Introduction
Carbon capture and storage (CCS) comprises separation of CO2 from industrial sources, compression and transportation to a geologic site for storage, or to enhanced oil recovery (EOR). Its uses cover a variety of industrial applications, outstanding abatement of CO2 from process or exhaust gases and in natural gas (NG) processing. In the former, depending on the technology, CO2 is separated from H2 (pre-combustion), N2 (post-combustion) and H2O (oxy-combustion, which burns hydrocarbons with pure O2) [1], while in NG processing CO2 is separated from CH4 and light hydrocarbons [2].
Flue gas is released from carbon-fired power plants at moderate temperature (50–100°C) and low pressure (<1.5 bar). Post-combustion with chemical absorption or physical absorption are the technologies closest to full scale realization and preferred for retrofitting [3••]. Although post-combustion and NG processing may employ different technologies, capture of CO2 by chemical and physical absorption are their leading options, where the solvent loading (α, mol CO2 per mol of active solvent, AS) is a capture response while the capture ratio (CR, kg of total solvent per kg of fed CO2) and the solvent regeneration heat ratio (HR, kJ per kg of fed CO2) are input factors [4••]. In chemical absorption, CO2 and the AS chemically bond giving high selectivity and low hydrocarbon losses (NG processing) with maximum stoichiometric α of 1 molCO2/mol at CR10–15 kg/kgCO2 and reversibly requiring high solvent regeneration HR (2000–4500 kJ/kgCO2). In physical absorption, weak physical binding of CO2 to solvent reduces selectivity, but can give α > 1 molCO2/mol of AS at low CR (1–5 kg/kgCO2) and low HR (0–500 kJ/kgCO2) for solvent regeneration. In chemical and physical absorption, the equilibrium α increases with CO2 fugacity (CO2partial pressure) and decreases with increasing temperature [86••].
Pre-combustion firstly reforms fossil fuel to synthesis gas (syngas, H2 + CO), and, in a second step, to H2 and CO2 via water-gas shift (WGS) reaction. H2 is purified via chemical or physical absorption of CO2 (easy separation due to high CO2 partial pressure) and can fuel supercritical boilers, gas turbine (in H2-fired power plants) or promisingly used in integrated gasification-combined cycle (IGCC) power plants [5]. In H2-IGCC, high capital expenditure (CAPEX) of syngas, WGS and capture units are drawbacks, and H2 as fuel requires development of new power machines, another H2-IGCC risk [6]. In typical coal-fired power plants, the power efficiency reduces from 38.4% without CO2 capture to 31.2% with CO2 capture [7], a susceptibility eliminated by changing to full Coal-H2-IGCC. The capture energy penalty in a carbon-fired power plant is the fraction of power output lost by implementing CO2 capture.
Oxy-combustion eliminates N2 in oxidizer of carbon-fired power plants [5], substituting CO2–N2 post-combustion separation by O2–N2 fractionation via cryogenic distillation, the most cost-effective commercially available route, though with refrigeration energy penalties, in the same range as that for fossil fuel de-carbonization [8]. As oxy-combustion flame temperature [5]. Oxy-combustion is not yet commercial, posing greater technical risks than pre-combustion or post-combustion for large-scales [6]. Porter et al. [9] discuss cost and CO2 purity variations for oxy-combustion and pre-combustion scenarios.
In NG processing, CO2 must be removed to comply with treated gas specifications. A determinant change in the technological scenario is pulled by offshore NG processing, mainly at ultra-deep waters on FPSO (Floating Production, Storage & Offloading) platforms. Membrane permeation offers advantages over conventional chemical or physical absorption for NG processing: small footprints, modularity and easy scale-up. The treated NG is the membrane permeation retentate at high pressure, which fits the final compression for pipeline dispatch.
Considering the state-of-the-art, Figure 1 depicts the CCS scenario, contemplating CO2-EOR and other CO2 sources (e.g., fertilizers, cement and steel production), including bioethanol plants producing food grade CO2 from fermenters, which can be directed to downstream CCS. This work analyzes the main technologies involved, focusing in identifying technological gaps requiring innovations and technology drivers in the big CCS scenario. Table 1 presents a compilation of state-of-the-art and advanced processes, including those at lower Technology Readiness Level (TRL) [72], from proof-of-concept to small pilot plants.
 Figure 1. The big CCS picture filtered by state-of-the-art CO2 capture routes. The expression ‘energy penalty’ refers to the decrease in energy generation efficiency resulting from electric and thermal power demand from CO2 capture processes.
Figure 1. The big CCS picture filtered by state-of-the-art CO2 capture routes. The expression ‘energy penalty’ refers to the decrease in energy generation efficiency resulting from electric and thermal power demand from CO2 capture processes.Table 1. CO2 capture technologies
| STATE OF THE ART (high technology readiness level—TRL, large-scale demonstration projects and/or commercial use) | |||
|---|---|---|---|
| Technology | Benefits and application | Gaps and challenges | References |
| Chemical absorption | Mature technology for NG processing and post-combustion; capture-ready standard for carbon-fired power plant; high capture efficiency and selectivity; low hydrocarbon losses; adequacy via CO2 partial pressure | High capture ratio (CR) and heat ratio (HR, energy penalty for solvent regeneration); high capture energy penalty (≈20–30%) for coal-fired power plants; corrosion, emissions and solvent degradation; new solvents challenges to: increase thermochemical stability; reduce CR, HR and stripping temperatures allowing use of waste heat | [104, 29•, 48•, 85, 3••] |
| Physical absorption | Mature technology for NG processing and post-combustion; capture-ready standard for carbon-fired power plants; high capture efficiency; low heat ratio (HR) for regeneration; adequacy via CO2 partial pressure | Low selectivity; hydrocarbon losses | [86••, 3••] |
| Membrane permeation | Used in NG processing of large-scale FPSOs; no regeneration; no chemicals; low footprint; adequacy via CO2 partial pressure | Demands compression of fed NG and permeate; hydrocarbon losses; trade-off permeability-selectivity; low CO2 partial pressure forbids it in post-combustion | [87•, 88] |
| Pre-combustion | Applicable to coal-fired power plants; potential lower cost; commercial for H2 production; high efficiency; low capture energy penalty (≈10–15%); large-scale in H2production | Complex scheme; novel materials for high temperature CO2 capture; high capital expenditure (CAPEX); still in development; insufficient large-scale H2-fired power plant experience | [89, 90•, 91, 86••, 3••] |
| Cryogenic distillation | Mature technology for processing NG with high CO2 content; high selectivity; low hydrocarbon losses; CO2obtained as liquid with benefits in CO2 transport (no compressors needed, pumps used instead); appropriate for high CO2content | Refrigeration energy penalties; avoidance of CO2 freeze-out required | [35, 3••] |
| ADVANCEMENTS (low technology readiness level—TRL, inadequate large-scale experience) | |||
|---|---|---|---|
| Technology | Advanced features | Claimed benefits | References |
| Hybrids | Bulk CO2 removal by cryogenic distillation or membrane permeation with polishing via chemical or physical absorption | Lower costs and capture energy penalty | [92, 2] |
| Enhanced chemical or physical absorption | Complex flowsheets; mixed solvents | 12% less equivalent work than a simple stripper; heat ratio (HR) reductions via mixed solvents instead of MEA solution. Increased thermochemical stability | [93, 85] |
| High efficiency solvents | Reduced HR | Luo et al.[71] | |
| Hybrid solvents | Reduced HR | [95] | |
| Anhydrous solvents, outstanding TSILs (Task Specific Ionic Liquids) and CO2BOLs (CO2-bonding Organic Liquids) | Low vapor pressure eliminating fugitive stripping emissions in regeneration. In addition, being anhydrous, the problem of high parasitic energy consumption associated with water | [96••, 97] | |
| Ionic liquids | Reduced HR, reduced loss by evaporation | [98, 99] | |
| Phase changing solvents | Reduced HR, phase switch triggered by CO2 loading | [100, 101, 102, 103, 76, 97] | |
| Addition of inert solvent for CO2 solventing-out | Use of waste heat in solvent regeneration | [104] | |
| Metal-organic solvents | Lower HR | [30] | |
| Membrane permeation | New materials | While having the benefit of high flux [87•], the low pressure of exhaust gases requires highly CO2permeable and selective membranes for application | [105] |
| Metal oxide framework (MOF) membranes | Superior thermal and chemical stability | [106, 107] | |
| Dense mixed-conducting membranes (MCMs) | Superior thermal and chemical stability | [92] | |
| Integrated membrane material and process development for gas separation | Sustained membrane permeability | [108] | |
| Multi-stage schemes | Higher efficiency | [109] | |
| Steam as sweep agent. For lean CO2 flue gas, driving force is low, unless compressed fed (≈2–4 bar) and/or permeate vacuum are used | Efficient permeate removal, avoiding CO2 buildup, reducing membrane area for exhaust gases even at low CO2 content | [110] | |
| Solvent supported membranes | Solvent with negligible volatility (ionic liquids and deep eutectic solvents) to increase selectivity | [118] | |
| Gas–liquid membrane contactors | Synthesis, characterization and performance of various membrane materials, contactors and their design aspects | Higher efficiency; higher modularity; independence of gravity; no flooding effects | [111••, 112] |
| Adsorption | New sorbent materials (e.g., residues from industrial and agricultural activities; Metal-organic frameworks) | With higher surface area, high selectivity and high regeneration ability, reducing energy penalty | [113•, 3••, 114•, 115, 86••] |
| Amine-functionalized solid sorbents | Reduced energy for regeneration | [116•, 94, 117] | |
| Oxy-combustion | Simplifies post combustion capture; high efficiency | High efficiency; low capture energy penalty | [3••, 86••, 90•] |
| Chemical looping combustion | Use of metal oxide as oxygen carrier, which is reduced to oxidize fuel to CO2 and water, being regenerated in a second stage | Low capture energy penalty | [3••] |
| Mineralization | Conversion to a solid material | Commercialization | [86••] |
Carbon capture from exhaust gases
Capture energy penalty on carbon-fired power plants is significant (≈15–30%) [10] representing 65–80% of CCS costs [11,12••]. To retrofit carbon-fired power plants with 33% power efficiency, a decrease of 12% of efficiency represents more than 1/3 of power output [13], with a capital expenditure (CAPEX) increase of ∼77% [14]. Carbon-fired power plants face large variations in CO2emissions due to differences in efficiency and employed fuel: coal-fired power plants emit 1116 gCO2/kWh at 30% and 669 gCO2/kWh at 50% of efficiency [15, 5].
Despite coal being the most CO2 intensive option, capacity expansion plans [67] indicate that carbon mitigation initiatives are insufficient to outweigh the economic incentives of a relatively cheap fuel. Concerning CAPEX, NG-fired power plants configure the best alternative with half CAPEX of coal-fired power plants and 1/5 of nuclear plants [16]. Impacts on operational costs (OPEX) are quantified mainly by simulation [17]. Uncertainties in overall performance are estimated probabilistically [18]. CAPEX estimation uncertainties are high (±40%), though variability has little influence on the levelized cost of energy (LCOE) [19], suggesting that OPEX dominates CCS.
Boot-Handford et al. [20••] present extensive review on leading CO2 capture technologies, available in the short and long term and their maturity. Post-combustion CO2 capture employing chemical absorption remains the most efficient and cost-effective capture [21], with heat demand (OPEX) for solvent regeneration as main drawback, reducing power capacity (capture energy penalty ≈10–30%), despite recent improvements lowering heat ratio (HR, energy penalty for solvent regeneration) from 5.5 to 2.6 GJ/tCO2. Carbon-fired power plant repowering or hybridization using solar-assisted post-combustion may conciliate capture and power plant load targets [22]. Limitations of driving force indicate that state-of-the-art membrane permeation are unlikely to compete with chemical absorption in capturing CO2 from exhaust gases [21].
The deployment of renewable energy substitutes partially the need of (fossil) carbon-fired power plants, reducing the amount of fossil-fuel burned. However, renewable energy dispatch is intermittent, demanding flexible operation of the capture unit to improve the economics of CCS power plants [23]; flexibility allows exploring this transient pattern to reduce CAPEX up to 28% [73]. With chemical absorption, flexibility can be achieved by solvent storage, exhaust gas venting (decoupling energy generation from CO2 capture, to meet peak energy prices) and time-varying solvent regeneration (allowing CO2 to accumulate in the solvent at peak energy prices) [24]. Variable capture aligned to energy demand and dispatch [25] results in temporary reduction of capture energy penalty, increasing net efficiency and capacity [26]. For instance, the absorber sized for a time-average condition costs ∼4% less then when sized for peak energy generation [27].
Capture energy penalty can be minimized by new solvents or flowsheetmodifications, reducing power losses by 25% [28••], conciliating the tradeoff of sensible heat loss (to raise the temperature of the stripper feed) at high solvent rate (high lean loading) and stripping steam use at low solvent rate (low lean loading) [20••]. Additionally, low solvent thermochemical stability [29•] leads to accumulation of degradation products and toxic emissions [30].
Evolving from the first commercial plant (first of a kind, FOAK) to the nth commercial plant (nth of a kind, NOAK) reduces OPEX and CAPEX [31]. Alternative technologies are sought, posing greater risk because of their earlier stage of development [6]. Emerging technologies (e.g., new membranes and solvents) with potential for ‘game-changing’ improvements are still scheduled to large-scale testing by 2025 and complete demonstration scale testing by 2030 [64]. Besides low TRL (Technology Readiness Level), a major issue in post-combustion technologies is the huge scale-up in size required for full scale (10X) and integration with carbon-fired power plants.
CO2 capture from natural gas (NG)
Zahid et al. [32] list eight large scale CCS projects (six for EOR and two in saline aquifers): four applications use physical absorption with Selexol (the largest facilities, notedly Century Plant and Shute Creek Gas Processing Facility, with 8 and 7 Mtpa, respectively), two use chemical absorption with MDEA (N-methyl-diethanolamine) (Sleipner and Snøhvit CO2 Storage Projects), one uses chemical absorption with DGA (diglycolamine) (Uthmaniyah CO2-EOR Project) and one uses membrane permeation (Petrobras Lula Field, ∼1 Mtpa). Analogous to post-combustion in exhaust gases, NG processing is dominated by absorption, mainly physical absorption.
A ‘game-changing’ scenario is found in offshore NG processing on FPSOs, where limited area creates a technology niche for membrane permeation due to its low footprint and modularity. In Brazil Pre-Salt oil and gas fields, the first FPSO started operation in 2010, employing membrane permeation for separating CO2, used in early CO2-EOR. In 2016, seven FPSOs where in operation [33], with six using membrane permeation. By the end of the decade, there will be sixteen FPSOs processing gas with membrane permeation, each processing 4–7 MMscmd of NG with ≈20% CO2 [2].
The main factors in selecting NG processing technologies are the partial pressure of CO2 in raw NG and plant location (onshore versus offshore). Chemical absorption is best suited to low CO2 feeds (<20%, as higher CO2content increases solvent recirculation rate and heat-duty). From medium to high CO2 partial pressure, membrane permeation outperforms chemical absorption [34••]. For ultra-high CO2 contents – Libra (48%) and Jupiter (78%) offshore Pre-Salt fields in Brazil and La Barge gas field (65%) in Wyoming (USA) – cryogenic distillation comes to scene. It has the advantage of producing liquid CO2, suitable for pipeline transportation. At low temperatures and high pressures, freeze-out of CO2 may occur, demanding special technologies as in the Ryan Holmes process [35].
Extreme scenarios demand NG processing innovations inspired in Ormen-Lang Project, where raw NG and monoethylene glycol as anti-hydrate, are transported through two subsea 120 km pipelines [36]. Raw NG may be sent to onshore facilities for separation of CO2 and fractionation of NG liquids, where CO2 is pipelined back to offshore CO2-EOR [37]. Hybrid processes can use cryogenic distillation for bulk separation, reducing CO2 composition so that chemical or physical absorption is usable [38]. Hybrid NG processing using membrane permeation for bulk removal and chemical absorption for polishing was evaluated [2], with superior economic performance and smaller footprint when compared to conventional alternatives (chemical and physical absorption) and membrane permeation alone.
CO2 transportation
Excepting cases where the CO2 source is located above a suitable geological formation (e.g., offshore NG processing), CO2 must be transported from capture points to destination sites [39]. Relevant aspects of transportation are CO2compression to supercritical state, pipeline corrosion and the impacts of fluid composition on power consumption [11••]. A design aspect of CO2 pipelines is that CO2 should remain above its critical pressure. This can be achieved by recompression along the pipeline, which is needed for distances above 150 km.
Cost of CO2 transport does not limit penetration of CCS, but impacts site choice [40•]. Transporting large quantities of CO2 is most economical with pipelines, a mature technology in CCS chain with 40 years of age in the USA, transporting 50 Mtpa CO2 through 3600 miles [41]. To reduce costs, shared transport network must be encouraged [42•], as pipeline transport costs benefit from economies of scale [43].
The knowledge of thermodynamic and transport properties of CO2 mixtures is important for designing CCS [44]. Since a major cost of the transportation/storage stages of CCS is compression of the CO2 stream, any opportunity to carry out higher pressure capture reduces downstream compression power.
CO2 geological storage
Storage sites (saline aquifers, depleted basins and EOR) to support CCS development are vast and higher than projected capacity requirements over the coming decades [45, 65]. To be suitable for CO2 storage, formations must be porous and permeable to allow injection of large volumes of CO2, and bear impermeable rock caps for CO2 imprisonment. Storage in abandoned oil–gas fields is geologically appropriate as they are likely to be impermeable after holding oil and gas for millions of years. A drawback of such reservoirs is that they were penetrated by many wells that may have damaged the reservoir or seal [46].
CO2 is retained through trapping mechanisms: (a) stratigraphic and structural (primary trapping, occurs beneath seals of low permeability rocks, dominant at early stage); (b) residual (trapped in rock pores by water capillary pressure); (c) solubility (residual gas trapping), and (d) mineralization (changing the pore-space topology and connectivity) [46]. At later stages of the storage process, precipitation of carbonates may cause blockage of fluid pathways and loss of storage pore volume [21].
Relationship between long-term injection and induced seismicity is reported [47], suggesting probability of earthquakes triggered by large injections of CO2into the brittle rocks found in continental interiors, threatening the seal integrity [77]. However, CO2 leakage uncertainties do not seem to pose a major barrier to scaling up CCS [48•].
Typical injection costs have been reported as ≈0.5–8 $/tCO2 [68••]. Combining storage with EOR may offset costs [49], as EOR is beneficial (decreasing oil viscosity and density, improving fluidity and enhancing lifting), resulting in ≈1–3 bbl oil/tCO2 [50]. Additionally, 60% of the injected CO2 can be retained in the reservoir [51], with room for improvement (e.g., use of polymers to adjust the mobility ratio) [52].
Carbon price
Demand-pull innovations arise in response to market stimulation, the most obvious being carbon price. A survey of OECD countries using environmental taxes shows positive effect on innovation [53]. There is a growing convergence of policy-makers that establishing a carbon price is the most effective way to reduce carbon footprint [54]. In the long-term, when CCS technologies are mature, carbon price should be the main driver to reduce emissions through CCS deployment, besides avoiding technology locking [55]. Despite increasing marginal costs of CO2 emissions [56], carbon taxation increases prices of energy and energy-intensive goods [57].
The two key factors affecting decisions are the future carbon price and the future CCS cost [6]. If the carbon price is sufficiently high, CCS is more economical than paying CO2 emissions taxes, and installing CCS in anticipation could be costlier. If a disruptive technology substantially reduces CCS costs, delayed adoption of CCS (paying carbon taxes) could be costlier [6]. Carbon prices of ≈$60–65/tCO2 are needed to make CCS economical [48•].
In the short-term, CCS costs will decrease benefitting from expansion of deployments (from the first to the nth commercial plant, that is, from FOAK, first of a kind, to NOAK, nth of a kind) reaching ≈$65/tCO2 [58]. In the mid- to long-term, costs decline slowly with technology maturity. The impact of innovations on CCS costs is unpredictable. On the other hand, carbon prices will start at low values and escalate with the years, pulling innovations. Nykvist [59] reports 10X increase in carbon price among efforts needed to mature CCS technologies.
Large scale CCS projects
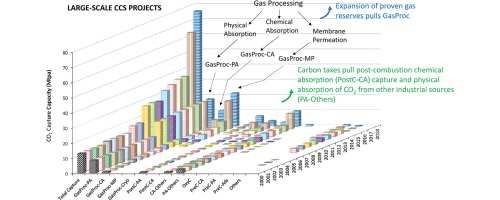
Figure 2 presents a pictured review of CO2 capture technologies in large-scale CCS covering exhaust gases, NG processing and other CO2 sources, including projects starting operation in 2018. Clearly, NG processing dominates the scene in 2010 pulling large-scale membrane permeation applications. Post-combustion capture is boosted by the end of the decade, with the increase of carbon price, dominated by chemical and physical absorption. Offshore EOR and new post-combustion plants respond for a 5X increase in capacity in the decade 2008–2018 (from 17 to 76 Mtpa).
 Figure 2. Large scale CCS projects in operation or to start operation until 2020. GasProc-PA = gas processing with physical absorption, GasProc-CA = gas processing with chemical absorption, GasProc-MP = gas processing with membrane permeation, GasProc-Cryo = gas processing with cryogenic distillation, Post-PA = post-combustion capture with physical absorption, PostC-CA = post-combustion capture with chemical absorption, CA-Others = chemical absorption from industrial sources, PA-Others = physical absorption from industrial sources, OxyC = oxy-combustion, PreC-CA = pre-combustion capture with chemical absorption, PreC-PA = precombustion capture with physical absorption, Others = other capture technologies and sources, PreC-Ads = pre-combustion capture with adsorptiom. Data was compiled from Ref. [78, 65, 1, 25, 26, 91, 8, 66, 75, 79, 80, 81, 82, 83, 84, 2] (on line Supplementary material); [7, 84].
Figure 2. Large scale CCS projects in operation or to start operation until 2020. GasProc-PA = gas processing with physical absorption, GasProc-CA = gas processing with chemical absorption, GasProc-MP = gas processing with membrane permeation, GasProc-Cryo = gas processing with cryogenic distillation, Post-PA = post-combustion capture with physical absorption, PostC-CA = post-combustion capture with chemical absorption, CA-Others = chemical absorption from industrial sources, PA-Others = physical absorption from industrial sources, OxyC = oxy-combustion, PreC-CA = pre-combustion capture with chemical absorption, PreC-PA = precombustion capture with physical absorption, Others = other capture technologies and sources, PreC-Ads = pre-combustion capture with adsorptiom. Data was compiled from Ref. [78, 65, 1, 25, 26, 91, 8, 66, 75, 79, 80, 81, 82, 83, 84, 2] (on line Supplementary material); [7, 84].Lessons learned in 2000–2010 paved the road for the accelerated growth, but ‘game-changers’ came to play: increased reserves of non-conventional NG (high %CO2) demand CO2 separation, and increased carbon taxes induced CCS initiatives (noticeable expansion of post-combustion chemical absorption). Ultra-deepwaters NG processing on FPSOs pulled membrane permeation technology to become an unpredicted co-adjuvant actor with chemical and physical absorption (in post-combustion and NG processing).
Andersen [60] listed StatoilHydro’s learning experiences from 1997 to 2008: (a) mature suppliers exist for pre-combustion (Syngas Plants) and oxy-combustion (Air Separation Units), resulting in abandonment of membrane permeation for separating H2 (pre-combustion) and O2 (oxy-combustion); (b) post-combustion showed no real improvement (pulled by carbon taxes, Figure 2 shows that last decade proves this learning inaccurate for forecasts); (c) limited capital and high risk restricted supplier industry from investing in CCS technologies, resulting in key investors being government, large emitters and oil and gas producers (pulled by carbon taxes); and (d) CCS market uncertainties impact industry.
Final remarks
CCS chain processes differ in TRL (Technology Readiness Level): (a) compression and transportation are mature, (b) EOR is well-proved and sequestration in geological formations have more than a decade of demonstration in large scale (Sleipner project in Norway); and (c) CO2 capture presents mature alternatives, mainly chemical absorption (amine based) and physical absorption (Selexol™ and Rectisol™), and amenable to post-combustion and NG processing applications. Technical risks derive from the ‘systems level’ integration of multiple processes [6], and the huge scale demanded [48•]. NG processing with membrane permeation dominates the high %CO2 offshore scene.
In exhaust gas applications, capture energy penalty (resulting from heat consumption for solvent regeneration) drives technology developments, but low maturity and high cost of pilot and demonstration scale processes move large-scale application to long-term horizons. A scenario-changer is carbon pricing. Anticipating technology costs and carbon price escalations determines when and whether to adopt CCS. CO2 transport costs do not limit penetration of CCS, but impact site choice. CO2 pipeline hubs are a key support to this decision, as shared transport network reduces CCS costs.
Regarding the storage step in CCS chain, relationship between long-term injection and induced seismicity suggests the need for monitoring leakages. Injecting large quantities of CO2 into geological reservoirs creates risks that need to be addressed within a regulatory framework, mainly considering the timeframe of storage. Internationally accepted guidance for monitoring the integrity storage sites and mitigation safeguards in place are required [61].
Literature generally argues for the necessity of disruptive changes to meet energy needs. This review shows that the current system mainly supports incremental changes. In this sense, Figure 3 summarizes the outcomes including CCS from bioethanol production as an immediate bioenergy CCS application (BECCS), mainly due to the grade of CO2 from fermentation [62], counterpointing the view that CCS is necessarily oriented to fossil carbon. Without BECCS, the goal may be substantially costlier to meet [63].
 Figure 3. CCS technologies and drivers versus timeline (boldface: mature technologies; dashed-lines: driver-technology connections).
Figure 3. CCS technologies and drivers versus timeline (boldface: mature technologies; dashed-lines: driver-technology connections).