1. Introduction
1.1. Background
The application of biomedical materials in reconstructive surgery for repair of surgical or traumatic defects has involved significant research in the field of biomaterial science with the aim to achieve faster and better biological healing outcomes. Currently, autograft and allograft remain the gold standard for bone replacement therapy. However; disadvantages such as limited supply of donor bone graft and a secondary trauma for autograft as well as the issue of immune response of the allograft challenge their clinical applications. This has stimulated interest in the development of synthetic materials for bone replacement and more recently novel biomaterials with similar properties to native bone [1].
A major advance in biomaterial science has been the development of bioceramics as bone substitutes. The most common bioceramics in use are calcium phosphate-based (CaP) biomaterials which include: hydroxyapatite (HA), α- and β-tricalcium phosphates (α-TCP, β-TCP), octacalcium phosphate (OCP), amorphous calcium phosphate (ACP) and biphasic calcium phosphates (BCP) which is a combination of two different CaP phases [2], [3], [4]. All types of CaP biomaterials can be manufactured in both porous and dense forms as bulk, granules, and powders or in the form of coatings. Their biocompatibility, safety, availability, low morbidity and cost effectiveness are important advantages over autografts and allografts. CaP bioceramics are now in common use for different medical and dental applications such as treatment of bone defects and fracture, total joint replacement, spinal surgery, dental implants, periodontal therapy and cranio-maxillofacial reconstruction [5], [6].
The majority of research on CaP based biomaterials has focused on HA (Ca5(PO4)3OH) and β-TCP (Ca3(PO4)2) [7]. CaP based biomaterials are bioactive and have a composition and structure similar to the mineral phase of bone and can be processed to have osteoconductive properties [8]. Furthermore, they have a high affinity for protein adsorption and growth factors [9] that in turn influence osteoinductivity. The osteoinductive properties can be achieved in two ways; intrinsic and extrinsic. Intrinsic is by structural or chemical optimization of the biomaterials themselves and the extrinsic is by the addition of osteoinductive signal molecules, such as bone morphogenetic proteins (BMP) or osteogenic cells [10], [11].
Although the CaP bioceramics have many advantages, they also suffer from disadvantages such as; poor mechanical strength, lack of organic phase (i.e. collagen), presence of impurities, micro-scale grain size and non-homogenous particle size and shape. Furthermore, the processing techniques for preparation of bioceramics suffer from prolonged fabrication time, low-yield final product and difficult porosity control [2]. However, over recent years, several modifications of fabrication parameters such as sintering temperature, sintering soaking time, pH and purity of the initial materials have given rise to biomaterials with improved physicochemical properties such as specific surface areas, surface energy, surface charge, surface topography and roughness, grain size and porosity [12].
Another area of recent interest in the literature is to produce bioceramics at the nanoscale level. This is because the bone matrix is also a precise composition of two major phases at the nanoscale level namely, the organic phase (proteins) and inorganic phase (minerals, mainly CaP nanocrystals). The conventional synthetic CaP ceramics are composed of large grain size at microscale level, which seems to possess different biological behaviors of bioactivity, biodegradability and mechanical properties than nanoscale alternatives. Therefore, fabrication of nanoscale bioceramics may improve the biological behavior of CaP bioceramics [13]. In addition, it has been shown that nano-HA promotes osteoblast cells adhesion, differentiation, and proliferation thereby enhancing osseointegration and deposition of calcium containing minerals on its surface better than microcrystalline HA [14]. Moreover, the superiority of biological calcified tissues (e.g., bones) are also due to the presence of biopolymers (mainly, collagen type I fibers) which confer strength and partial elasticity. Therefore, one of the most promising ideas is to apply biomaterials with similar composition and nanostructure to that of natural bone tissue. In this regard, development of composite organic–inorganic biomaterials may provide better opportunities for optimizing the conventional bone substitutes [13].
1.2. Biphasic calcium phosphate
Another major area of research on CaP based biomaterials has focused on BCP [15]. According to the definition, BCP consists of two individual CaP phases: most commonly from a more stable phase (HA) and more soluble phase (β-TCP) in different proportions. This combination has presented significant advantages over other types of CaP bioceramics by allowing a better control over bioactivity and biodegradation which guarantees the stability of the biomaterial while promoting bone ingrowth. BCP ceramics are osteoconductive with the possibility of acquiring osteoinductive properties [16], [17].
Different researchers have attempted to achieve desirable biological responses by modification of different parameters during biomaterial fabrication or by incorporation of different biocompatible polymers. The biological response to BCP ceramics varies according to their chemical compositions and physical properties which can give rise to different rates and patterns of bone regeneration. To the best of our knowledge, a consensus of the ideal physicochemical properties of BCP scaffolds such as; composition ratio, pore size, total porosity and interconnected porosity has yet to be determined. In addition, there are no generally accepted standards for in vitro and in vivo studies with regards to experimental protocol and interpretation of results; this most likely appears to contribute to the variation of results and findings on the properties and effectiveness of BCP.
For the purpose of this review, it is divided into two main sections. First, 3.1 Concept and history, 3.2 BCP synthesis and preparation, 3.3 Physicochemical properties and characterization, 3.4 The optimal composition ratio provide a general overview on BCP (HA/β-TCP) elaborating the concept, synthesis and importance of physicochemical properties and characterization. This section also includes a systematic review of animal studies comparing application of different composition ratios of BCP (Section 3.4). Second, 4.1 The controversies on composition ratio of BCP, 4.2 Recommendations for characterization and documentation of biomaterials, 4.3 Rationale and duration of pre-clinical animal study, 4.4 Sample size calculation; importance and impact, 4.5 Critical size defect; a clarification. provide discussion regarding controversies on BCP composition ratio followed by general guidelines for characterization and documentation of BCP. This section also presents recommendations to help in reducing the inconsistencies in protocols and reporting strategies of future studies. Of course, the provided guidelines and recommendations can also be applied to study of other biomaterials in the field of bone tissue engineering.
2. Review of literature
It is well accepted that the best representative model of biological condition are animal studies. Therefore, for systematic review on application of different composition ratios of BCP, we restricted our search to animal studies as the best models that represent the real biological condition with less diversity compared to in vitro models. As long as the human trials of BCP are limited to only few composition ratios, we decided to exclude human studies from our systematic review.
2.1. Search strategy
An electronic search was performed from 1989 to 2015, using MEDLINE (National Library of Medicine) and PubMed following the criteria as below:
2.2. Inclusion criteria
1. articles in English, 2. use of biphasic calcium phosphate (BCP, HA/β-TCP) ceramics, 3. animal studies, 4. the keyword “bone substitutes”, 5. use of BCP for intraosseous implantation, 6. use of pure BCP (only inorganic phases).
2.3. Exclusion criteria
1. literature review, 2. use of single composition ratio of BCP, 3. use of BCP in extraosseous implantation sites (subcutaneous or intramuscular), 4. presence of other polymeric phase within BCP, 5. in vitro studies, 6. human trials, 7. non-English articles.
After the review process, the available papers were studied carefully to determine the following data: 1. author/year, 2. studied HA/β-TCP ratios, 3. animal type and number, 4. site of implantation of BCP, 5. osseous defect dimension, 6. characterization of BCP (including particle/granule size, pore size and porosity), 7. follow up time and analysis intervals, 8. type of investigations and 9. main findings.
All identified studies were assessed independently and in detail to ensure meeting the review's inclusion criteria. A total of 15 articles (out of 101) meet the inclusion criteria and underwent data extraction independently and in duplicate. Data were registered in a table and arranged by year chronologically.
3. BCP bioceramics
3.1. Concept and history
The majority of biomedical materials usually consist of a single phase referred to as a monophasic system e.g. HA bioceramics. However, the term multiphasic is referred to the mixture of two or more individual phases with almost similar physical properties, for example a combination of HA and TCP will be called biphasic as it consists of only two phases. The distinction should be made from the term “composite” that is made from two or more constituent materials with significantly different physicochemical properties [18].
Nery et al. [19] in 1975 were the first to report on clinical application of a “tricalcium phosphate” preparation as bone substitutes in surgically created “infrabony” periodontal defects in animals. This preparation was subsequently analyzed by X-ray diffraction (XRD) and found to be actually a mixture of 20% HA and 80% β-TCP [20]. Later, the term “biphasic calcium phosphate” (BCP) was first introduced by Moore et al. [21] and Anuta et al. [22] in 1985 presentations at the 11th annual meeting of the society for biomaterials and later on, in 1986 by Ellinger et al. [23] who reported its application in periodontal osseous defects in a case report. LeGeros [24] (1986) and later on Daculsi [16] (1989) started basic research on preparation and clinical application of BCP.
BCP formulations are of two major types; 1) BCP consisting of CaP phases with similar molar Ca/P ratio (e.g. α-TCP and β-TCP, Ca/P = 1.5 for both); 2) BCP consisting of CaP phases with different molar ratio (e.g. β-TCP and HA, Ca/P = 1.5 and 1.67, respectively). Table 1 summarizes the different composition phases of BCP and their properties.
Table 1. BCP composition phases (HA and TCP) and their major properties.
| Material | Stoichiometry | Crystallography | Molar ratio (Ca/P) | Solubility at 25 °C | Molar mass g/mol | Density g/cm3 | |
|---|---|---|---|---|---|---|---|
| − log Kps | mg L− 1 | ||||||
| Hydroxyapatite (HA) | Ca10(PO4)6(OH)2 | Hexagonal | 1.67 | 116.8 | 0.00010 | 988.62 | 3.156 |
| Beta-tricalcium phosphate (β-TCP) | β-Ca3(PO4)2 | Rhombohedral | 1.5 | 28.9 | 0.20 | 310.17 | 3.066 |
| Alpha-tricalcium phosphate (α, α′-TCP) |
α-Ca3(PO4)2 α′-Ca3(PO4)2 |
α = monoclinic α′ = hexagonal |
1.5 | 25.5 | 0.97 | 310.17 |
α = 2.866 α′ = 2.702 |
Compiled from references [2], [3], [4].
BCP (HA/β-TCP) appear to be the focus of many research papers in the field of bioceramics and therefore they are investigated extensively [25], [26]. Selection of appropriate phases is of great importance to overcome the shortcomings of a single phase formulation. HA is used because of its similarity to the mineral phase of bone and a better mechanical properties compared to α- and β-TCP. However, to overcome its poor biodegradation rate, HA is combined with other more biodegradable bone phases at an appropriate ratio. For the second phase, usually β-TCP is selected because of its higher chemical stability (compared to α-TCP) and more favorable biodegradation rate. The BCP scaffolds proved to be highly biocompatible and could support attachment, proliferation and differentiation of osteoblast cells [12], [27].
The main concept behind the use of BCP is to improve the biological properties of the bioceramics such as bioactivity, bioresorbability, osteoconductivity and osteoinductivity so as to enhance bone tissue formation [26]. It has to be highlighted that a delayed or fast biodegradation rate may interfere with rate and pattern of new bone formation. Therefore, the main advantage of BCP is the possibility to manipulate the composition ratio of more stable phase and more biodegradable phase to optimize biodegradation rate and enhance bone repair process for specific application. This is achieved by selection of an optimum composition ratio of the HA and the α- or β-TCP, where increasing the ratio of latter, improves the bioactivity and biodegradability of BCP [16].
The biodegradation process initiates after BCP implantation by dissolution of CaP crystals and precipitation of ion-substituted carbonated calcium deficient-HA (CDHA) which will be replaced by new bone ingrowth during bone healing [28]. Greater biodegradation of TCP and partial dissolution of the HA facilitate an increase in the supersaturation of calcium (Ca2 +) and phosphate (HPO42 −, PO43 −) ions concentration in the local microenvironment. This results in precipitation of released ions and formation of CDHA microcrystals [27], which incorporates other ions (CO32 −, Mg2 +, Na+) present in the biological fluid. This process results in subsequent mineralization of extracellular matrix (ECM) and incorporation of CDHA in the newly generated bone, forming a strong interface through direct chemical bonds [25].
The biodegradation process of BCP depends on several factors such as; chemical composition, particle sizes, crystallinity, specific surface area and porosity. It is known that both in vitro and in vivo biodegradation process of BCP follow a common pattern. A general accepted pattern of biodegradation of different phases of BCP in increasing order is as follow: HA < β-TCP < α-TCP; where α-TCP manifests the fastest biodegradation rate [12], [27], [29], [30]. The biodegradation kinetics of BCP with similar particle size and porosity depends on types of available chemical phases (HA/TCP) and their percentage ratio, where the higher the ratio of TCP, the higher the biodegradation rate of BCP. However, the biodegradation process is also influenced by other factors, for example, a lower porosity and surface area or a higher crystallinity and larger particle sizes are associated with a lower rate of biodegradation process [31]. The biodegradation process of BCP is characterized in vivo by decrease in crystal size and increase in total porosity [27].
Currently, there are over 30 commercially available BCP bone substitute products for various orthopedic and maxillofacial applications. Table 2summarizes the available BCP products based on composition ratio of HA and TCP. Furthermore, BCP has been used as carrier or delivery system for therapeutic drugs, antibiotics, hormones and growth factors to enhance bone tissue engineering [16], [17].
Table 2. The commercially produced BCP and percentage ratio of composition phases.
| HA % | TCP % | Brand name |
|---|---|---|
| > 96 | < 4 | Calciresorb (Ceraver, France) |
| 80 | 20 | Osteosynt (Einco, Brazil) |
| 75 | 25 | TCH (Kasios, France) |
| 70 | 30 |
Ceratite (NGK Spark Plug, Japan) OrthoCer HA TCP (Baumer, Brazil) |
| 65 | 35 |
CuriOs (Progentix Orthobiology BV, Netherlands) Ceraform (Teknimed, France) Calcicoat (Zimmer, IN) |
| 60 | 40 |
BCP (Depuy Bioland, France) BCP BiCalPhos (Medtronic, MN) BonaGraft (Biotech One, Taiwan) BoneMedik-DM(Meta Biomed, Korea) CellCeram (Scaffdex Oy, Finland) GenPhos HA TCP (Baumer, Brazil) Graftys BCP (Graftys, France) Hatric (Arthrex, US) Hydros (Biomatlante SA, France) Kainos (Signus, Germany) MasterGraft Granules (Medtronic Sofamor Danek, US) MBCP (Biomatlante SA, France) OpteMx (Exactech, FL) Ossceram nano (Bredent Medical, Germany) Osteosynt (Einco, Brazil) Ostilit (Stryker Orthopaedics, NJ) SBS (ExpanScience, France) TriOsite (Zimmer, IN) 4Bone (MIS, Israel) |
| 55 | 45 |
CuriOs (Progentix Orthobiology BV, Netherlands) Eurocer (FH, France) |
| 30–50 | 50–70 | Indost (Polystom, Russia) |
| 20 | 80 |
BoneCeramic (Straumann, Switzerland) BoneSave (Stryker Orthopaedics, NJ) Kainos (Signus, Germany) MBCP + (Biomatlante SA, France) OsSatura BCP (Integra Orthobiologics, CA) Osteosynt (Einco, Brazil) ReproBone (Ceramisys, UK) Tribone 80 (Stryker, Europe) |
3.2. BCP synthesis and preparation
For bone tissue engineering, the fabricated scaffolds should simulate the physicochemical properties of the bone ECM. BCP bioceramics best represent the inorganic phase of the bone ECM with mineral composition similar to that of natural bone. More stable HA phase could function as a structural framework that support the scaffold and newly formed bone, while the less stable TCP phase create space for new bone ingrowth during biodegradation process.
Various types of CaP bioceramics have been used in medicine as bone replacement materials due to their chemical similarity to the inorganic mineral phase of natural bones. Therefore, several synthetic routes have been developed to prepare BCP bioceramics of variable HA/β-TCP ratios simulating the physical and biological properties of natural bones. Nevertheless, up to now, there has been only a relative success in the fabrication of bone substitute materials similar to natural bone; this emphasizes the complexity of the natural structures. Among the available BCP preparation techniques, the most usual one consists of sintering of non-stoichiometric CaP, such as ACP and CDHA, at temperatures above ~ 750 °C [32], [33]. However, commonly, this procedure is made at temperatures exceeding ~ 1000 °C [34], [35], [36], [37], [38], [39]. The process is simple, more economic and bypasses the time-consuming purification process compared to other preparation techniques. Various modifications of sintering, such as a two-step sintering [40] and a microwave heat processing [41], [42], [43], [44] of both ACP and CDHA have been applied as well. Namely, the chemical reaction of a thermal decomposition of “Ca10 − x(HPO4)x(PO4)6 − x(OH)2 − x (CDHA)” with x = 0.5 is represented as:(1)
This reaction results in to formation of a BCP, consisting of HA and β-TCP. For this decomposition, a reasonable solid-state transformation mechanism based on the diffusion of OH− and Ca2 + ions was proposed in 2002 [45], [46]. In 2015, that mechanism was further itemized by discovering a fully separated growth of microscopic HA and β-TCP crystals, which could be adjacent, forming particles but without mutual intergrowth [47]. It is important to notice, that under strictly equal conditions, the numerical value of the Ca/P ratio of the initial CDHA influences the grain sizes of the sintered BCP, where the average grain size decreases with increasing Ca/P ratio [48].
Another preparation approach is based on solid-state reactions between two solid compounds, performed at elevated temperatures. The examples comprise solid state reactions between calcium hydrogen-phosphate dihydrate (brushite, CaHPO4·2H2O) and calcium carbonate (calcite, CaCO3) [49], [50], TCP and Ca(OH)2 [51], monocalcium phosphate monohydrate (Ca(H2PO4)2·H2O) and CaCO3 [52], [53]. Thus, to prepare BCP, initially, the chosen Ca- and P-containing compounds are mixed at known proportions to get the desired Ca/P ratio (1.50–1.67) followed by heating and sintering. When milling of the initial reagents was applied, the proportion of HA and β-TCP phases in the prepared BCP was found to depend on the milling time [50]. Furthermore, BCP could be prepared by other methods, such as a flame spray pyrolysis [54], a liquid mix [55] and a sol-gel [56], [57] techniques; both latter processes must be followed by sintering [55], [56], [57]. Regarding the initial raw materials, BCP could also be manufactured from the natural CaP containing recourses, such as bovine [58], [59], [60] and cuttlefish [61] bones, corals [62] and algae [63]. The various processing techniques yield diverse compositions and properties of the final BCP formulations, which is crucial for further applications.
In addition, a mechanical blending of the desired amounts of either the individual powders of HA and β-TCP [64], [65], [66], [67], [68], [69] or their precursors might also be used to produce BCP. For instance, one can mix two types of CDHA powders, such as one with x = 0.1 (almost HA) and another one with x = 0.9 (almost TCP) and sinter the mixture. In this case, the first CDHA will decompose to HA with a small admixture of β-TCP, while the second CDHA will decompose to β-TCP with a small admixture of HA, resulting in BCP (HA + β-TCP). Synthesis conditions of the precursive non-stoichiometric CaP were found to influence the phase ratio in the final BCP [70]. However, due to inability to get homogenous crystal distributions of the mixed phases, a blending of both individual CaP and their precursors is not suggested for production of BCP formulations with reproducible structure and composition. Furthermore, such types of mechanically blended BCP bioceramics were found to exhibit both an elevated extent of dissolution [71] and an inferior sintering performance [72] if compared to those manufactured by a thermal decomposition of a CDHA powder of the equal Ca/P ratio. On the other hand, when soaking in simulated body fluid, the weight increase for the mechanically blended BCP was found to be greater than that for precipitated BCP [64]. Besides, in mechanical blends with β-TCP, HA could be partly decomposed at sintering, changing the HA/β-TCP ratio in the final BCP [67]. Moreover, if sintering is performed at temperatures exceeding ~ 1250 °C, formation of α-TCP phase becomes possible, which results in transformation of the initially biphasic blends of HA + β-TCP into triphasic (HA + β-TCP + α-TCP) ones [73], [74]. The latter process is reversible because dwelling of the HA + β-TCP + α-TCP formulations for several hours at ~ 900 °C results in a reverse change of α-TCP to β-TCP, and, thus, transformation of the triphasic formulations into BCP (HA + β-TCP) again [73].
Furthermore, in the presence of dopants, various ion-substituted forms of BCP might be synthesized [75], [76], [77], [78]. Depending on the nature of dopants, in such formulations the doping elements could be present in either phase. For example, F [79], Cl [80], Si [81] and carbonates [82], [83] appeared to enter more readily into the HA phase, Zn [84] and Sr [85], [86] were found to enter into both phases, while Na [77], K [87], Mg [88], [89], [90], [91], Mn [92], [93], [94] and Nb [95] entered preferably into the β-TCP phase [96]. Furthermore, addition of MgO to BCP was found to suppress a phase transition from β-TCP to α-TCP [88].
3.3. Physicochemical properties and characterization
BCP chemical formulation and phase compositions should be stable to fit the purpose of clinical applications. BCP is vulnerable to moisture contamination that could transform it to single phase products such as CDHA. For this reason they should be stored in a clean and dry condition away from heat to maintain its chemical nature prior to clinical application [18].
The major physicochemical properties of BCP are similar to the single phase bioceramics (HA and β-TCP) and depending on the composition ratio of each phase their properties may differ. However, the critical issue is their in vivo differences such as biodegradation, precipitation of apatite crystal on their surface, protein adsorption and cellular behavior [25], [97], [98].
The most common method for characterization of BCP is X-ray diffraction (XRD) technique. XRD is used for crystallography and phase identification and quantification of bioceramics materials using X-ray diffractometer machine (with Cu-Kα radiation). For BCP the usual XRD scan range is from 20° to 60° because most of the CaP phases have the strongest peaks at this range [99]. XRD analysis is the only technique that can provide a mean of approximating the HA/β-TCP ratio in the BCP. This ratio is determined using the ratio of intensities of the most intense diffraction peaks of the HA phase to those of the most intense diffraction peaks of β-TCP phase compared with the ratios obtained from calibrated standard mixtures of pure HA and β-TCP [27] (standard XRD card, JCPDS Card 09–0432 for HA and 09-0169 for β-TCP).
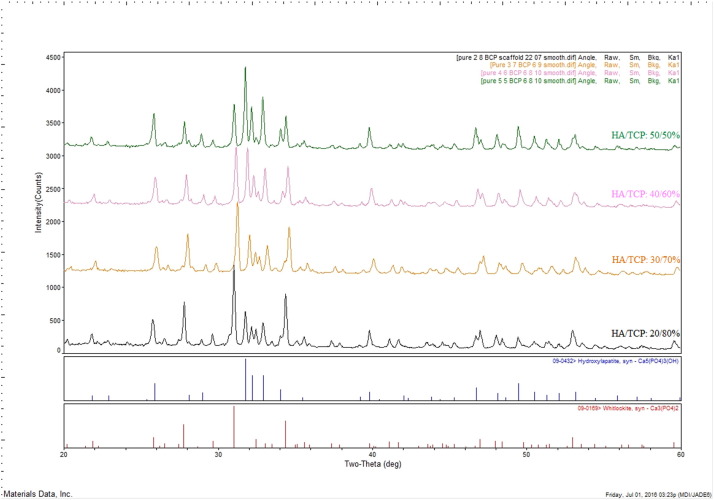
XRD pattern of BCP (HA/β-TCP) indexes main peaks corresponding to HA (JCPDS no. 09-0432) and β- β-TCP (JCPDS no. 09-169) in accordance to ICDD standard (The International Centre for Diffraction Data). Fig. 1 shows the main characteristic peaks of HA at 2-Theta diffraction angles and the absolute intensity (a.u.) relevant to each peak as follow (°/a.u.); 25.9°/628, 31.8°/1469, 32.2°/816, 33°/900, 34.1°/425, 46.8°/442 and 49.6°/519. For β-TCP, the main peaks are indexed at; 25.8°/315, 26.7°/495, 29.7°/380, 31.1°/887, 32.6°/350 and 34.5°/639 (Fig. 1) [100]. The presence of other secondary phases (i.e. α-TCP or calcium oxide) can also be evaluated to determine phase decomposition or transformation during biomaterials processing such as sintering process [12], [27], [99], [100]. It is obvious that by decrease of β-TCP ratio in BCP, a lower intensity of β-TCP and a higher intensity of HA peaks are indexed which is relevant to each HA/β-TCP ratio (Fig. 1).
 Fig. 1. XRD pattern of sintered BCP scaffolds with different HA/β-TCP composition ratios.
Fig. 1. XRD pattern of sintered BCP scaffolds with different HA/β-TCP composition ratios.The Reference Intensity Ratio (RIR) is a general method for quantitative phase analysis by scaling all diffraction data to the diffraction of the standard reference materials [101]. Software programs such as “ICDD's Sleve” allow identification of materials and calculation of peak intensity and concentration of each phase by RIR method. For complete structural description of a material, use of Rietveld structural refinement techniques and powder pattern indexing are complementary. These methods allow accurate phase identification by lattice matching techniques [102].
Fourier transform infrared spectroscopy (FTIR) is useful for chemical characterization of major functional groups. This technique can be used for chemical analysis of BCP and other organic components if present (Fig. 2). FTIR is a very sensitive technique for determining phase composition and transformation of one phase to another [99], [103]. Analyzing the chemical structure using FTIR reveals that both HA and β-TCP show the absorption bands corresponding to PO43 − group at approximately 1090–1120 (ν3), 1040–1050 (ν3), 945–970 (ν1), 600–610 (ν4) and 550–570 (ν4) cm− 1, and bands corresponding to CO32– group at 870–880 (ν2) and 1445–1455 (ν3) cm− 1 (Fig. 2) [100]. Furthermore, for HA, additional absorption band corresponding to OH− group can also be detected at 630 and 3572 cm− 1 due to stretching vibration of OH−ions. However, this peak cannot be seen in TCP since chemical structure of TCP lack OH− group. It is worth mentioning that adsorbed H2O molecules may express extra broads at 3433 and 1642 cm− 1 [104]. Therefore, in FTIR pattern of BCP, in addition to peaks of PO43 − and CO32 −, the peaks of OH− groups can also be detected relevant to the ratio of HA.
 Fig. 2. FTIR pattern of HA and β-TCP showing major corresponding peaks of PO43 − and CO32 −.
Fig. 2. FTIR pattern of HA and β-TCP showing major corresponding peaks of PO43 − and CO32 −.Other available techniques such as X-ray fluorescence (XRF) and inductively coupled plasma-optical emission spectrometry analysis (ICP-OES) can also be used for analyzing the calcium phosphate molar ratio [105].
Physical characterization of bioceramics including BCP can be performed to determine the shape, size and distribution pattern of bioceramics particles using transmission electron microscopy (TEM), laser diffraction particle size analyzer (PSA) and scanning electron microscopy (SEM).
3.4. The optimal composition ratio
The abundance of CDHA crystals on the surface of BCP implant appears to be inversely related to HA/β-TCP ratio. The lower the ratio, the greater the abundance of CDHA crystals on the surface [106]. This in turn influences the bioactivity of BCP therefore, the control of the ratio of the BCP is crucial to achieve desired biological outcomes.
Various HA/β-TCP ratios have been tested in order to improve BCP properties. An ideal balance between these two phases may improve mechanical strengthand enhance the biological behaviors of BCP scaffolds [27]. The first studies of BCP with multiple ratios of HA/β-TCP reported by Daculsi et al. (1989) demonstrated that manipulating the HA/β-TCP ratio could control the bioactivity of these bioceramics [16].
However, similar physicobiological properties of BCP may not be the distinctive feature of a single ratio of HA/β-TCP but rather applies to a range of close ratios. For example, in comparison to HA/β-TCP ratio of 20/80, similar results were also found with HA/β-TCP ratios of 15/85 [107], 25/75 [108] and 30/70 [109]. Furthermore, some studies reported that formulations containing HA/β-TCP ratio of 50/50 [110] and 60/40 [111] enhanced cell proliferation to a higher extent. Therefore, the relationship between the composition ratio of BCP and cellular behavior is complicated and requires further development of standardized protocols for material preparation, characterizations, and analysis of biological behaviors.
The literature is not clear with regards to the effect of each particular ratio on bone tissue regeneration, particularly in animal studies. The findings in the literature on biological in vivo performance of different ratios of BCP can be categorized into three groups; 1) those who reported the beneficial effects of a particular ratio compared to other ratios in enhancing bone regeneration, 2) those who did not find any differences between various studied ratios, and 3) those who reported detrimental effects of BCP on healing and bone regeneration in comparison to autograft and control groups (no graft). Table 3summarizes the comparison of different parameters among animal studies, including all the available and reported data by authors.
Table 3. Summary of literature on animal studies used different composition ratios of BCP (HA/β-TCP), their investigated parameters and main findings.
| Authors/year/reference | HA/TCP % | Animal/no. age. wt. | Site of implant | Defect dimension | BCP characterization | Investigation times and follow up period | Type of investigations | Main findings |
|---|---|---|---|---|---|---|---|---|
| Daculsi G et al. 1989 [16] |
85/15 65/35 15/85 |
Beagle dogs no: NR age: NR wt: NR |
Surgically created periodontal defects? | NR | Macroporosity 150-200 μm, porosity: NR mechanical property: NR | 6 & 12 months | Histologic, TEM, electron microdiffraction, microanalysis X-ray |
|
| Nery EB et al. 1992 [107] |
100/0 85/15 65/35 50/50 35/65 15/85 0/100 |
Adult beagle dog, no:21 age: NR wt: NR |
Mesial to maxillary & mandibular first molars and canines | NR 3-wall intrabony defects? | NR | 6-months | Histologic |
|
|
Schopper C et al. 2005 [115] |
30/70 50/50 100/0 |
Female sheep, no: 6 24–48 months 58.5 kg |
Rib corticocancellous defects 6 holes/rib |
5 × 5 mm |
Granulates, particle sizes 0.5–1 mm, porosity: NR mechanical property: NR |
6 & 12 months | Histologic, histomorphometric, backscattered SEM |
|
| Bodde EWH et al. 2007 [132] |
75/25 0/100 |
Female sheep, No: 9 24–36 months 50 kg |
Trabecular bone of the femoral medial condyles, bilaterally 2 holes/side |
Ø 6 × 9 mm depth |
0/100 (Conduit™); irregular granules, Ø 1.5–3 mm. Interconnectivity 70%, pores 1–600 μm. 75/25 (Biosel®); 3 mm cube-shaped particles, interconnectivity 70%, pore 200–500 μm. Mechanical property: NR |
3, 12, 26 weeks | Histologic, histomorphometric, radiologic |
|
| Jensen SS et al. 2007 [119] |
60/40 100/0 0/100 |
Adult Göttingen minipig no: 16 age: NR 55 kg |
lateral mandibular body and ramus 4 holes/side |
Ø 7 × 4 mm depth |
Particle size of 0.5–1 mm, macropore 100–500 μm, total porosity of 90%, crystallinity 100%. Mechanical property: NR |
2, 4, 8, 24 weeks | Histologic, histomorphometric, radiologic |
|
| Balçik C et al. 2007 [117] |
60/40 100/0 |
White rabbit no: 38 4 months 1.94 kg |
Central third of the right and left tibia | 10 mm long segment |
Macropore 150–200 μm, 2nd pores 20–100 μm. Microporosity 1–2 μm, compressive strength 4.9–5.3 MPa, elastic modulus 3.1–3.5 GPa, porosity: NR |
4, 6, 8, 12, 18 weeks | Histologic, radiologic, DXA, Q-CT scan (quantitative), 3-point bending test |
|
| Fariña NM et al. 2008 [112] |
85/15 15/85 |
Beagle dog no: 6 24 months 20 kg |
Right and left mandibular bone, 4 holes/side | Cylindrical 2 × 6 mm |
Particle size 5 μm porosity: NR mechanical property: NR |
4,12, 26 weeks | Histologic, histomorphometric, SEM, X-ray microanalysis |
|
| Chissov VI et al. 2008 [133] |
100/0 80/20 0/100 |
Wistar rat no: 108 age: NR wt: NR |
Fenestral defect in rat shin bone | 5–7 mm fenestral defect in shin bone |
Granule size 300–600 μm, Pore 10–30 μm, porosity: NR mechanical property: NR |
3,6,9,12 weeks, & 6, 9 months | Histologic |
|
| Jensen SS et al. 2008 [120] |
80/20 60/40 20/80 |
Göttingen Minipig no: 24 age: NR 59.2 kg |
Mandibular body, 3 holes/side | Ø 9 × 4 mm depth | Macropores 100–500 μm, total porosity 90%, crystallinity 100%, crystal size < 5 μm, particle sizes 0.5–1 mm, mechanical property: NR | 4, 13, 26, 52 weeks | Histologic, histomorphometric |
|
| Park JW et al. 2010 [113] |
60/40 0/100 |
Adult male New Zealand white rabbit no: 30 age: NR 3.7 kg |
Transosseous defect in the mid-portion of parietal bone | Ø 8 mm? |
60/40A: donut shaped, 300–400 μm central pore, particle size 0.8 mm. 60/40B: rod shaped, no central pore, particle size 0.6 × 2 mm. Micropore 20–60 μm, small micropore 1–8 μm, grain size 300–400 nm, porosity: NR, mechanical property: NR |
4, 8 weeks | Histologic, histomorphometric |
|
| Hung C-L et al. 2011 [122] |
70/30 60/40 0/100 |
Mature beagle dog no: 4 24–36 months 10 kg |
All Premolars extraction site max. 2 holes man. 4 holes |
Cylindrical Ø 3 × 6 mm length | Granules, particle size 0.5–1 mm, macropore 400–600 μm, porosity: NR, mechanical property: NR | 2, 4, 6, 8 weeks | Histologic, histomorphometric |
|
| Yun P-Y et al. 2014 [114] |
30/70 20/80 |
Pilot 1: rabbit age: NR 2.8 kg no: 12 pilot 2: beagle dog no: 6 5–6 months 9 kg |
Pilot 1: calvarial defects 4 holes/rabbit pilot 2: mandibular sockets of extracted premolars |
Pilot 1: Ø 7 × 7 mm pilot 2: 6 × 6 × 3 mm |
Macropore 250-400 μm, macroporosity 70–75%, mechanical property: NR | 2, 4, 8 weeks | Histologic, histomorphometric, micro-CT |
|
| Hong JY et al. 2014 [121] |
100/0 60/40 0/100 |
Male beagle dog no: 8 18 months 15 kg |
Extraction socket of all third premolars of maxilla & mandible. 4 exo/jaw |
-NR |
100/0 (Calcitite® 2040); particle size 420–840 μm. 0/100 (Cerasorb®); particle size 0.5–1 mm. BCP (BoneMedik-DM®) particle size 0.5–1 mm. Porosity: NR mechanical property: NR |
2, 4, 8 weeks | Histologic, histomorphometric |
|
| Kunert-Keil C et al. 2015 [116] |
60/40 0/100 |
Lewis rat no: 24 2 months 300 g |
Parietal region of the cranium | Ø 5 mm? | Round granules, total porosity 70%, mechanical property: NR | 4 weeks | Histologic, immunohistochemistry, RT-PCR |
|
| Lim H-C et al. 2015 [118] |
70/30 30/70 |
Adult New Zealand white rabbit no: 8 age: NR 3 kg |
Bilateral sinus lift | Ø 6 mm? |
Porosity 77%, pore size; BCP 70/30: 300–500 μm, BCP 30/70: 250 μm. Mechanical property: NR |
2, 8 weeks | Histologic, histomorphometric, micro-CT |
|
Abbreviations and symbols: DXA: Dual-energy X-ray absorbtiometry, Exo: extracted tooth, NR: not reported, RT-PCR: reverse transcription polymerase chain reaction, TEM: transmission electron microscopy, wt: weight, Ø: diameter, ?: not fully reported.
An in vivo study reported a greater gain in probing attachment levels and bone regeneration in periodontal defects when lower ratios of TCP were used (HA/β-TCP ratios of 85/15 and 65/35 versus 0/100 and 50/50) [107]. On the other hand, it has been reported that faster and greater quantity of bone is formed in 15/85 ratio due to the greater osteoinductive potential compared to 85/15 ratio [112]. JW Park et al. [113]. reported that 60/40 ratio exhibited a significantly greater percentage of newly formed bone when compared with 0/100 ratio at both 4 and 8 weeks with direct ingrowth of bone tissue and blood vessels. However, other researchers have reported that 30/70 ratio (compared to 20/80) showed a faster bone resorption and more favorable result in new bone formation and space maintenance, particularly at 8 weeks [114].
In contrast to previous findings, some studies reported no statistical significant differences (p > 0.05) between ratios in terms of new bone formation, bone volume, volume stability, bone mineral density and osteoconductive capacity in the defect site (HA/β-TCP ratios of 30/70 vs. 50/50 [115]; 60/40 vs. 0/100 [116], 60/40 vs. 100/0 [117] and 70/30 vs. 30/70 [118]). However, a high β-TCP ratio did not impair the volume stability of an implant biomaterial before bone ingrowth.
In comparing different BCP ratios with autograft at 8 weeks, Jensen et al. [119]. reported the greatest bone formation in defects with autograft. This was then observed to be reduced over the different BCP with decreasing TCP ratios - 0/100 > 60/40 > 100/0, respectively. However, at the end of the study (24 weeks), they failed to detect any difference in bone formation between the study groups. HA/β-TCP ratios of 100/0 and 60/40 demonstrated similar resorption patterns which was at a slower rate, whereas the autograft and 0/100 ratio showed faster biodegradation and substitution with newly formed bone. In a later report by same group [120] they revealed that 20/80 ratio showed bone formation and biodegradation similar to autografts whereas 60/40 and 80/20 ratios were similar to DBBM (deproteinized bovine bone mineral). They concluded that the amount of bone formation and biodegradation of BCP is inversely proportional to the HA/TCP ratio.
The majority of studies have reported that regardless of the ratio, BCP in general enhances the rate and quality of bone regeneration when compared to empty control groups. However, Hong et al. [121]. reported that bone formation in extraction sockets was delayed in the sockets grafted with BCP and showed different healing process according to the biodegradation patterns. In addition, the percentage of new bone was significantly higher in the control group (no graft) compared with the grafted (BCP) groups at all healing periods (2, 4, and 8 weeks). Furthermore, they reported higher numbers of multinucleated cells in 60/40 and 0/100 ratios, followed by 100/0 ratio and smallest number in the control group. This was contributed to a lower percentage of residual bone of 0/100 ratio than 100/0 and 60/40 ratios at all healing periods. Contrasting to these results, in a similar study of dental extraction sites of beagle dogs, Hung et al. [122] reported that ratios of 70/30 and 60/40 provided better new bone regeneration rate than 0/100 ratio and empty control groups without any toxic or inflammatory reactions.
In general, BCP scaffolds are osteoconductive, but as mentioned earlier, some studies reported their possible osteoinductive property. This is due to the evidence of ectopic bone formation upon implantation in non-osseous sites (e.g. subcutaneously or in intramuscular sites). It has been shown in ectopic sites that an optimal balance between the ratio of the HA and β-TCP can induce mesenchymal stem cells (MSC) for enhanced rate of new bone tissue formation. HA/β-TCP 20/80 scaffolds seeded with human MSC have been shown to have a highest rate of bone formation compared to other HA/β-TCP ratios (76/24, 63/37, 56/44), pure HA and pure β-TCP [123]. However, osteoinductive property may not be inherent in nature but could be attributed to the critical geometry, topography and ratio which allow local concentration of endogenous BMPs or growth factors on materials' surface to induce osteoinductivity [124], [125].
Currently, there is no general agreement on an ideal ratio of each phase of BCP for clinical applications. Various HA/β-TCP ratios have been evaluated in the literature in order to determine the best ratio for optimum bone regeneration, however, only BCP ratios of HA/β-TCP 65/35, 60/40 and 50/50 have been applied successfully in human clinical trials [126], [127], [128], [129], [130]. Interestingly, two ratios of HA/β-TCP; 30/70 and 20/80 have also been reported to reveal some osteoinductive properties [2], [29], [131].