HA coated Ti-30Ta-15Zr NTs (4.823 × 10–6 A/cm2) [78]. Additionally, the surface current density of uncoated Ti-30Nb-15Zr NTs (1.765 × 10–5 A/cm2) was higher than that of HA coated Ti-30Nb-15Zr NTs (1.366 × 10–7 A/cm2) [84].
Pulsed laser deposition technique has been employed to deposit a HA layer with Ag NPs on TiO2 NTs [85].The Ag-HA NP/TiO2 NTs composite contains both anti-bacterial and biocompatibility properties [85].
4.2.4. Hydrothermal treatment
Hydroxy-apatite has also been hydrothermally grown on TiO2 NTs at 200 °C from Ca (NO3)2 and (NH4)2HPO4 using urea as a precipitating agent [86]. Hexagonal HA crystals of 1 to 2 μm diameter and 2 to 3 μm length grew after 3 h, with an increase in reaction time to 12 h yielding HA crystals of 10 to 20 μm length, (Fig. 10a,b) [86].
 Fig. 10. FESEM images of HA grown on TiO2 NTs at 200 °C after (a) 3 h and (b) 12 h (reprinted from Ref. [86]).
Fig. 10. FESEM images of HA grown on TiO2 NTs at 200 °C after (a) 3 h and (b) 12 h (reprinted from Ref. [86]).Hydroxy-apatite has also been deposited on Ti-35Nb-xHf NTs by a radio-frequency magnetron sputtering technique [91]. For NTs with a high Hafnium(Hf) content, HA formed a uniform thin film on the Ti-35Nb-xHf NTs, with HA coated Ti-35Nb-xHf NTs more hydrophilic than uncoated Ti-35Nb-xHf NTs [87]. HA deposited Ti-35Nb-10Zr NTs fabricated using laser texturing, were hydrophilic, compared to uncoated Ti-35Nb-10Zr. Additionally, the corrosion potential (Ecorr) of the HA coated Ti-35Nb-10Zr NTs was higher (− 1100 mV/SCE) than for both the uncoated Ti-35Nb-10Zr and Ti-35Nb-10Zr NTs (− 410 mV/SCE and − 1070 mV/SCE, respectively) [88].
4.2.5. Wet chemistry synthesis
Titanium screw implants have been precalcified using a cyclic precalcification process [89]. Firstly, the screw was blasted by bioabsorbable HA powder and anodized in fluoride containing media to form a nanotubular surface. Following this, the screw was immersed continuously in 0.05 M NaH2PO4 solution at 80 °C for 20 min, and following that, in saturated Ca (OH)2 solution at 100 °C for 20 min. This process is named the continuous immersion treatment (CIT) [89]. Another approach was attempted, named the alternative immersion treatment (AIT), by which the as-anodized surface was immersed in 0.05 M NaH2PO4solution at 80 °C for 1 min, then in deionized water for 5 s. Following that it was immersed in saturated Ca (OH)2 solution at 100 °C for 1 min and eventually in deionized water for 5 s [89]. The AIT treated sample induced a higher HA formation than on the CIT treated sample after immersion in SBF for 3 days (Fig. 11a,b). The removal torque measurement and Energy dispersive X-ray spectroscopy (EDS) analysis after implantation in rat tibia for 4 weeks, illustrated that the AIT treated sample stimulated higher bone ingrowth than the CIT treated sample [89]. For example, the removal torque value of the AIT treated sample was 28.1 N cm, while it was 17.5 N cm for CIT treated sample. Furthermore, the AIT treated sample contained 29 wt% Ca and 13.6 wt% P while the CIT treated sample contained 12 wt% Ca and 6.9 wt% P [89].