Highlights
-
•
E-waste is one of the fastest-growing solid wastes in the globe.
-
•
E-waste co-processing with ore minerals and their products is feasible.
-
•
Some e-waste is co-processed in integrated pyro-hydrometallurgical facilities.
-
•
Niche e-waste co-processing based on leaching can be developed with further studies.
Abstract
E-waste generation has been exponential due to technological advancements, urbanisation and modern lifestyles, reduced replacement intervals of consumer electronics, lack of design-for-environment components in electrical and electronic equipment, lack of repairs and high costs of repairs. 62 million tonnes of e-waste was generated in 2022, equivalent to 7.8 kg of e-waste per person per year. E-waste management has also been challenging, and a significant fraction ends up in landfills despite the presence of precious, base and critical metals. In addition, another substantial fraction is processed by the informal sector employing sub-standard methods without considering approved environmental, health and safety aspects. Since only 22.3 % of e-waste has been collected and properly recycled, extensive e-waste recycling frameworks, including niche methods, are needed. E-waste co-processing with ore minerals, including mine waste, and metal concentrates and other waste materials in smelters, is thus identified as a promising area to address the e-waste management and value recovery challenge. This is supported by the availability of low-grade ores to produce critical metals and copper and mine tailing management requirements. Integrated pyro-hydrometallurgical facilities in developed countries already process some e-waste with metal concentrates and other waste materials. However, niche e-waste co-processing techniques utilising heap leaching, tank leaching and acid-generating mine tailings are needed since the best available techniques depend on local socio-techno-economic considerations. This study on e-waste co-processing with natural resources and their products could be beneficial to developing the relatively unexplored research area with future studies.
Keywords
Copper
Co-processing
Critical metals
E-waste recycling
Low-grade ores
Niche minerals processing
Abbreviations
Best available techniques
Base Metals Operations
Electrical and electronic equipment
Environmental, social and governance
Kayser recycling system
Printed circuit boards
Polychlorinated dibenzo-p-dioxins
Polychlorinated dibenzofurans
Platinum group metals
Precious Metals Operations
Persistent organic pollutants
Waste electric and electronic equipment
1. Introduction
Conventional energy systems with combustion technologies utilise substantial fossil fuel volumes. Thus, these are associated with key challenges, including high environmental impact, resource depletion, price volatility, and geopolitical instability (Figueres et al., 2017). Therefore, transitioning to more sustainable and renewable energy systems, such as solar power, hydropower, and wind power, has become a global necessity as these energy sources have been sustainable for a long period of time. However, transitioning to green energy sources creates a high demand for certain metals and minerals, including critical metals, and it becomes one of the major challenges to achieving the planned sustainable energy transition (Ilankoon et al., 2022). For example, a 3-megawatt (MW) wind turbine consumes 4.7 tonnes of copper, 2 tonnes of rare earth elements (REEs), 3 tonnes of aluminium, and a considerable amount of zinc and molybdenum (World Bank Group, 2017).
Considering the widespread green energy implementation targets, including electric vehicles (EVs), critical metals are paramount. According to Hache et al. (2019), a metal is identified as critical due to its necessity and scarcity for green energy technologies and geological occurrence, respectively. The European Commission (EC) assessed the critical raw materials based on two factors, namely, economic importance and supply risk (European Commission, 2023). Moreover, factors such as the potential for substitution, recycling rates, geopolitical concerns, available mining technologies, political instabilities, and economic policies are typically considered when assessing critical metals (Poulton et al., 2013). Different international organisations have defined lists of critical metals based on the estimations of demand for modern technologies and supply risk. The EC and the US Geological Survey (USGS) have two lists of critical materials/minerals, and the latest lists contain 34 and 50 materials, respectively (USGS, 2022; European Commission, 2023). These lists consist of several base metals and REEs. Copper is a base metal highly utilised in almost every electrical and electronic equipment (EEE) and other high-tech industries, whilst not still defined as a critical metal by USGS. However, the latest critical metal list, which is the fifth list of critical raw materials for the European Union (EU) in 2023, includes copper (and nickel) as a strategic raw material (European Commission, 2023).
The demand for these critical metals has increased due to factors such as the rapid growth of renewable energy development, supply disruption, regionalisation of supply chains, and geopolitical concerns. For example, China significantly controls the mining, processing, and refinery of many critical metals, including REEs (Ilankoon et al., 2022), tellurium (Yin et al., 2023), germanium (Patel and Karamalidis, 2021), indium (Lin et al., 2019), and aluminium while consuming between 20 % to 85 % for their production (Gulley et al., 2018). As a result, China has a controlling power to influence the global supply of many critical metals. Furthermore, critical metals and copper continue to be mined from existing low-grade ores, and some ores are not amenable to mineral processing. This could create disruptions to the supply chains of several metals (Northey et al., 2014, Ilankoon et al., 2022). In addition to low-ore grades, conventional mining of critical metals using natural resources is hampered by excessive environmental impacts and environmental, social and governance (ESG) concerns, waste generation, and higher operational costs. Therefore, it is necessary to develop alternative, reliable and steady critical metals and copper supply chains to overcome supply chain uncertainties in the future. In this context, more intense value recovery strategies, including niche co-processing strategies, using low-grade ores and secondary resources, such as e-waste and mine tailings, are essential.
Secondary sources are becoming an alternative and attractive resource to diversify the supply chains of critical metals and copper. Secondary sources of metals can be defined as materials reprocessed to generate raw materials by substituting primary materials (Ongondo et al., 2015). However, most of these secondary sources and waste materials end up in stockpiles (e.g. mine tailings) and landfills (e.g. e-waste) due to the constraints of the economic extraction, inefficiencies in waste management and loss of metals by the dilution. Despite that, the volume of secondary resources is extremely high (European Parliament Briefing, 2015). Therefore, the economic extraction of critical metals and copper from these secondary sources is essential. In addition, the co-processing of ore minerals and secondary resources (e.g. processing e-waste at a mine site) could be a future prospect, though current studies at the lab-scale (e.g. Guezennec et al., 2015, Fernando et al., 2019, Bryan et al., 2020) demand large-scale studies, such as modified heap leaching and tank leaching.
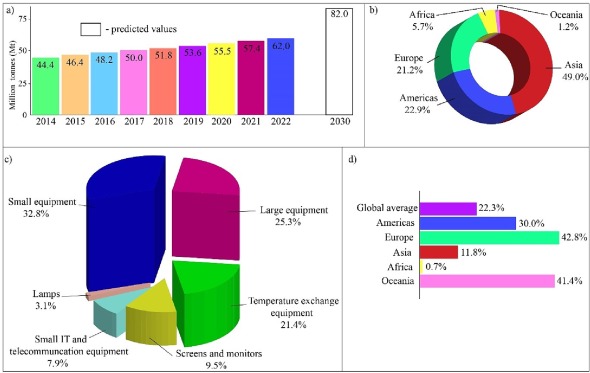
Waste electric and electronic equipment (WEEE) or e-waste is discarded EEE by their consumers due to a number of factors, including rapid obsolescence, shorter replacement intervals, high repair costs and lack of interest in reuse (Tesfaye et al., 2017, Ilankoon et al., 2018, Sengupta et al., 2022). 62 million tonnes (Mt) of e-waste were generated in 2022, and it is envisaged that e-waste generation will be 82 Mt by 2030 (Fig. 1a). Asia (49 %) and the Americas (22.9 %) are the largest contributors to this accumulation (Fig. 1b). Besides, China (12.07 Mt), the USA (7.19 Mt), and India (4.14 Mt) are the top three countries of e-waste generation in 2022 (Baldé et al., 2024). There are 6 classes of e-waste defined by the EU (Forti et al., 2018), and small (e.g. radios, video cameras) and large (e.g. washing machines, electric stoves) equipment are the main e-waste segments generated in 2022 (Fig. 1c). In addition, e-waste possesses a non-metal fraction (e.g. plastics, ceramics) and hazardous components (e.g. brominated flame retardants) (Işıldar et al., 2019, Sengupta et al., 2022). Currently, only about 22.3 % of e-waste is properly collected and recycled, and Europe is leading in this with a percentage of 42.8 % (Fig. 1d) (Baldé et al., 2024). One of the main challenges related to e-waste management is the lack of proper e-waste collection and recycling infrastructure in many parts of the world, which can lead to e-waste being improperly disposed of in landfills, subjected to improper recycling and exported to developing countries with inadequate environmental and labour regulations (i.e. transboundary movement of e-waste) (Ilankoon et al., 2018).
 Fig. 1
Fig. 1In the context of value recovery, significant accumulation of e-waste facilitates urban mining operations due to the presence of different metals in e-waste, including precious (e.g. gold and silver), base (e.g. copper, tin), and critical metals (e.g. REEs, gallium) in concentrations higher than the same in ore minerals (Table 1) (Ilankoon et al., 2018).
✓ Critical metals defined in the list of EU in 2023 and USGS in 2022.


Regarding e-waste value recovery operations, current techniques employed in developing countries extract a few metals, including gold, silver, and copper (section 2). Most of the remaining e-waste in developing countries is managed by the informal sector (Sengupta et al., 2022) by performing artisanal recycling operations (section 2.1). E-waste value recovery operations in developed countries extract several metals, though these are not readily applicable in developing countries (section 2.2). Thus, more concentrated efforts are required to extract and recover critical metals, including copper, using e-waste. Processing of e-waste at mineral processing sites or co-processing of e-waste with ore minerals, including mine waste, has received some attention (e.g. Guezennec et al., 2015, Fernando et al., 2019, Bryan et al., 2020), though considered a largely unexplored area in minerals processing. Thus, research studies addressing the co-processing of e-waste with ore minerals are paramount. Considering these research gaps, the objective of this work is to critically evaluate the potential of co-processing of e-waste with natural ores and their products, mine waste and tailing materials, including the challenges in terms of the mine sites and processing facilities modifications, supply chains of e-waste and downstream metal recovery operations. Future studies to develop this research field will also be highlighted in this work, and this work could advance the co-processing of e-waste in the context of critical metals.
2. E-waste-based value recovery: The status quo
Current e-waste-based value recovery activities are two main categories, namely, informal and formal operations. Informal value recovery operations are carried out to extract more valuable precious metals (e.g. gold and silver) and concentrated base metals in e-waste, such as copper, and these do not follow established and local waste management and recycling practices and legislative frameworks. However, formal e-waste refurbishing and recycling operations are based on waste management-related legislative frameworks in a particular country, and established value recovery flowsheets are used to extract the maximum value (i.e. a number of metals). The value recovery techniques employed in formal operations depend on the local socio-techno-economic considerations. Hydrometallurgical operations are thus primarily carried out in developing countries, while state-of-the-art pyrometallurgical operations coupled with hydrometallurgical metal separation techniques or integrated pyro-hydrometallurgical operations are employed in developed countries (e.g. Umicore, Belgium).
2.1. Informal e-waste recycling
Informal waste collectors in developing countries often supply e-waste to informal recyclers (Sengupta et al., 2022), and thus, small tools-based value recovery activities are carried out targeting the easily extractable value (e.g. copper in copper cables and transformers) (Ilankoon et al., 2018). According to the Global E-waste Flows Monitor 2024 (Baldé et al., 2024), more than 88 % of the total e-waste generated in Asia and more than 99 % of the e-waste in Africa were not subjected to environmentally sound e-waste collection and recycling (i.e. a substantial fraction is handled by the informal collectors and subsequently processed by informal recyclers). The strong presence of informal e-waste recycling approaches in Asia (Yong et al., 2019), including India (Sengupta et al., 2023) and Malaysia (UNODC, 2022) was demonstrated. For example, open burning of e-waste to remove the plastic fraction is sometimes practised. Air pollution due to the release of persistent organic pollutants (POPs), such as polychlorinated dibenzofurans (PCDFs) and polychlorinated dibenzo-p-dioxins (PCDDs) thus occurs (Alabi et al., 2021), and the POPs are classified by the Stockholm Convention (Stockholm Convention, 2023). In addition, some organised informal value recovery operations are reported, and chemicals-based gold and silver recoveries are the primary objectives in these backyard activities (Ilankoon et al., 2018). However, final liquid and solid waste disposal to the soil, drains, and nearby water bodies are the environmentally polluting pathways in informal value recovery operations (Yong et al., 2019).
2.2. Formal e-waste recycling
Formal e-waste recycling operations, including hydrometallurgy methods and integrated pyro-hydrometallurgical techniques, require a myriad of mechanical and physical pre-treatments depending on the scale of operation.
In hydrometallurgy-based e-waste recycling (section 2.2.1), e-waste is firstly subjected to physical sorting, dismantling and mechanical pre-treatment operations to remove large non-metallic fractions (e.g. extract PCBs by removing plastic components) and the precious metal-containing parts (e.g. gold contacts). The sorted e-waste is crushed and milled to produce a feed of 1–2 mm in size for hydrometallurgical operations (Yong et al., 2019, Tabelin et al., 2021). Physical separation techniques, such as gravity, magnetic and electrostatic separation (Tuncuk et al., 2012), yield the metallic fraction for subsequent metal leaching.
Initial e-waste physical sorting, dismantling and separation processes are practised for integrated pyro-hydrometallurgical operations (section 2.2.2), though extensive PCB crushing and milling operations are not required (Fig. 2), similar to hydrometallurgical processes. Separated e-waste components, such as PCBs, are also purchased from developing countries to maintain the required e-waste supply chains.
 Fig. 2
Fig. 22.2.1. Hydrometallurgy-based e-waste recycling
Acids-based metal leaching is employed for copper extraction using sulphuric (Guo et al., 2020, Ishak et al., 2022), nitric (Kamberović et al., 2009, Ajiboye et al., 2019), and hydrochloric acids (Birloaga and Vegliò, 2022) with (e.g. ferric, hydrogen peroxide) or without oxidants, and ammonia-ammonium salt solution (Kamberović et al., 2009). Gold extraction with dilute sulphuric acid and thiourea with the presence of ferric (Ippolito et al., 2021), cyanide (Kamberović et al., 2009, Panda et al., 2020) and iodine-iodide (Lucheva et al., 2017) is typically practised, while chloride leaching facilitates palladium extraction (Kamberović et al., 2009). In numerous lab-scale studies, low solid–liquid ratios were employed, and those are not necessarily practical when large-scale metal leaching tests are designed.
In addition, base metal extraction, followed by gold leaching, is employed to minimise metal losses (Tuncuk et al., 2012, Yong et al., 2019). The resultant metal-enriched solution in hydrometallurgical operations is known as pregnant leach liquor (PLS), and the metal recovery techniques (e.g. ion exchange, precipitation, solvent extraction, and electrowinning) are employed to recover individual metals.
Low capital costs favour this technique in developing countries, though final liquid and solid waste management aspects are not explicitly discussed in a myriad of studies in the literature (Yong et al., 2019). The coupling of bio-leaching to existing hydrometallurgical operations is also considered (Arya and Kumar, 2020), and the industrial applications are not explicitly reported.
2.2.2. Integrated pyro-hydrometallurgical e-waste recycling
Initial pyrometallurgical operations produce metal bullion and slag fractions, and the non-metallic fraction in the e-waste feed (e.g. plastic) contributes to energy savings in these integrated pyro-hydrometallurgical operations (Ilankoon et al., 2018). The resultant metal fraction is the feed for subsequent hydrometallurgical techniques to extract and separate individual metals (Ilankoon et al., 2018, Chen et al., 2023, Umicore, 2023). Highly capital-intensive and state-of-the-art facilities in developed countries (e.g. Umicore, Belgium; Kosaka smelting and refining facility, Japan) target many metals present in different e-waste streams (Table 2). High metal recovery, purity, and energy recovery are the key advantages. Although these state-of-the-art facilities are currently practised on the industrial scale in developed countries, technical and economic factors, including high capital costs and the difficulties of maintaining continuous e-waste supply chains, have limited their application in developing countries (Ilankoon et al., 2018).
| Company | Recycling facility | Location | Processing technique | Recovered metals (precious metals, base metals and metalloids) |
|---|---|---|---|---|
| Umicore | Hoboken reccyling plant | Hoboken, Belgium | Integrated base metals and precious metal operations |
Precious: Au, Ag, Pd, Pt, Rh, Ru, Ir Base: Cu, Pb, Ni, Sn, Sb, Bi, In Metalloid: Se, Te |
| Aurubis | Kayser recycling system (KRS) | Lünen and Hamburg, Germany | Smelting and electrorefining |
Precious: Au, Ag Base: Cu, Pb, Ni, Zn, Sn, Sb, Bi Metalloid: Te |
| DOWA Group | Kosaka smelting and refining facility | Kosaka, Japan | Smelting and electrorefining |
Precious: Au, Ag Base: Cu, Pb, Ni, Sn, Sb, Bi Metalloid: Te |
| Boliden | Boliden Rönnskär smelter | Skellefteå, Sweden | Kaldo furnace produces black copper and refining; Capacity of 120,000 tonnes of e-waste per year |
Precious: Au, Ag, Pd, Pt, Rh, Ru, Ir Base: Cu, Pb, Ni, Sn, Sb, Bi, In Metalloid: As, Se |
| Glencore | Horne smelter | Quebec, Canada | Process e-waste in a copper smelter; Upgrade in converters and an anode furnace, and electrorefining; Capacity of 110,000 tonnes per year |
Precious: Au, Ag, Pd, Pt Base: Cu, Ni Metalloid: Se, Te |
3. Co-processing of e-waste: Current processes and niche concepts
Considering e-waste as a valuable secondary resource, and the availability of mineral processing facilities, including metal smelters, co-processing of e-waste with ore minerals, including mine waste (note: still at development stage) and in metal concentrators (note: this is already implemented) could be a potential and niche alternative for improving e-waste recycling rates and reducing the substantial e-waste volumes processed by the informal sector, such as in India, Southeast Asia and African countries.
3.1. Definitions and opportunities
The co-processing technique is a combination of different unit operations for the recovery of valuables by integrating primary sources (e.g. ore minerals and their derivatives) and secondary sources (e.g. e-waste). There are several similarities between primary mining and mineral processing, and urban mining or recycling of e-waste. Adapting the co-processing of e-waste in primary mine sites is thus a potential solution considering the socio-techno-economic aspects of current e-waste management and value recovery operations.
Large-scale e-waste value recovery operations and primary mining and mineral processing facilities require a large area distally from densely populated regions (Simoni et al., 2015), and this helps to alleviate social challenges arising in primary mining and mineral processing and urban mining. The presence of high metal concentrations (e.g. gold and copper) in e-waste compared to the same in low-grade ores favours the co-processing of e-waste with ore minerals. In addition, metal extraction and recovery techniques employed in conventional mineral processing, such as communition, leaching and smelting, are similar to e-waste processing techniques (Acuña, 2015). Moreover, ore pre-treatment equipment (e.g. crushers and mills) could be used for some e-waste pre-treatment operations, though size reduction of e-waste is efficiently achieved by shredders with a focus on collecting shredder dust that contains precious metals. Regionalisation of technology and natural resources results in artisanal mining operations with natural resources mainly to extract precious and critical metals. This is also true for e-waste recycling, where informal recyclers dominate urban mining operations, especially in developing countries (Moyo et al., 2022). Considering these similarities, the co-processing of e-waste with ore minerals, including mine waste and tailings, at primary mining and mineral processing sites is feasible for achieving the objectives of the circular economy and maintaining economic stability and supply chain diversifications in the metal industry by redefining the operational parameters.
3.2. Existing e-waste co-processing operations
Some large-scale facilities already co-process e-waste in smelters with metal concentrates derived from primary mining operations (Table 3a). Boliden Rönnskär smelters in Sweden process e-waste with copper and lead concentrates in the Kaldo furnace (Boliden, 2023b). In addition, once the Noranda mine in Canada is closed, its Horne smelter (currently owned by Glencore) processes a feed consisting of e-waste and copper ore concentrates (Fig. 3) (Glencore Canada, 2023).
| Study/Experiment | Targeted Metals | Key Features | Current Status | Performance Criteria | Reference |
|---|---|---|---|---|---|
| Simulations: Environmental indicators, exergy, recycling and recovery rates, qualities and quantities of recyclates, losses, and emissions | E-waste (LED lamps) and nickel-pig iron |
Process simulations and environmental software were used to predict recyclates, stream compositions, and grades. Process simulations of e-waste co-processing with copper scrap were carried out in a top submerged lance furnace, including the environmental footprint. |
Simulation-based results for best available techniques (BAT) and design for recycling (DfR) |
BAT for e-waste processing. Estimating the true environmental impacts. Frameworks for product redesigns. |
Reuter et al., 2015, Reuter and van Schaik, 2015 |
| Jiangxi Huagan Nerin Rare & Precious Metal Technology Co Ltd in China | Cu and precious metals from e-waste |
Nerin recycling technology is employed. Feed materials include e-waste, copper concentrates and other waste streams. |
Ongoing |
For 585 days between January 2018 and June 2021, the processed raw material amount was 156,040.9 tonnes. Crude copper generation was 29,784.4 tonnes. 181,975.22 tonnes of smelting slag were produced. |
Ye et al., 2021 |
| Glencore Canada: Processing of e-waste in Horne smelter, which was for metal concentrates from ore minerals | Cu and precious metals from e-waste |
Horne smelter in Rouyn-Noranda, Quebec started recycling in the 1940s. One of the first smelters to process e-waste in the 1980s. Recycling is now a core functionality for Glencore. |
Ongoing | 100,000 tonnes of recycling materials annually. | Glencore, 2022 |
| Aurubis plants in Germany: Processing of e-waste with other copper concentrates and scraps in smelters | Cu, Ni, Zn, Sn, Pb, Bi, Te, Sb, and precious metals |
KRS enables the processing of complex feed materials. The submerged lance furnace produces a copper alloy of 80 % copper. A top blown rotary converter increases the copper content to 95 % (unwrought copper), and lead and tin are separated into a slag. Unwrought copper is processed to produce 99 % copper in an anode furnace, while precious metals are enriched in anode slimes. |
Ongoing |
1 million tonnes of recycling materials per year. 19 different metals are recovered by recycling complex feed materials, including e-waste and copper scraps. |
Aurubis, 2023 |
| Boliden Rönnskär smelter in Sweden: Co-processing of copper and lead concentrates and e-waste | Cu, Zn, Pb, Au, and Ag from concentrates and e-waste |
Established in 1930. Low-carbon copper is produced from natural resources-based copper concentrates. Recycled copper is produced from secondary sources, such as e-waste. A new leaching plant was built in 2021 to handle the residual materials generated in smelters. |
Ongoing |
Production figures in 2022: Copper: 218,000 tonnes Zinc: 33,000 tonnes Lead: 29,000 tonnes Silver: 467 tonnes Gold: 12 tonnes Annual Capacity of 120,000 tonnes of e-waste. Operating profit: USD 120 million in 2022. |
Boliden, 2023a, Boliden, 2023b |
| Umicore, Hoboken, Belgium: Recycling of e-waste and complex waste streams in an integrated pyro-hydrometallurgical facility | Cu, Pb, Ni, Sb, Sn, Bi, Ag, Au, Pd, Pt, Rh, Ru, Ir, In, Se, Te |
Evolution into a materials-technology company from a number of mining and smelting facilities in the last 200 years. Feed materials include e-waste, scrap, spent auto catalysts, spent industrial catalysts, sweeps, bullions, drosses, mattes, speiss, and anode slimes. Two recycling processes are precious metals operations (PMO) and base metal operations (BMO). |
Ongoing |
Adjusted earnings before interest, taxes, and depreciation in 2022 is Euro 532 million. The capacity of the metal recycling plant is 500,000 tonnes per year. |
Umicore, 2023 |
 Fig. 3
Fig. 3The existing e-waste co-processing facilities are thus mainly smelters, and these are primarily located in developed countries. E-waste management and value recovery legislative frameworks in those countries are designed to achieve the circular economy objectives, and these drive the smelters that process e-waste, other waste materials, and metal concentrates derived from ore minerals. Thus, high percentages of environmentally sound e-waste collection and recycling resulted in, for example, 42.8 % in 2022 in Europe (Baldé et al., 2024). Furthermore, the critical metal-related developments in the USA and EU, including e-waste recycling for maximum value recovery, are predominantly designed to diversify critical metals and copper supply chains without adverse effects of geopolitics (Ilankoon et al., 2022).
In addition to the collected e-waste in developed countries, sorted e-waste, such as PCBs, from developing countries is imported to carry out large-scale e-waste-based value recovery operations. Considering the hazardous components in e-waste discussed earlier, the presence of state-of-the-art equipment in these smelters that capture toxic components has been advantageous. Final solid waste management has also been addressed in these operations (Fig. 2and Umicore, 2023), and the applications of copper slag waste were discussed in detail (Phiri et al., 2021, Phiri et al., 2022).
3.3. Niche e-waste co-processing concepts
Apart from existing e-waste co-processing with metal concentrates in smelters, niche e-waste co-processing concepts at a mine site are rather limited (Table 3b). In addition, the proposed ideas are mainly restricted to lab studies, and pilot-scale feasibility projects have not been performed.
| Study/Experiment | Targeted Metals | Key Features | Current Status | Performance Criteria | Reference |
|---|---|---|---|---|---|
| Employ a lixiviant produced from sulphidic mining waste to process e-waste by bio-leaching |
Cu, Ni, Zn, Pb, Ga, Sn from PCBs |
Sulfidic mine wastes are biologically oxidised. Flotation tailing with high pyrite produces a sulphuric acid with enough ferric ion concentration (18 g/L). Ferric ion-sulphuric acid lixiviant facilitates copper extraction from e-waste. Bacterial activities that oxidise ferrous to ferric determine the feasibility of the bio-leaching process. |
E-waste co-processing concept and lab-scale two-step bioleaching experiments |
Bioleaching is more effective than chemical leaching for PCBs. Higher ferric concentration increases the extraction efficiency of copper. Extraction efficiency: 98.3 % in 2 days with an increment of 20 % compared to the tests without bacterial activities. |
Guezennec et al., 2015 |
|
Co-processing of low-grade copper ores and PCBs in a heap leaching system |
Co-extraction of copper from low-grade copper ores and PCBs |
A significant e-waste co-processing study compared to existing e-waste co-processing in smelters. A 2-D packed bed at lab scale. The packed bed was prepared by mixing low-grade ores and coarse PCBs at different ratios. Coarse PCBs in the packed bed modify the heap structure compared to the heaps with only ores. Out-flow liquid distribution was measured to evaluate the improvements in terms of heap flow behaviour. |
E-waste co-processing concept and lab-scale experiments on heap hydrodynamics |
Packed beds of coarse PCBs and low-grade ores produce improved heap flow behaviour compared to ore-only beds. Further experiments are required to validate the co-processing concept with chemical or bio-leaching. |
Fernando et al., 2019 |
| Employ a lixiviant produced from coal mining waste to process e-waste by bio-leaching | Cu, precious metals, Sn, Pb, REEs, Ga and Ta from PCBs |
Coal mine waste containing pyrite contributes to AMD over time. Value recovery from coal mine waste does not generate economic benefits. Ferric ion (10 g/L)-sulphuric acid lixiviant facilitates copper extraction from PCBs. PCB pre-treatment produces a metallic char fraction, and it will be bio-leached. |
E-waste co-processing concept and lab-scale two-step bioleaching experiments |
Amount of coal mine waste repurposed. Mass of copper cathode production using pre-treated PCBs. Power consumption for e-waste co-processing flowsheet. Handling of the final solid waste fraction after leaching. |
Bryan et al., 2020 |
Fernando et al. (2019) presented an excellent proposal for e-waste co-processing with low-grade ores at a mine site (Table 3b). The presence of high copper concentrations in PCBs was the basis for this study, and thus, low-grade copper ore heap leaching was suggested to process e-waste (Fig. 4). This requires modifications to heap construction procedures in conventional heap leaching. The heaps can be constructed by adding a certain fraction of coarse PCBs (e.g. 1 cm by 1 cm particles). Compared to −25 mm particles employed in conventional heap leaching, coarse PCBs have a flat sandwiched structure with very low porosity values. Thus, pre-treatment of PCBs before adding them to the heap is paramount in increasing porosity and facilitating improved mass transfer mechanisms. Organic swelling of PCBs could be a potential pre-treatment solution to expand the PCB structure and, by then, to increase the metal leaching efficiency. Kang et al. (2023) discussed organic swelling-based PCB swelling mechanisms for multiple copper layer PCBs in detail. In addition to the contributions to the overall copper grade by coarse PCBs, Fernando et al. (2019)showed that the PCBs in the modified heap improve active flow paths through the particles (i.e. the shape of the particles in the modified heap is different compared to conventional heap leaching). Thus, preferential flow or liquid channelling through the particles was reduced using a 2-D packed bed in laboratory conditions, and the same will be expected in a heap. Improved heap hydrodynamics is considered an added advantage when processing e-waste and copper ores together in a modified heap leaching approach. The optimum PCB particle size can be selected considering the copper liberation and porosity of the particles. As long as the requirement of having coarse particles that alter the characteristic length scale of the heap and the resultant improved inter-particle hydrodynamics is satisfied, lower PCB sizes (i.e. less than 1 cm by 1 cm) and pre-treatments could be employed to liberate the encapsulated copper layers.
 Fig. 4
Fig. 4Employing the waste liquid generated in primary mining operations (e.g. acid mine driange or AMD, pyrite containing coal mining waste) in e-waste co-processing was also attempted. For example, Guezennec et al. (2015)conducted a study to extract metals from milled e-waste using sulphidic mining waste, which was the raw material to produce the lixiviant with a high ferric ion concentration (Table 3b). Bio-leaching facilitates improved metal oxidation reactions, and thus, base and critical metal extraction from e-waste is possible (copper, nickel, zinc, lead, gallium and tin), though selective metal leaching offers advantages during metal recovery. In addition, Bryan et al. (2020)discussed the applications of acidic mine waste in coal mines in extracting metals from e-waste biologically. Pyrite-containing coal mine waste was employed to produce the lixiviant enriched in ferric and sulphuric acid (Table 3b). Flowsheets were also suggested to integrate mine tailings and e-waste (Fig. 5). Usage of the pyrite fraction in mine waste facilitates base and critical metal extraction from PCBs, and thus, the resultant mine waste with reduced pyrite content offers additional advantages in mine tailing management (i.e. it does not produce AMD).
 Fig. 5
Fig. 5The discussed co-processing studies (e.g. Guezennec et al., 2015, Bryan et al., 2020) are limited to lab-scale and primarily employed a lixiviant produced in AMD or mine tailing facilities. The applications of tank leaching to co-process e-waste and ore minerals or mine tailings, either by chemical leaching or bio-leaching, are potential future areas to manage the increasing volumes of e-waste and mine tailings. Mine tailing management is a crucial and current issue for mining and mineral processing companies in addressing ESG frameworks. In addition to the presence of metals in low concentrations (Kinnunen and Kaksonen, 2019), microorganisms in mine tailings (Gagnon et al., 2020) will help to extract metals in e-waste biologically (Fig. 6). In this potential method, selective leaching from e-waste and mine tailings is a prerequisite rather than producing a PLS containing many metal ions, which invariably causes metal recovery problems in downstream operations. The addition of milled e-waste into the tank leaching feed will have either the catalyst effect that increases the metal extraction efficiency or the inhibitor effect retarding the metal extraction efficiency. These possibilities may vary with the available metals in mine tailings and milled e-waste. These leave a lot of scope for improvement in terms of controlling the particle size of e-waste, viable blending ratios of e-waste and ore particles, maintaining suitable solid–liquid ratios in designing industrial-scale reactors, on-site sorting and automation in composition identification for improved process efficiencies, selective leaching and reaction kinetic models, identifying microorganism cultures and assessing their effectiveness, final waste generation and waste disposal strategies, and life cycle assessments evaluating overall impacts of co-processing methods. The studies on these fronts will identify the co-processing potential of milled e-waste and mine tailings via tank leaching chemically or biologically and will drive the research area forward, answering the challenges.
 Fig. 6
Fig. 64. Limitations and risks of e-waste co-processing
4.1. Technical challenges
Emerging concepts and laboratory-scale experiments of co-processing e-waste and ore minerals or their derivatives (e.g. Guezennec et al., 2015, Fernando et al., 2019, Bryan et al., 2020) demand advanced knowledge for integrating primary mineral processing and e-waste recycling techniques. This is mainly due to the high degree of heterogeneity in ore minerals, the complexity of e-waste and its composition, and the time evolution of the material composition in e-waste and types (e.g. photovoltaic panels, e-bikes). Moreover, ongoing co-processing facilities (e.g. Aurubis Hamburg, Germany) employ already matured techniques, such as integrated pyro-hydrometallurgical operations, and thus, metal extraction and recovery processes do not possess any major technical challenges (Reuter and van Schaik, 2015, Reuter et al., 2015). The same is not necessarily true for proposed niche techniques. For example, the composition of PLS generated by e-waste co-processing in heap leaching and tank leaching with lixiviants sourced from AMD is largely unknown, and it may consist of a wide range of metal ions compared to conventional heap and tank leaching only with ore minerals. A complex PLS could make recovery processes, such as solvent extraction, complex and costly. The generated waste liquid handling could be challenging as well. Therefore, extensive lab and pilot-scale studies are suggested for the niche co-processing methods (Table 3b) to solve these technological challenges.
E-waste co-processing based on integrated pyro-hydrometallurgical methods or hydrometallurgical processes requires energy balance, exergy analysis, waste disposal mechanisms and environmental footprint assessments to formulate BAT (Reuter, 2013, Reuter et al., 2015, Reuter and van Schaik, 2015). Furthermore, DfR frameworks govern the effectiveness of e-waste co-processing (Reuter et al., 2015, Reuter and van Schaik, 2015). Considering these, the extraction of critical metals, such as indium and gallium, from e-waste is not feasible (Chancerel et al., 2015), and e-waste co-processing methods should be developed to address the technical constraints of critical metal extraction.
4.2. Economic challenges
If e-waste co-processing with ore minerals at mine sites requires major modifications to existing facilities and equipment, higher operating and capital costs are undoubtedly expected. Furthermore, the existing co-processing methods primarily employ PCBs, considering the economic benefits. Separation of PCBs is thus required prior to co-processing, and PCB pre-treatment (e.g. organic swelling, removal of electronic components) should be carried out on a large-scale. Those also increase the costs of e-waste co-processing operations when large-scale operations are considered.
Depending on selective metal extraction operations, e-waste co-processing would generate significant liquid and solid waste volumes, which must be handled by appropriate waste management strategies. Since waste management costs are identified as economic constraints, one of the ways to reduce those will be to maximise value extraction from e-waste in any e-waste co-processing methods rather than limiting to fewer metals.
The plastic fraction of PCBs contributes to reducing energy costs in integrated pyro-hydrometallurgical operations, though it will not be the case in other e-waste co-processing techniques, such as hydrometallurgical operations (Table 3b). Since energy consumption is one of the key challenges in both primary mining and e-waste recycling, the targeted e-waste co-processing flowsheets must be formulated to yield the intended benefits at affordable energy costs.
In the case of e-waste co-processing at mine sites, transportation of separated and sorted e-waste, such as PCBs, incurs significant costs, which must be accounted for when full flowsheets are assessed. This economic challenge is exacerbated considering the fact that primary mining and processing operations are located away from populated areas, though significant e-waste is generated in larger cities.
Furthermore, research and development costs for trial runs of emerging e-waste co-processing concepts (Table 3b) will be high, especially in developing countries.
4.3. Supply chain challenges
As the co-processing of e-waste demands a continuous feed of e-waste, the collection of e-waste should be performed perpetually. However, the uninterrupted collection of e-waste, especially household e-waste, in both developed and developing countries has been difficult because of the absence of proper legislative frameworks in some countries and inefficiencies in the collection systems (Yong et al., 2019, Forti et al., 2020, Baldé et al., 2024). In addition, a substantial fraction of collected e-waste in developing countries is primarily processed by informal e-waste recyclers (Sengupta et al., 2022), and thus, it would be challenging to form new e-waste supply chains pertaining to co-processing of e-waste with ore minerals. Large-scale e-waste collection strategies and improved legislative frameworks targeting the household e-waste fraction currently handled by informal recyclers and disposed of in landfills could alleviate e-waste supply chain constraints. For example, banning e-waste disposal in landfills by the Government in Victoria helps to collect e-waste effectively in Australia (Victoria State Government, 2022), and thus, being predominantly a mining nation, e-waste co-processing can be targeted.
4.4. Assessment of risks of e-waste co-processing
Pilot-scale experiments on the co-processing of e-waste and ore minerals, including mine waste and tailings, are essential for evaluating the factors that affect the industrial-scale application of co-processing techniques, and using those technical challenges, capital and operation costs can be assessed. Although the emerging e-waste co-processing strategies (Table 3b) address current socio-environmental challenges pertaining to e-waste and low-grade resources management, including mine waste, the ideas are still conceptual or experimental. Thus, pilot-scale experiments would facilitate the assessment of the process viability and sustainability of e-waste co-processing techniques, including final waste disposal strategies and costs.
Detailed risk assessments of the available and emerging e-waste co-processing techniques are paramount to further developing these methods, and a simplified form is presented in Table 4. Since integrated pyro-hydrometallurgical techniques are already employed to co-process e-waste with metal concentrates, their direct or modified applications possess relatively lower technical risks in the case of deployment in developing countries despite high capital and operating costs. On the other hand, e-waste co-processing based on heap and tank leaching is considered high risk due to unknown technical and economic challenges.
| Co-processing method | Technical risks | Economic risks | Supply chain risks |
|---|---|---|---|
| Integrated pyro-hydrometallurgical |
Low: Matured technology and applied at an industrial scale |
Low: A number of metals are extracted by combining pyrometallurgical and hydrometallurgical processes |
Low: Improved legislative frameworks in developed countries and separated PCB shipments from other countries facilitate supply chains |
| Heap leaching |
High: Upstream challenges: Physical treatment of PCBs to remove electronic components, particle size reduction, improving porosity by organic swelling or pyrolysis, maintaining a suitable blending of e-waste and ores Process risks:Selective metal leaching, maintaining the desired microbial population in case of heap bio-leaching Downstream challenges:Suitability of the PLS for metal recovery, solid–liquid separation |
High: Co-processing research studies are limited to lab-scale experiments, and thus, technology is immature, research and development costs will be high |
Medium: Forming new e-waste supply chains for the co-processing of e-waste could be challenging, large-scale e-waste collection strategies are needed, improved legislative frameworks are needed in countries having a strong informal e-waste sector |
| Tank leaching |
High: Upstream challenges: Physical treatment of PCBs to remove electronic components, crushing and milling costs, physical separation of metals and non-metals, maintaining a suitable blending of e-waste and ores Process risks:Selective metal leaching, maintaining the desired microbial population in case of tank bio-leaching Downstream challenges:Suitability of the PLS for metal recovery, solid–liquid separation |
High: Co-processing research studies are limited to lab-scale experiments, and thus, technology is immature, research and development costs will be high |
Medium: Forming new e-waste supply chains for the co-processing of e-waste could be challenging, large-scale e-waste collection strategies are needed, improved legislative frameworks are needed in countries having a strong informal e-waste sector |
It is important to note that the development of the BAT should be carried out considering local socio-techno-economic considerations. Thus, an e-waste co-processing method applicable in a developed country may not be the appropriate method in a developing country. Based on the assessment of local mining and mineral processing developments, suitable e-waste co-processing methods should be formulated. For countries without established mining and mineral processing infrastructure, standalone formal e-waste recycling can be applied or separated e-waste, such as PCBs, can be exported to the countries that operate co-processing facilities.
5. Conclusions
Current renewable energy developments, including EVs, add massive strains on critical metals and copper supply chains based only on natural resources. Existing e-waste volumes are very high, though a substantial fraction is disposed of in landfills. In addition, informal e-waste management and value recovery are other challenges that pollute soil, air, surface, and groundwater. Since more organised e-waste management and value recovery processes are paramount, co-processing e-waste with ore minerals and their products could diversify the existing critical metal and copper supply chains, often connected to geopolitics. Despite certain e-waste co-processing in integrated pyro-hydrometallurgical facilities, the fraction of e-waste processed by these facilities is limited by a predefined blend of e-waste and metal concentrates. Thus, the processing of increased e-waste volumes translates to new facilities to yield the required critical metals. Since integrated pyro-hydrometallurgical facilities are extremely cost-intensive, more concentrated efforts are required to develop niche e-waste co-processing methods, such as heap leaching, tank leaching and bio-leaching. Thus, e-waste co-processing routes that align with local socio-techno-economic considerations could be developed to extract the maximum value from e-waste, and e-waste management, mine waste and tailing challenges in waste recycling and mining industries could be effectively addressed. The authors believe this study will help drive more research studies.
CRediT authorship contribution statement
I.M.S.K. Ilankoon: Writing – original draft, Investigation, Formal analysis, Data curation, Conceptualization. R.A.D.P. Dilshan: Writing – original draft, Methodology, Formal analysis, Data curation, Visualization. Nimila Dushyantha:Writing – original draft, Methodology, Data curation, Visualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
© 2024 The Author(s). Published by Elsevier Ltd.