Highlights
-
•
The rapid industrialization and energy transition demands on great amount of metals.
-
•
Deep Eutectic Solvents (DES) are emerging as a “green” alternative for metal recovery.
-
•
DESs properties could be adjust according to their application in metal recovery.
-
•
DES are adequate leaching and extraction agent for hydrometallurgycal methods.
Abstract
The rapid industrialization and energy transition, as well as social pressure is increasing the current needs for metals. This results in an increasing demand, so the exploitation of more natural resources. In this scenario, a current alternative to minimise the lack of resource is the recycling of industrial waste and by-products, such as electrical and electronic waste, lithium batteries, solar panels, blast furnace slag, etc. Many articles focus on recovery of metals by hydrometallurgical methods are published. Currently, one of the great scientific challenges is to minimize the environmental impact of these recovery methods. Deep Eutectic Solvents (DESs) are emerging as an environmentally friendly alternative for the recovery of metals from industrial waste and by-products due to their greener properties. This is entailing an increasing number of scientific publications in the area. This review article collects the publications and highlights the relevance of the use of these solvents in metal recovery from industrial waste and by-products by hydrometallurgical methods, showing the main disadvantages of its use in the area. As well as, the possibility to adjust their physico-chemical properties according to their application.
Graphical abstract
Keywords
Properties of deep eutectic solvents
Deep eutectic solvents
Leaching
Liquid–liquid extraction
Metal recovery
Abbreviations
deep eutectic solvents
hydrogen bond acceptor
methyltriphenyl phosphonium bromide
tetra-n-butyl ammonium bromide
tetra-n-butyl ammonium chloride
benzyltriphenyl phosphonium chloride
benzyltriphenyl phosphonium bromide
N,N-diethylethanol ammonium chloride
2,2,2-trifluoroacetamide
lithium nickel manganese cobalt oxides
end-of-life lithium ion batteries
p-Toluene sulfonic acid
hydrophobic deep eutectic solvents
tetrabutylammonium chloride
trioctylphosphine oxide
thenoyltrifluoroacetone
Di-(2-ethylhexyl) phosphoric acid
1. Introduction
The development and social necessity of new technologies and the energy transition leads to an increasing consumption of metals, contributing to risks of scarcity of various metals (Mo, Co, Li, Zn, Cu, Sn, Ni, Au, etc.). The extraction of metals from primary geological sources, as well as waste and by products, have to adapt to meet the growing demand. Supply risk of critical raw materials can harm a country's economy. Different techniques have been used to perform metal recovery. Pyrometallurgy process is one of most used. High purity alloys can be recovered with this process, however this process requires high temperatures, being an energy-intensive process (Dias et al., 2022). In addition, metal losses can be produced in the slag with lower recovery rates than with other techniques. Hydrometallurgical recovery is also widely used for separation of metals in aqueous medium (F. J. Alguacil et al., 2003). With this process, higher metal recovery rates are achieved with lower energy consumption, but more research is needed to reduce the environmental impact of hydrometallurgical processes (F. J. Alguacil et al., 2019) DESs are a potential tool that can contribute to the design of cleaner processes, due to good thermal and chemical stability, low melting point, easy synthesis, low vapour pressure and low or practically negligible toxicity. Most of them can be considered biodegradable solvents, showing themselves to be excellent “green” solvents. The aim of this review article collects the publications and highlights the relevance of the use of these solvents in metal recovery from industrial waste and by-products by hydrometallurgical methods. It is necessary to present a summary of the main properties, because them have a great influence on the use of DES in hydrometallurgical processes. To complete the review types, classification and preparation methods of these solvents, as well as the advantages and disadvantages (or limitations) of their use are also included.
2. Definition and history of DESs
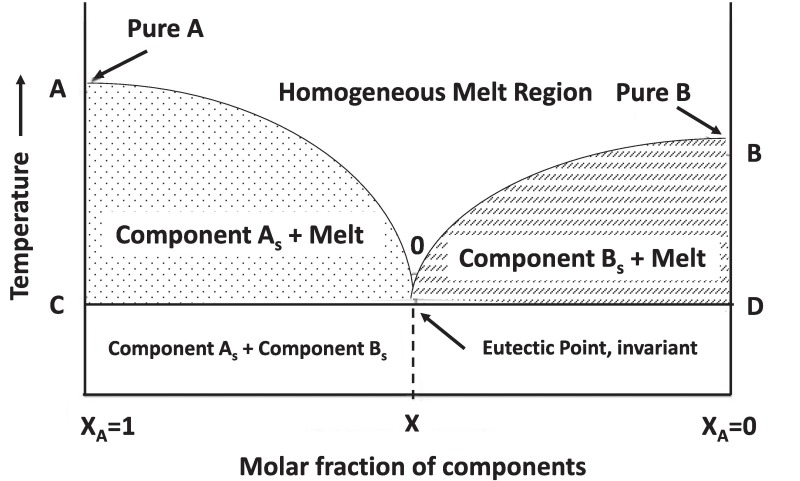
The term eutectic has its origin in the Greek word “eutēktos” which means easy to melt, and which is interpreted as reducing the melting point of the mixture (D. Yu et al., 2021). An eutectic mixture is “an approximately reversible isothermal mixture which does not react between different components during the cooling of a liquid system, causing the system’s freezing point to decrease compared to the melting points of the pure components” (Singh et al., 2021) as shown in Fig. 1.
 Fig. 1
Fig. 1This “deep” nature was first explained by Abbot et al. (Abbott et al., 2002),(Abbott, Capper, et al., 2004a), prepared different melts using metal chlorides (MCl2, M = Zn y/o Sn) with quaternary ammonium salts of formula [Me3NC2H4Y]Cl (Y = OH, Cl, OC(O)Me, OC(O)Ph) and abbreviated as “liquid ionic Lewis acids”. These authors indicated that the ChCl-MCl2 (1:2) (M = Zn o Sn) are a suitable medium for Diels-Alder reactions to take place, the main accelerating effect is associated with the Lewis acidity of the ionic liquid, these are facially recyclable by decanting and washing with hexane, have a reusability of five times (Abbott et al., 2002),(Abbott, Capper, et al., 2004a). This same idea was extended to other hydrated metal salts (CaCl26H2O, LaCl36H2O, CoCl26H2O, LiNO34H2O y Zn(NO3)24H2O) and more specifically for the system ChCl/CrCl36H2O (Abbott, Capper, et al., 2004b).
Abbot et al. (Abbott et al., 2003) studied a mixture formed from choline chloride and urea in a 1:2 ratio and observed that they had unusual properties with a eutectic point of 285 K, this mixture is currently known as reline. The melting point of the mixture is less than each of its individual components corresponding to 575 K and 406 K respectively; the decrease in the freezing point may be due to the interaction between urea molecules and chloride ions. This depression at the melting point gave rise to the term “Deep”, these compounds being known as Deep Eutectic Solvent, DES (Abbott et al., 2003).
DESs are obtained from the mixture of two or three substances with a given composition where the melting points of each of the individual components are higher than that of the mixture, consisting of the appropriate combination of hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBA). The decrease of the freezing point is due to the extensive interspecific hydrogen bonds and the offshoring of the charges (E. L. Smith et al., 2014), Fig. 2. Typical HBDs used are alcohols, carboxylic acids, amides and amino acids and HBAs are ChCl, tetraalkyl halides, tetrabutylphophonium bromide and hydrated metal halides/halides.Fig. 3..
 Fig. 2
Fig. 2 Fig. 3
Fig. 3DESs are compounds with low vapour pressure, a relatively wide liquid range, low or negligible toxicity, low reactivity and non-flammability. These properties make them a new kind of emerging ecological solvent, which is currently in its infancy (Steudte et al., 2014).
Although DESs have physical properties similar to ionic liquids (IL), the terms DESs and ionic liquids are used to refer to different compounds. IL is usually defined as ionic compounds having melting temperatures below 373 K (F. J. Alguacil et al., 2019). The DESs are obtained from the combination of acids and bases of Lewis or BrØnsted, which differs from the IL formed by systems entirely of discrete ions. Other differences between IL and DES are that DES are easier to synthesize than IL, less expensive and are considered as ecological solvents (Q. Zhang et al., 2012).
3. Types and classification
According to the literature, DESs can be classified into five types according to their chemical structure. The general formula for the first type of DES is, Cat+X-x(MCln), where, X- and x refer to a Lewis base and the number of MCln in the DES unit, respectively. This type of compounds is achieved by the combination of non-hydrated metal chlorides (MCln) and quaternary ammonium salts (HBA), within this type of structures include choline chloride (ChCl), carboxylic acidsand amines. The metal elements normally used, represented by M are Ga, Sn, In, Zn, Al, and Fe. The magnitude of n in formulas is restricted to reach DES with a low melting point, ZnCl2, FeCl2, AgCl, CuCl2; CdCl2; LiCl, SnCl2 y SnCl4 (J. Wang et al., 2017)(Kalhor & Ghandi, 2019)(Ijardar et al., 2022). One way to solve this problem is to use hydrated halide metals, instead of anhydrous ones, which correspond to type II DES. Where the melting point is further reduced due to hydration water, further decreasing network energy (E. L. Smith et al., 2014).
The second type of DES is obtained from the same HBA but the metallic chloride is hydrated (MCln·zH2O), where z represents the number of water molecules in the unit cell of salt. The general formula for this type of DES is Cat+X-x(MCln)zH2O y M corresponds to metals such as Fe, Ni, Cu, Co, Cr.
Type III DES has been extensively studied. This type of DES is obtained from the combination of quaternary ammonium salts such as HBA and HBD (carboxylic acid, alcohols, amides and carbohydrates, etc.) (Abbott et al., 2003)(Abbott, Boothby, et al., 2004). Fig. 4 shows the HBAs normally used for the preparation of DESs. These DESs have great importance due to their ability to dissolve different transition metals (Plechkova & Seddon, 2008).
 Fig. 4
Fig. 4Type IV DES is obtained from metallic salts or hydrated metal salts, namely transition metal chlorides and HBD, such as ZnCl2:urea, these metal salts can also form DESs with compounds such as ethylene glycol, acetamide and 1,6 hexanodiol (Ijardar et al., 2022); (Abbott, Capper, et al., 2004b); (Gambino & Bros, 1988). Type V DES is a new type of DES, which has recently been described (Abbott et al., 2007). They consist of non-ionic molecular substances such as donors and acceptors of hydrogen bonds. They are non-ionic DESs formed by compounds such as thymol, menthol in a 1:2 M ratio. Although ionic contribution is not present in DESs type V have the characteristics of the DESs melting point. This could be due to the large number of hydrogen bonds present in these DESs (Abranches et al., 2019).
Natural eutectic solvents (NADES): those DESs derived from cellular metabolites such as alcohols, amino acids, organic acids and sugars (Xie et al., 2019). NADES have a fundamental role in cell metabolism and in many biological processes (germination, resistance to follow, dehydration, etc.) (Shaibuna et al., 2022). They also play an important role in the cryopreservation of organs in living organisms (Gertrudes et al., 2017). In addition, they are used in extraction, chromatography, biomass pre-treatment and enzymatic saccharification (Liu et al., 2018).
The DESs mentioned above are reviewed in this paper for the hydrometallurgical recovery of metals. However, in recent times another kind of DESs are gaining more attention. Therefore, it is considered necessary to mention in this review (Shaibuna et al., 2022).
Therapeutic DESs: those in which one of its active ingredients is a pharmaceutical compound (Duarte et al., 2017). This type of DES is used to reduce problems related to drug solubility, bioavailability, difficulty in manufacturing, handling and permeability (Aroso et al., 2016).
Polyuasi-eutectic solvents (PQESs): The term PEGylated was proposed by Jiang et al. (Jiang et al., 2017), called these systems as poly-quasi-eutectic solvents (PQESs) (Jiang et al., 2019). These are obtained from polymers such as polyethylene glycol (PEG) poly(ethylene glycol)-block-poly(propylene glycol)- block poly(ethylene glycol) (P123), poly(propylene glycol)bis(2- aminopropyl ether) (PPG-NH2), and poly(ethylene glycol) dimethyl ether (DMPEG) and donors of hydrogen bonds as carboxylic acids and amides (Jiang et al., 2019). Its application consists of the evolution reaction of oxygen and for the processing of metal oxides.
Deep eutectic polymeric solvents (PDESs) (Ren’ai et al., 2018): those DESs are considered to be the hydrogen donor part is polymerizable. They are obtained from various ammonium salts and acrylic/acrylic acids such as HBDs (Mota-Morales et al., 2013). Reactivity and frontal polymerization capability is based on the choice of ammonium salt the fully converted polymer can have medical applications for drug administration. PDESs have been successfully applied in nanotechnology (K. Zhang et al., 2020), gas separation (Isik et al., 2016) and catalysis (Ishaq et al., 2020).
4. Preparation methods
DESs synthesis methods are simple because they do not require multiple steps or separation methods, such as organic solvents. In addition, it should be noted that most DESs components are cheap and natural (Singh et al., 2021). DESs are obtained from the mixture of HBA and HBD in the appropriate proportions, do not involve chemical reactions and can therefore be called preparation methods and not synthesis methods (Farooq et al., 2020). There are different synthesis methods, including mixing components with mortar and stirring components with heating.
The grinding method was introduced to prepare DESs without heat. It consists of crushing the mixture of the compounds (HBA and HBD) at room temperature, with a mortar or a mortar hand, until the formation of a clear and homogeneous liquid, usually carried out under nitrogen atmosphere or in a glovebox. This method was introduced by Florindo et al. (Florindo et al., 2014) for the preparation of DESs based on choline chloride and carboxylic acids (Florindo, Oliveira, et al., 2017) (Florindo et al., 2018).
The most commonly used method for the preparation of DESs are the method of heating (between 323 and 373 K), under agitation of the mixture of the compounds until the formation of a homogeneous liquid (Abbott et al., 2001)(Shaibuna et al., 2022). The temperature is selected according to the melting point, the boiling point of the reagents and the stability of the reagents. High temperature may lead to potential DESs degradation due to esterification reactions (Abbott, Boothby, et al., 2004)(Abbott, Capper, et al., 2004b), it is essential to identify the appropriate temperature and preparation time.
Apart from these two more traditional methods of preparing DES, other methods based on lyophilization are also included in the literature, which involves the dissolution of the DESs components separately in the minimum amount of bi-distilled water the aqueous solutions were frozen between 77 and 253 K, subsequently freeze-dried obtaining viscous and clear liquids (Gutiérrez et al., 2010) (Nam et al., 2015).
The vacuum evaporation method was first used for the preparation of NADES. Dai et al. (Dai et al., 2013) (Dai et al., 2015) studied a method of DESs synthesis from evaporation methods, first dissolve the DESs components in water and then undergo evaporation at 323 K. Then the DESs is stored in a silica geldesiccator. This method uses relatively lower temperatures compared to the heating and stirring method and is frequently used for components with higher melting points. Considering the optimization of time and energy consumption Gómez et al. (Gomez et al., 2018) prepared Natural DESs from a more environmentally friendly method assisted by microwaves. With this objective Santana et al. (Santana et al., 2019) developed an unconventional NADES synthesis method assisted with an ultrasound bath, previously the mixtures were homogenized with a vortex.
The twin screw extrusion method is a preparation method that is used to overcome the limitations of heating and stirring methods in the preparation of DESs (Shaibuna et al., 2022)(Crawford et al., 2016). This method consists of two screws that rotate in the opposite direction, are housed in a stainless-steel barrel, where multiple transport and kneading sections are located. In the transport sections the materials are moved forward and the kneading section high shear and compression forces are applied on the material as it passes through. HBA and HBD are incorporated in the appropriate proportions after preheating of the double screw sections.
The method that is usually cheaper and faster for DESs production is the microwave irradiation method (Farooq et al., 2020). HBAs and HBDs are microwave irradiated for 20 s (Gomez et al., 2018)(Farooq et al., 2020). The application of this method requires careful optimization of heating time, power and component selection. Another method of preparation is the use of ultrasound (Calvo‐Flores & Mingorance‐Sánchez, 2021); (Farooq et al., 2020). Stoichiometric amounts of HBD and HBA are mixed in a glass vial and sealed and introduced into an ultrasound bath. The time and temperature for DES formation is based on pure constituents.
Solvent addition is not necessary in preparation methods, therefore no purification steps are necessary, increasing their potential as economic substitutes for traditional organic solvents (Hansen et al., 2021).
5. DESs properties in relation with hydrometallurgy process
The physical properties of DES, such as density, viscosity, surface tension and conductivity are essential to determine their applications. Its application in the different industrial sectors depends on these properties, being interesting that they present a low density, viscosity and surface tension (Qin et al., 2020).
The physico-chemical characteristics of DESs depend among other factors on the nature of the hydrogen bond acceptor and donor components, which form the eutectic mixture. It is possible to adjust the physico-chemical characteristics by modifying the molar ratio and the anion size of HBA and HBD (Omar & Sadeghi, 2021). The following sections will analyse the main properties of interest to the DESs.
5.1. Melting point
Deep eutectic solvents can be identified by their depression at the melting point (Tm), Fig. 1. As noted above, DESs have melting points lower than their pure components. To date, specific data on eutectic DESs compositions and accompanying binary phase diagrams have been virtually non-existent. This further underlines the importance of obtaining phase diagrams of all DESs under investigation as they provide information on the temperature and composition range that can be expected from a liquid, which will help other researchers design DESs systems for their specific applications (Hansen et al., 2021). Most papers on DESs analyse the mixtures in their assumed eutectic compositions, but diagrams justifying the choice of composition are not provided.
The depression of the eutectic melting point is due to the strong interaction between the hydrogen bond acceptor (halide anion) and the hydrogen bond donor (E. L. Smith et al., 2014). DEs with melting points below 323 K are the most researched and of great interest due to their low cost and could be used as alternative solvents in a wide variety of industrial applications (Singh et al., 2021). The molar ratio of organic salts, the alkyl chain length and the hydrogen bond donor have a major impact on the melting point of DES, as shown in Table 1.
| HBA | HBD |
HBA:HBD (molar ratio) |
Tm (K) | Reference |
|---|---|---|---|---|
| ChCl | Urea | 1:2 | 285 | (Shah & Mjalli, 2014) |
| ChCl | Glycerol | 1:2 | 290 | (AlOmar et al., 2016) |
| ChCl | Glycerol | 1:3 | 274 | (Shahbaz et al., 2012a) |
| ChCl | Glycerlo | 1:4 | 275 | (Shahbaz et al., 2012b) |
| ChCl | EG | 1:2 | 237 | (Ibrahim et al., 2019a) |
| ChCl | Imidazole | 3:7 | 329 | (Hou et al., 2008) |
| ChCl | Acrylic acid | 1:1.6 | 269 | (Mota-Morales et al., 2011) |
| ChCl | Pyrogallol | 1:1 | −346 | (Omar & Sadeghi, 2021) |
| ChCl | Pyrogallol | 1:2 | 236 | (Omar & Sadeghi, 2021) |
| ChCl | Oxalic, pheny acetic, phenyl propionic, tricarballyclic, succinic and acid citric | 1:1 or 1:2 | 283 (malonic), 293 (phenylpropionic) − 363 (tricarballylic) | (Abbott et al., 2004) |
| ChCl | levulinic acid, itaconic acid, xylitol, D-sorbitol, L-(+)-tartaric acid, D-isosorbide, 4-hydroxybenzoic acid, caffeic acid, p-coumaric acid, trans-cinnamic acid, suberic acid, gallic acid | Varies (1:05. 1:1 or 1:2) | RT* (levulinic) − 366 (suberic) | (Maugeri & Domínguez de María, 2012) |
| ChCl | Phenol, o-cresol, 2, xylenol | 1:3 | 249 (o-cresol) − 290 (2,3-xylenol) | (Guo et al., 2013) |
| Triphenylphosphunium bromide | Etilen glycol | 1:3 | 227 | (Shahbaz et al., 2011b) |
| Triphenylphosphunium bromide | Etilen glycol | 1:4 | 223 | (Shahbaz et al., 2011a) |
| Triphenylphosphunium bromide | Etilen glycol | 1:5 | 265 | (Shahbaz et al., 2011a) |
| Tetraethylammonium bromide | Benzilic acid | 1:1 | 162 | (Omar & Sadeghi, 2020a) |
| Tetraethylammonium bromide | Benzilic acid | 1:1 | 265 | (Omar & Sadeghi, 2020b) |
| Tetrabutylammonium hydrogen sulfate | Benzilic acid | 1:1 | 254 | (Omar & Sadeghi, 2020b) |
| ZnCl2 | EG, urea | 1:4 (1:3.5 urea) | 243 (EG) | (Abbott et al., 2007) |
| Halide salts: MTPB, TBAB, BTPC, DEAC | EG, glycerol | (1:1–1:4) | 242 (EG)-276(MTPB:glycerol) | (Ibrahim et al., 2019b)(Shahbaz et al., 2012b) |
* = observed to be liquid at room temperature.
Bibliography.
Abbott, A. P., Barron, J. C., Ryder, K. S., & Wilson, D. (2007). Eutectic-Based Ionic Liquids with Metal-Containing Anions and Cations. Chemistry - A European Journal, 13(22), 6495–6501. https://doi.org/10.1002/chem.200601738.
Abbott, A. P., Boothby, D., Capper, G., Davies, D. L., & Rasheed, R. K. (2004). Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. Journal of the American Chemical Society, 126(29), 9142–9147. https://doi.org/10.1021/ja048266j.
AlOmar, M. K., Hayyan, M., Alsaadi, M. A., Akib, S., Hayyan, A., & Hashim, M. A. (2016). Glycerol-based deep eutectic solvents: Physical properties. Journal of Molecular Liquids, 215, 98–103. https://doi.org/10.1016/j.molliq.2015.11.032.
Guo, W., Hou, Y., Ren, S., Tian, S., & Wu, W. (2013). Formation of Deep Eutectic Solvents by Phenols and Choline Chloride and Their Physical Properties. Journal of Chemical & Engineering Data, 58(4), 866–872. https://doi.org/10.1021/je300997v.
Hansen, B. B., Spittle, S., Chen, B., Poe, D., Zhang, Y., Klein, J. M., Horton, A., Adhikari, L., Zelovich, T., Doherty, B. W., Gurkan, B., Maginn, E. J., Ragauskas, A., Dadmun, M., Zawodzinski, T. A., Baker, G. A., Tuckerman, M. E., Savinell, R. F., & Sangoro, J. R. (2021). Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chemical Reviews, 121(3), 1232–1285. https://doi.org/10.1021/acs.chemrev.0c00385.
Hou, Y., Gu, Y., Zhang, S., Yang, F., Ding, H., & Shan, Y. (2008). Novel binary eutectic mixtures based on imidazole. Journal of Molecular Liquids, 143(2–3), 154–159. https://doi.org/10.1016/j.molliq.2008.07.009.
Ibrahim, R. K., Hayyan, M., AlSaadi, M. A., Ibrahim, S., Hayyan, A., & Hashim, M. A. (2019a). Physical properties of ethylene glycol-based deep eutectic solvents. Journal of Molecular Liquids, 276, 794–800. https://doi.org/10.1016/j.molliq.2018.12.032.
Ibrahim, R. K., Hayyan, M., AlSaadi, M. A., Ibrahim, S., Hayyan, A., & Hashim, M. A. (2019b). Physical properties of ethylene glycol-based deep eutectic solvents. Journal of Molecular Liquids, 276, 794–800. https://doi.org/10.1016/j.molliq.2018.12.032.
Maugeri, Z., & Domínguez de María, P. (2012). Novel choline-chloride-based deep-eutectic-solvents with renewable hydrogen bond donors: levulinic acid and sugar-based polyols. RSC Adv., 2(2), 421–425. https://doi.org/10.1039/C1RA00630D.
Mota-Morales, J. D., Gutiérrez, M. C., Sanchez, I. C., Luna-Bárcenas, G., & del Monte, F. (2011). Frontal polymerizations carried out in deep-eutectic mixtures providing both the monomers and the polymerization medium. Chemical Communications, 47(18), 5328. https://doi.org/10.1039/c1cc10391a.
Omar, K. A., & Sadeghi, R. (2020a). Novel benzilic acid-based deep-eutectic-solvents: Preparation and physicochemical properties determination. Fluid Phase Equilibria, 522, 112752. https://doi.org/10.1016/j.fluid.2020.112752.
Omar, K. A., & Sadeghi, R. (2020b). Novel benzilic acid-based deep-eutectic-solvents: Preparation and physicochemical properties determination. Fluid Phase Equilibria, 522, 112752. https://doi.org/10.1016/j.fluid.2020.112752.
Omar, K. A., & Sadeghi, R. (2021). Novel Deep Eutectic Solvents Based on Pyrogallol: Synthesis and Characterizations. Journal of Chemical & Engineering Data, 66(5), 2088–2095. https://doi.org/10.1021/acs.jced.1c00023.
Omar, K. A., & Sadeghi, R. (2022). Physicochemical properties of deep eutectic solvents: A review. Journal of Molecular Liquids, 360, 119524. https://doi.org/10.1016/j.molliq.2022.119524.
Shah, D., & Mjalli, F. S. (2014). Effect of water on the thermo-physical properties of Reline: An experimental and molecular simulation based approach. Phys. Chem. Chem. Phys., 16(43), 23900–23907. https://doi.org/10.1039/C4CP02600D.
Shahbaz, K., Baroutian, S., Mjalli, F. S., Hashim, M. A., & AlNashef, I. M. (2012a). Densities of ammonium and phosphonium based deep eutectic solvents: Prediction using artificial intelligence and group contribution techniques. Thermochimica Acta, 527, 59–66. https://doi.org/10.1016/j.tca.2011.10.010.
Shahbaz, K., Baroutian, S., Mjalli, F. S., Hashim, M. A., & AlNashef, I. M. (2012b). Densities of ammonium and phosphonium based deep eutectic solvents: Prediction using artificial intelligence and group contribution techniques. Thermochimica Acta, 527, 59–66. https://doi.org/10.1016/j.tca.2011.10.010.
Shahbaz, K., Mjalli, F. S., Hashim, M. A., & AlNashef, I. M. (2011a). Using Deep Eutectic Solvents Based on Methyl Triphenyl Phosphunium Bromide for the Removal of Glycerol from Palm-Oil-Based Biodiesel. Energy & Fuels, 25(6), 2671–2678. https://doi.org/10.1021/ef2004943.
Shahbaz, K., Mjalli, F. S., Hashim, M. A., & AlNashef, I. M. (2011b). Using Deep Eutectic Solvents Based on Methyl Triphenyl Phosphunium Bromide for the Removal of Glycerol from Palm-Oil-Based Biodiesel. Energy & Fuels, 25(6), 2671–2678. https://doi.org/10.1021/ef2004943.
Anions also have a significant impact on the DESs melting point. Therefore, the DESs formed from choline and urea salt decreased in the order F > NO3 > Cl > BF4, which indicates that there is a strong correlation with the strength of the hydrogen bond as shown in Table 2. According to the above the melting points of eutectic mixtures depends on the way the salt anion interacts with HBDs, the grid energy and the change of entropy in the result of the formation of the liquid phase.
Bibliography.
Abbott, A. P., Capper, G., Davies, D. L., Rasheed, R. K., & Tambyrajah, V. (2003). Novel solvent properties of choline chloride/urea mixturesElectronic supplementary information (ESI) available: spectroscopic data. See https://www.rsc.org/suppdata/cc/b2/b210714g/. Chemical Communications, 1, 70–71. https://doi.org/10.1039/b210714g.
Omar, K. A., & Sadeghi, R. (2022). Physicochemical properties of deep eutectic solvents: A review. Journal of Molecular Liquids, 360, 119524. https://doi.org/10.1016/j.molliq.2022.119524.
5.2. Density
Density is one of the fundamental properties of DESs to consider in the selection of the solvent and separation performance in the hydrometallurgy processes considering the biphasic nature of this methods. In general the density of DESs are higher than that of water, the values oscillate between 1.0 and 1.3 g cm−3 a 298 K, DESs based on metallic salts have a slightly higher density in the range of 1.3–1.6 g cm−3 (Tang & Row, 2013). For example, ethaline has a density of 1.14 g cm−3 and glycine from 1.19 g cm−3 a 293 K. An exception is the case of some hydrophobic DESs, where its density is lower than that of water (Florindo et al., 2019). Table 3 shows the different densities of these compounds.
| HBA | HBD | HBA:HBD(molar ratio) | Density values (g /ml) at 298 K | Reference |
|---|---|---|---|---|
| ChCl | Urea | 1:2 | 1.21 | (Mjalli & Abdel Jabbar, 2014) |
| ChCl | Urea | 1:2 | 1.25 | (Abbott et al., 2006) |
| ChCl | glycerol | 1:1 | 1.16 | (Abbott et al., 2006) |
| ChCl | glycerol | 1:2 | 1.18 | (AlOmar et al., 2016) |
| ChCl | glycerol | 1:3 | 1.20 | (Abbott, Harris, et al., 2007) |
| ChCl | EG | 1:2 | 1.12 | (Zhang et al., 2020) |
| ChCl | EG | 1.3 | 1.12 | (Abbott, Harris, et al., 2007) |
| ChCl | Malic acid | 1:1 | 1.185 (293 K) | (Yadav et al., 2015) |
| ChCl | Acetic acid | 1:1 | 1.12 | (Zhu et al., 2016) |
| ChCl | D-(+)-glucose, citric acid, D-(+)-sucrose; L-(+)-tartaric acid, D-(+)-xylose | 1:1 | 1.22 (sucrose) − 1.27 (glucose) | (Craveiro et al., 2016) |
| ChCl | Oxalic acid | 1:1 | 1.15 | (Florindo et al., 2014) |
| ChCl | amines: MEA, DEA, MDEA | 1:6 | 1.056–1.102 (293 K) | (Adeyemi et al., 2018) |
| ChCl | phenol, o-cresol, 2,3-xylenol | 1:3 | 1.071 (cresol), 1.095 (phenol) | (Guo et al., 2013) |
| ChCl | Glycolic acid | 1:1 | 1.259 | (Florindo et al., 2014) |
| ChCl | Malonic acid | 1:1 | 1.231 | (Florindo et al., 2014) |
| ChCl | 1-(trifluoromethyl) urea | 1:1.15 | 1.324 | (Abbott et al., 2006) |
| ChCl | Glucose | 2:1 | 1.2423 | (Mjalli & Ahmad, 2017) |
| ChAc | Urea | 1:2 | 1.206 | (Abbott et al., 2006) |
| ZnCl2 | Acetamide | 1:4 | 1.36 | (Abbott et al., 2004) |
| ZnCl2 | EG | 1:4 | 1.45 | (Abbott, Barron, et al., 2007) |
| ZnCl2 | Urea | 1:3.5 | 1.63 | (Abbott, Barron, et al., 2007) |
| MTPB, BTPC, DEAC, TBAB | EG | 1:2 (1:11 BTPC) | 1.07 (TBAB) − 1.24 (MTPB) | (AlOmar et al., 2016) |
| MTPB, BTPC, ATPB, DEAC, TBAB | Glycerol | 1:3 (MTPB), 1:4 (TBAB), 1:16 (BTPC) | 1.17 (TBAB) − 1.30 (MTPB) | (AlOmar et al., 2016) |
| Decanoic acdi | lidocaine, atropine, menthol | 2:1 (1:1 menthol) | 0.899 (menthol) − 1.026 (atropine) | (van Osch et al., 2019b) |
| dodecanoic acid | lidocaine, atropine | 2:1 | 0.949 (lidocaine) − 1.008 (atropine) | (van Osch et al., 2019a) |
| Menthol | lidocaine | 2:1 | 0.939 | (van Osch et al., 2019a) |
| Thymol | lidocaine, coumarin, menthol | 1:1 | 0.936 (menthol) − 1.091 (coumarin) | (van Osch et al., 2019a) |
Bibliography.
Abbott, A. P., Barron, J. C., Ryder, K. S., & Wilson, D. (2007). Eutectic-Based Ionic Liquids with Metal-Containing Anions and Cations. Chemistry - A European Journal, 13(22), 6495–6501. https://doi.org/10.1002/chem.200601738.
Abbott, A. P., Capper, G., Davies, D. L., & Rasheed, R. K. (2004). Ionic Liquid Analogues Formed from Hydrated Metal Salts. Chemistry - A European Journal, 10(15), 3769–3774. https://doi.org/10.1002/chem.200400127.
Abbott, A. P., Capper, G., & Gray, S. (2006). Design of Improved Deep Eutectic Solvents Using Hole Theory. ChemPhysChem, 7(4), 803–806. https://doi.org/10.1002/cphc.200500489.
Abbott, A. P., Harris, R. C., & Ryder, K. S. (2007). Application of Hole Theory to Define Ionic Liquids by their Transport Properties. The Journal of Physical Chemistry B, 111(18), 4910–4913. https://doi.org/10.1021/jp0671998.
Adeyemi, I., Abu-Zahra, M. R. M., & AlNashef, I. M. (2018). Physicochemical properties of alkanolamine-choline chloride deep eutectic solvents: Measurements, group contribution and artificial intelligence prediction techniques. Journal of Molecular Liquids, 256, 581–590. https://doi.org/10.1016/j.molliq.2018.02.085.
AlOmar, M. K., Hayyan, M., Alsaadi, M. A., Akib, S., Hayyan, A., & Hashim, M. A. (2016). Glycerol-based deep eutectic solvents: Physical properties. Journal of Molecular Liquids, 215, 98–103. https://doi.org/10.1016/j.molliq.2015.11.032.
Craveiro, R., Aroso, I., Flammia, V., Carvalho, T., Viciosa, M. T., Dionísio, M., Barreiros, S., Reis, R. L., Duarte, A. R. C., & Paiva, A. (2016). Properties and thermal behavior of natural deep eutectic solvents. Journal of Molecular Liquids, 215, 534–540. https://doi.org/10.1016/j.molliq.2016.01.038.
Florindo, C., Oliveira, F. S., Rebelo, L. P. N., Fernandes, A. M., & Marrucho, I. M. (2014). Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. ACS Sustainable Chemistry & Engineering, 2(10), 2416–2425. https://doi.org/10.1021/sc500439w.
Guo, W., Hou, Y., Ren, S., Tian, S., & Wu, W. (2013). Formation of Deep Eutectic Solvents by Phenols and Choline Chloride and Their Physical Properties. Journal of Chemical & Engineering Data, 58(4), 866–872. https://doi.org/10.1021/je300997v.
Hansen, B. B., Spittle, S., Chen, B., Poe, D., Zhang, Y., Klein, J. M., Horton, A., Adhikari, L., Zelovich, T., Doherty, B. W., Gurkan, B., Maginn, E. J., Ragauskas, A., Dadmun, M., Zawodzinski, T. A., Baker, G. A., Tuckerman, M. E., Savinell, R. F., & Sangoro, J. R. (2021). Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chemical Reviews, 121(3), 1232–1285. https://doi.org/10.1021/acs.chemrev.0c00385.
Ijardar, S. P., Singh, V., & Gardas, R. L. (2022). Revisiting the Physicochemical Properties and Applications of Deep Eutectic Solvents. Molecules, 27(4), 1368. https://doi.org/10.3390/molecules27041368.
Mjalli, F. S., & Abdel Jabbar, N. M. (2014). Acoustic investigation of choline chloride based ionic liquids analogs. Fluid Phase Equilibria, 381, 71–76. https://doi.org/10.1016/j.fluid.2014.08.017.
Mjalli, F. S., & Ahmad, O. (2017). Density of aqueous choline chloride-based ionic liquids analogues. Thermochimica Acta, 647, 8–14. https://doi.org/10.1016/j.tca.2016.11.008.
van Osch, D. J. G. P., Dietz, C. H. J. T., van Spronsen, J., Kroon, M. C., Gallucci, F., van Sint Annaland, M., & Tuinier, R. (2019a). A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustainable Chemistry & Engineering, 7(3), 2933–2942. https://doi.org/10.1021/acssuschemeng.8b03520.
van Osch, D. J. G. P., Dietz, C. H. J. T., van Spronsen, J., Kroon, M. C., Gallucci, F., van Sint Annaland, M., & Tuinier, R. (2019b). A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustainable Chemistry & Engineering, 7(3), 2933–2942. https://doi.org/10.1021/acssuschemeng.8b03520.
Yadav, A., Kar, J. R., Verma, M., Naqvi, S., & Pandey, S. (2015). Densities of aqueous mixtures of (choline chloride + ethylene glycol) and (choline chloride + malonic acid) deep eutectic solvents in temperature range 283.15–363.15 K. Thermochimica Acta, 600, 95–101. https://doi.org/10.1016/j.tca.2014.11.028.
Zhang, Y., Poe, D., Heroux, L., Squire, H., Doherty, B. W., Long, Z., Dadmun, M., Gurkan, B., Tuckerman, M. E., & Maginn, E. J. (2020). Liquid Structure and Transport Properties of the Deep Eutectic Solvent Ethaline. The Journal of Physical Chemistry B, 124(25), 5251–5264. https://doi.org/10.1021/acs.jpcb.0c04058.
Zhu, S., Li, H., Zhu, W., Jiang, W., Wang, C., Wu, P., Zhang, Q., & Li, H. (2016). Vibrational analysis and formation mechanism of typical deep eutectic solvents: An experimental and theoretical study. Journal of Molecular Graphics and Modelling, 68, 158–175. https://doi.org/10.1016/j.jmgm.2016.05.003.
The density of DESs depends on the organization and molecular packaging, it is affected by the existence of gaps and vacancies within liquid DESs. That is why the density of DESs urea/choline chloride is higher than that of the urea/acetylcholine chloride system due to the presence of a large hole in acetylcholine chloride (Vigier et al., 2012)(Ul Haq et al., 2022)(Abbott et al., 2007). Another parameter that affects the density of DESs is the molar ratio HBA and HBD.
Abbot et al. (Abbott, Capper, & Gray, 2006) indicated that the addition of choline chloride to glycerol reduces the density of DESs due to increased free volume. An increase in the length of the alkyl cation chain leads to a decrease in the density of DESs, as follows: tetraethyl ammonium bromide > tetra propyl ammonium bromide > tetra butyl ammonium bromide. This indicates that the increase that occurs in the free volume is due to the elongation of alkyl chain length (García et al., 2015)(Montalbán et al., 2015).
Basaiahgari et al. (Basaiahgari et al., 2018) measured the density of ethylene, diethylene and triethylene glycol and glycerol as HBD and benzyl ammonium chloride salts as HBA. The results showed that the DESs obtained from ethylene glycol has a lower density than the DESs obtained from glycerol. According to the authors this increase in density by replacing ethylene glycol with diethylene glycol, triethylene glycol and glycerol means that the increase in the number of OH functional groups in HBD increases the formation of more H bonds, resulting in a decrease in the average available volume.
The composition and molar ratio between HBA and HBD are ways of modifying the density of eutectic mixtures. DESs based on ChCl and citric acid were studied by Shafie et al. (Shafie et al., 2019), observed that as the amount of ChCl increases in relation to citric acid, density decreases and vice versa, an increase in citric acid means an increase in viscosity.
The density of the deep eutectic solvent shows a temperature-dependent behaviour, decreasing linearly as the temperature increases, due to the thermal expansion of DES (Cui et al., 2017)(Ibrahim et al., 2019)(Florindo et al., 2014)(Shahbaz et al., 2012). According to Hole theory thermal energy can generate fluctuations in local densities, which leads to the increase of space between the HBA and HBD of the liquid DESs system (Omar & Sadeghi, 2022). The effect of the temperature on the density of DESs could be expressed in terms of isobaric thermal expansion coefficients, Eq. (1), which defines the available volume of free DES (Ijardar et al., 2022). The calculation of this expansion coefficient may be useful for understanding the compressible behaviour of DES.(1)
So, the linear decrease in the density of the DESs is observed with an increase of the temperature, resulting in the availability more free space, the available space in DESs is related to change in α values. The value of α in DESs are minimal compared with the value of common solvents therefore the temperature showed a little effect on density and the isobaric thermal coefficient. DESS expanded or compressed less in comparison to ILs and other organic solvents.
5.3. Viscosity
Viscosity is another important factor of DESs due to its influence in separation application, such as leaching or liquid–liquid extraction. The DESs viscosity has extensively studied Table 4. Viscosity can be defined as the resistance of a fluid in response to a deformation at a given shear speed. This indicate that fluids with low viscosities flow easily, however, liquids with higher viscosities have a slower flow. Most DESs are recognized as viscous liquids at room temperature, with a viscosity ƞ>100 mPaּS (El Achkar et al., 2021), this makes it difficult to use in commercial applications such as catalysis, synthesis etc. compared to other types of compounds (Yadav & Pandey, 2014). The viscosity of DESs are higher than that of water, but comparable to that of ionic liquids. This increased viscosity is attributed to the presence of an extensive network of hydrogen bonds along with other interactions such as Van de Waals forces and electrostatic interactions between donor and acceptor of hydrogen bonds of DES components, which leads to high viscosity and lower ionic mobility in the small empty volume within the liquid DES (Omar & Sadeghi, 2022).
| HBA | HBD | HBA:HBD (molar ratio) | Viscosity (cP) at 298 K | Reference |
|---|---|---|---|---|
| ChCl | urea | 1:2 | 750 | (Mjalli & Abdel Jabbar, 2014) |
| ChCl | Glycerol | 1:2 | 281 | (Meng et al., 2016) |
| ChCl | Glycerol | 1:2 | 302 | (van Osch et al., 2015) |
| ChCl | Glycerol | 1:2 | 376 (323) | (Florindo et al., 2017) |
| ChCl | Glycerol | 1:2 | 259 | (D’Agostino et al., 2011) |
| ChCl | EG | 1:2 | 48 | (Zhang et al., 2020) |
| ChCl | EG | 1:2 | 44.4 | (Harifi-Mood & Buchner, 2017) |
| ChCl | EG | 1:2 | 36 (293) | (Leron & Li, 2012) |
| ChCl | EG | 1:2 | 37 | (D’Agostino et al., 2011) |
| ChCl | Acrylic acid | 1:1.6 | 115 (295 K) | (Mota-Morales et al., 2011) |
| ChCl | Acetic acid | 1:1 | 162 | (S. Zhu et al., 2016) |
| ChCl | levulinic, glutaric, and glycolic acid | 1:1(1:2 levulinic) | 2015 (glutaric) − 227 (levulinic) | (Florindo et al., 2014) |
| ChCl | citric, malonic, oxalic acid, succinic acid | 1:1 | 9126 (citric), 1638 (malonic), 1489 (succinic), 597 (oxalic) | (Abbott, Boothby, et al., 2004) |
| ChCl | malonic acid | 1:2 | 1124 | (D’Agostino et al., 2011) |
| ChCl | malic acid | 1:1 | 1100 | (Barzinjy & Zankana, 2016) |
| ChCl | phenol, o-cresol, 2,3 xylenol | 1:3 | 44 (phenol), 77 (cresol) | (Guo et al., 2013) |
| ChCl | phenol | 1:2 | 99.8 | (J. Zhu et al., 2017) |
| ChCl | phenol | 1:3 | 446 | (Mjalli & Naser, 2015) |
| ChCl | p-cresol | 1:2 | 102 | (J. Zhu et al., 2017) |
| ChCl | imidazole | 3:7 | 15 (343 K) | (Hou et al., 2008) |
| ChCl | amines: MEA, DEA, MDEA | 1:6 | 52 (MEA) − 567 (DEA) (293 K) | (Adeyemi et al., 2018) |
| ChCl | Triethylene glycol | 1:2 | 839 | (Bahadori et al., 2013) |
| ChCl | Triethylene glycol | 1:3 | 66 | (Mjalli & Naser, 2015) |
| ChCl | Fructose | 1:1 | 653 | (Abbott, Harris, et al., 2007) |
| ChCl | Fructose | 2:1 | 11.312 | (Hayyan et al., 2012) |
| ZnCl2 | Urea, ChCl | 1:3.5, 1:2 (ChCl) | 11,340 (urea); 85,000 (ChCl) | Urea (Abbott, Barron, et al., 2007), ChCl (Abbott, Capper, et al., 2004) |
| MTPB, BTPC, TBAB | EG | 1:2 (1:11 BTPC) | 57 (BTPC) − 177 (MTPB) (293 K) | (Ibrahim et al., 2019) |
| DEAC | EG, glycerol | 1:2 | 50 (EG), 513 (glycerol) | (Siongco et al., 2013) |
| MTPB, BTPC, TBAB | glycerol | 1:3 (MTPB), 1:4 (TBAB), 1:16 (BTPC) | 877 (TBAB) − 2220 (MTPB) | (AlOmar et al., 2016) |
| TBAB | imidazole | 3:7 | 810 (293 K) | (Hou et al., 2008) |
| TBAC | decanoic acid | 1:2 | 429 | (Ruggeri et al., 2019) |
| decanoic acid | lidocaine, atropine, menthol | 2:1 (1:1 menthol) | 20 (menthol) − 5985 (atropine) | (van Osch et al., 2019) |
| dodecanoic acid | lidocaine, atropine | 2:1 | 371 (lidocaine) − 5600 (atropine) | (van Osch et al., 2019) |
| thymol | lidocaine, coumarin, menthol | 1:1 | 29 (coumarin) − 177 (lidocaine) | (van Osch et al., 2019) |
| Diethylethanolammonium chloride | Glycerol | 1:2 | 433 | (Sarmad et al., 2017) |
| Benzyltrimethylammonium chloride | Glycerol | 1:2 | 716.6 | (Sarmad et al., 2017) |
| Triethylmethylammonium chloride | Glycerol | 1:2 | 236.6 | (Sarmad et al., 2017) |
| Tetrabutylammoniun hydrogen sulfate | Ninhydrin | 1:0.75 | 48,500 (323 K) | (Omar & Sadeghi, 2020) |
| ChCl | Ninhydrin | 1:0.75 | 30,500 (293 K) | (Omar & Sadeghi, 2020) |
Bibliography.
Abbott, A. P., Barron, J. C., Ryder, K. S., & Wilson, D. (2007). Eutectic-Based Ionic Liquids with Metal-Containing Anions and Cations. Chemistry - A European Journal, 13(22), 6495–6501. https://doi.org/10.1002/chem.200601738.
Abbott, A. P., Boothby, D., Capper, G., Davies, D. L., & Rasheed, R. K. (2004). Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. Journal of the American Chemical Society, 126(29), 9142–9147. https://doi.org/10.1021/ja048266j.
Abbott, A. P., Capper, G., Davies, D. L., & Rasheed, R. (2004). Ionic Liquids Based upon Metal Halide/Substituted Quaternary Ammonium Salt Mixtures. Inorganic Chemistry, 43(11), 3447–3452. https://doi.org/10.1021/ic049931s.
Abbott, A. P., Harris, R. C., & Ryder, K. S. (2007). Application of Hole Theory to Define Ionic Liquids by their Transport Properties. The Journal of Physical Chemistry B, 111(18), 4910–4913. https://doi.org/10.1021/jp0671998.
Adeyemi, I., Abu-Zahra, M. R. M., & AlNashef, I. M. (2018). Physicochemical properties of alkanolamine-choline chloride deep eutectic solvents: Measurements, group contribution and artificial intelligence prediction techniques. Journal of Molecular Liquids, 256, 581–590. https://doi.org/10.1016/j.molliq.2018.02.085.
AlOmar, M. K., Hayyan, M., Alsaadi, M. A., Akib, S., Hayyan, A., & Hashim, M. A. (2016). Glycerol-based deep eutectic solvents: Physical properties. Journal of Molecular Liquids, 215, 98–103. https://doi.org/10.1016/j.molliq.2015.11.032.
Bahadori, L., Chakrabarti, M. H., Mjalli, F. S., AlNashef, I. M., Manan, N. S. A., & Hashim, M. A. (2013). Physicochemical properties of ammonium-based deep eutectic solvents and their electrochemical evaluation using organometallicreference redox systems. Electrochimica Acta, 113, 205–211. https://doi.org/10.1016/j.electacta.2013.09.102.
Barzinjy, A. A., & Zankana, M. M. (2016). A Novel Application of the Quartz Crystal Microbalance for Determining the Rheological Properties of the Highly Viscous Liquids. Acta Physica Polonica A, 130(1), 239–244. https://doi.org/10.12693/APhysPolA.130.239.
D’Agostino, C., Harris, R. C., Abbott, A. P., Gladden, L. F., & Mantle, M. D. (2011). Molecular motion and ion diffusion in choline chloride based deep eutectic solvents studied by 1H pulsed field gradient NMR spectroscopy. Physical Chemistry Chemical Physics, 13(48), 21383. https://doi.org/10.1039/c1cp22554e.
Florindo, C., Oliveira, F. S., Rebelo, L. P. N., Fernandes, A. M., & Marrucho, I. M. (2014). Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. ACS Sustainable Chemistry & Engineering, 2(10), 2416–2425. https://doi.org/10.1021/sc500439w.
Florindo, C., Oliveira, M. M., Branco, L. C., & Marrucho, I. M. (2017). Carbohydrates-based deep eutectic solvents: Thermophysical properties and rice straw dissolution. Journal of Molecular Liquids, 247, 441–447. https://doi.org/10.1016/j.molliq.2017.09.026.
Guo, W., Hou, Y., Ren, S., Tian, S., & Wu, W. (2013). Formation of Deep Eutectic Solvents by Phenols and Choline Chloride and Their Physical Properties. Journal of Chemical & Engineering Data, 58(4), 866–872. https://doi.org/10.1021/je300997v.
Hansen, B. B., Spittle, S., Chen, B., Poe, D., Zhang, Y., Klein, J. M., Horton, A., Adhikari, L., Zelovich, T., Doherty, B. W., Gurkan, B., Maginn, E. J., Ragauskas, A., Dadmun, M., Zawodzinski, T. A., Baker, G. A., Tuckerman, M. E., Savinell, R. F., & Sangoro, J. R. (2021). Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chemical Reviews, 121(3), 1232–1285. https://doi.org/10.1021/acs.chemrev.0c00385.
Harifi-Mood, A. R., & Buchner, R. (2017). Density, viscosity, and conductivity of choline chloride + ethylene glycol as a deep eutectic solvent and its binary mixtures with dimethyl sulfoxide. Journal of Molecular Liquids, 225, 689–695. https://doi.org/10.1016/j.molliq.2016.10.115.
Hayyan, A., Mjalli, F. S., AlNashef, I. M., Al-Wahaibi, T., Al-Wahaibi, Y. M., & Hashim, M. A. (2012). Fruit sugar-based deep eutectic solvents and their physical properties. Thermochimica Acta, 541, 70–75. https://doi.org/10.1016/j.tca.2012.04.030.
Hou, Y., Gu, Y., Zhang, S., Yang, F., Ding, H., & Shan, Y. (2008). Novel binary eutectic mixtures based on imidazole. Journal of Molecular Liquids, 143(2–3), 154–159. https://doi.org/10.1016/j.molliq.2008.07.009.
Ibrahim, R. K., Hayyan, M., AlSaadi, M. A., Ibrahim, S., Hayyan, A., & Hashim, M. A. (2019). Physical properties of ethylene glycol-based deep eutectic solvents. Journal of Molecular Liquids, 276, 794–800. https://doi.org/10.1016/j.molliq.2018.12.032.
Leron, R. B., & Li, M.-H. (2012). High-pressure density measurements for choline chloride: Urea deep eutectic solvent and its aqueous mixtures at T= (298.15 to 323.15) K and up to 50 MPa. The Journal of Chemical Thermodynamics, 54, 293–301. https://doi.org/10.1016/j.jct.2012.05.008.
Meng, X., Ballerat-Busserolles, K., Husson, P., & Andanson, J.-M. (2016). Impact of water on the melting temperature of urea + choline chloride deep eutectic solvent. New Journal of Chemistry, 40(5), 4492–4499. https://doi.org/10.1039/C5NJ02677F.
Mjalli, F. S., & Abdel Jabbar, N. M. (2014). Acoustic investigation of choline chloride based ionic liquids analogs. Fluid Phase Equilibria, 381, 71–76. https://doi.org/10.1016/j.fluid.2014.08.017.
Mjalli, F. S., & Naser, J. (2015). Viscosity model for choline chloride-based deep eutectic solvents. Asia-Pacific Journal of Chemical Engineering, 10(2), 273–281. https://doi.org/10.1002/apj.1873.
Mota-Morales, J. D., Gutiérrez, M. C., Sanchez, I. C., Luna-Bárcenas, G., & del Monte, F. (2011). Frontal polymerizations carried out in deep-eutectic mixtures providing both the monomers and the polymerization medium. Chemical Communications, 47(18), 5328. https://doi.org/10.1039/c1cc10391a.
Omar, K. A., & Sadeghi, R. (2020). Novel ninhydrin-based deep eutectic solvents for amino acid detection. Journal of Molecular Liquids, 303, 112644. https://doi.org/10.1016/j.molliq.2020.112644.
Omar, K. A., & Sadeghi, R. (2022). Physicochemical properties of deep eutectic solvents: A review. Journal of Molecular Liquids, 360, 119524. https://doi.org/10.1016/j.molliq.2022.119524.
Ruggeri, S., Poletti, F., Zanardi, C., Pigani, L., Zanfrognini, B., Corsi, E., Dossi, N., Salomäki, M., Kivelä, H., Lukkari, J., & Terzi, F. (2019). Chemical and electrochemical properties of a hydrophobic deep eutectic solvent. Electrochimica Acta, 295, 124–129. https://doi.org/10.1016/j.electacta.2018.10.086.
Sarmad, S., Xie, Y., Mikkola, J.-P., & Ji, X. (2017). Screening of deep eutectic solvents (DESs) as green CO 2 sorbents: from solubility to viscosity. New Journal of Chemistry, 41(1), 290–301. https://doi.org/10.1039/C6NJ03140D.
Siongco, K. R., Leron, R. B., & Li, M.-H. (2013). Densities, refractive indices, and viscosities of N,N-diethylethanol ammonium chloride–glycerol or –ethylene glycol deep eutectic solvents and their aqueous solutions. The Journal of Chemical Thermodynamics, 65, 65–72. https://doi.org/10.1016/j.jct.2013.05.041.
van Osch, D. J. G. P., Dietz, C. H. J. T., van Spronsen, J., Kroon, M. C., Gallucci, F., van Sint Annaland, M., & Tuinier, R. (2019). A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustainable Chemistry & Engineering, 7(3), 2933–2942. https://doi.org/10.1021/acssuschemeng.8b03520.
van Osch, D. J. G. P., Zubeir, L. F., van den Bruinhorst, A., Rocha, M. A. A., & Kroon, M. C. (2015). Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chemistry, 17(9), 4518–4521. https://doi.org/10.1039/C5GC01451D.
Zhang, Y., Poe, D., Heroux, L., Squire, H., Doherty, B. W., Long, Z., Dadmun, M., Gurkan, B., Tuckerman, M. E., & Maginn, E. J. (2020). Liquid Structure and Transport Properties of the Deep Eutectic Solvent Ethaline. The Journal of Physical Chemistry B, 124(25), 5251–5264. https://doi.org/10.1021/acs.jpcb.0c04058.
Zhu, J., Yu, K., Zhu, Y., Zhu, R., Ye, F., Song, N., & Xu, Y. (2017). Physicochemical properties of deep eutectic solvents formed by choline chloride and phenolic compounds at T = (293.15 to 333.15) K: The influence of electronic effect of substitution group. Journal of Molecular Liquids, 232, 182–187. https://doi.org/10.1016/j.molliq.2017.02.071.
Zhu, S., Li, H., Zhu, W., Jiang, W., Wang, C., Wu, P., Zhang, Q., & Li, H. (2016). Vibrational analysis and formation mechanism of typical deep eutectic solvents: An experimental and theoretical study. Journal of Molecular Graphics and Modelling, 68, 158–175. https://doi.org/10.1016/j.jmgm.2016.05.003.
Different parameters such as the chemical nature of HBA and HBD (Abbott et al., 2007)(D’Agostino et al., 2015), the temperature (Abbott, Boothby, et al., 2004); (Abbott, Capper, et al., 2004b)(Du et al., 2016)(Kareem et al., 2010), molar mass (Abbott, Harris, et al., 2011) and the molar ratio (Q. Zhang et al., 2012), in addition to the water content (Wazeer et al., 2018)(D’Agostino et al., 2015)(Florindo et al., 2014)(Shah & Mjalli, 2014) affect the viscosity of DESs. For example, the viscosity of the DESs for the different HBA/glycerol decreases according to the following order:
Benzyltrimethylammonium chloride > Diethylethanolammonium chloride > Choline chloride > Triethylmethylammonium chloride > Choline chloride > Triethylmethylammonium chloride.
And that of choline chloride DES/HBDs decreases according to the order:
Zinc chloride > Malonic acid > Triethylene glycol > Urea > Levulinic acid > P-cresol > Phenol > Ethylene glycol.
The viscosity of DESs decreases with the increase of the molar salt ratio: HBD, for example, the viscosity of ChCl:glycerol mixtures with molar ratios of 1:4, 1:3 and 1:2 decreases when the glycerol content decreases, being 503, 450 and 376 cP (at 293 K) respectively. (Wazeer et al., 2018). This is due to the breakdown of the hydrogen bonds associated with the addiction of organic salts in the DESs (Abbott, Harris, et al., 2011).
Another parameter that influences the viscosity is the temperature, which decreases with the temperature increase, this is due to the breakdown of the network of hydrogen bonds between the HBA and HBD. Water increases the solubilizing power of DESs by decreasing its viscosity (García et al., 2015).
The most interesting DESs for industrial application are those with low viscosities, a specific DESs design can be achieved through the small size of HBD and cations (Abbott, Capper, & Gray, 2006).
The DESs obtained from choline chloride:sorbitol 1:1 have a viscosity of 12,730 mPa S to 303 K, for choline chloride:glucose 1:1 a viscosity of 34,400 mPa S to 323 K. Hydrophobic deep eutectic solvents based on DL-menthol have low viscosities, such as DLmentol:octanic acid 1:3 with a viscosity for 298 K of 7.61 mPa S (Nunes et al., 2019)(Ribeiro et al., 2015).
It should be noted that there are differences in the viscosity data obtained by different authors for the same eutectic solvent, for example, 152 mPa. s vs 527.28 mPa. s for 1:2 choline chloride:urea at 303 K and 202 mPa. s vs 2142 for 1:1 choline chloride:oxalic acid at 313 K (García et al., 2015). These large differences can be attributed not only to the method of preparation, Florindo et al. (Florindo et al., 2014), but also the experimental method and the presence of impurities and water content (García et al., 2015).
5.4. Conductivity
Conductivity is other important factor to study due to the use of electrochemical techniques to recovery of metals with deep eutectic solvents. DESs have a low ionic and electrical conductivity at room temperature due to their high viscosities, there is a strong relationship between conductivity and viscosity. Most DESs tend to have poor ionic conductivities (k < 2 mS cm−1 at room temperature) (El Achkar et al., 2021)(Lapeña et al., 2019)(Q. Zhang et al., 2012). The conductivity depends significantly on the temperature, so it can be predicted assuming a behaviour type Arrhenius (Wazeer et al., 2018). Abbot et al. (Abbott et al., 2003) carried out the first measurements of the conductivity of ChCl-Urea in 2003, revealing a significant increase with the increase in temperature as shown in Fig. 5. This increase in conductivity with temperature is due to the decrease in viscosity, by breaking the network of hydrogen bonds and increasing their ionic mobility (Lapeña et al., 2019)(Q. Zhang et al., 2012).
 Fig. 5
Fig. 5Studies have shown a dependence on conductivity with the molar ratio of HBDs/HBAs (Abbott, Boothby, et al., 2004); (Abbott, Capper, et al., 2004b), the alkyl cation chain length, temperature and water addiction (Dai et al., 2015).
For example, the conductivity of choline chloride and glycerol-based DES increases with the addition of a 33% chloride molar fraction which causes a change in conductivity a 1.047 mS cm−1 (Florindo, Oliveira, et al., 2017), see Table 5. In addition, the length of the alkyl chain cation has a slight influence on the DESs conductivity according to the following order, ethyl ammonium bromide > propyl ammonium bromide > butyl ammonium bromide.
| HBA | HBD |
HBA:HBD (molar ratio) |
Conductivity (mS cm−1) |
Reference |
|---|---|---|---|---|
| ChCl | Glicerol | 5%HBD | 0.1064 | (Abbott et al., 2007) |
| ChCl | Glicerol | 10%HBD | 0.243 | (Abbott et al., 2007) |
| ChCl | Glicerol | 15%HBD | 0.47 | (Abbott et al., 2007) |
| ChCl | Glicerol | 20%HBD | 0.58 | (Abbott et al., 2007) |
| ChCl | Glicerol | 25%HBD | 0.85 | (Abbott et al., 2007) |
| ChCl | Glicerol | 30%HBD | 0.964 | (Abbott et al., 2007) |
| ChCl | Glicerol | 33%HBD | 1.047 | (Abbott et al., 2007) |
| Ethylammonium bromide | Glicerol | 1:2 | 0.217 | (Hayyan et al., 2012) |
| Propylammonium bromide | Glicerol | 1:2 | 0.209 | (Hayyan et al., 2012) |
| Butylammonium bromide | Glicerol | 1:2 | 0.207 | (Hayyan et al., 2012) |
Bibliography.
Abbott, A. P., Harris, R. C., & Ryder, K. S. (2007). Application of Hole Theory to Define Ionic Liquids by their Transport Properties. The Journal of Physical Chemistry B, 111(18), 4910–4913. https://doi.org/10.1021/jp0671998.
Hayyan, A., Mjalli, F. S., AlNashef, I. M., Al-Wahaibi, T., Al-Wahaibi, Y. M., & Hashim, M. A. (2012). Fruit sugar-based deep eutectic solvents and their physical properties. Thermochimica Acta, 541, 70–75. https://doi.org/10.1016/j.tca.2012.04.030.
Omar, K. A., & Sadeghi, R. (2022). Physicochemical properties of deep eutectic solvents: A review. Journal of Molecular Liquids, 360, 119524. https://doi.org/10.1016/j.molliq.2022.119524.
Kareem et al. (Kareem et al., 2010) studied the conductivity of phosphonium-based DESs and Zhang et al. (Q. Zhang et al., 2012) showed that the conductivity of DESs increase with the increase in salt concentration (for example, for ChCl-EG). However, this behaviour is not true for all DESs (e.g., tetrabutylamonium chloride (TBAC)-EG), the change in conductivity depends on both the HBD and the nature of the salt.
5.5. Refractive index
The refractive index is one of the most important physical properties of DESs, providing useful information on their composition, checking the purity of materials and in measuring concentration of solutes in solution. The refractive index is one of the most important physical properties of DESs, providing useful information on their composition. The refractive index (nD) is a dimensionless value representing the relationship between the speed of light in a vacuum (c and the speed of light in a given material (ν) (Mjalli et al., 2023). Following the principles of Snell’s law:(2)
Few studies provide information on DESs refractive index (SU et al., 2015)(Leron et al., 2012)(Sánchez et al., 2019), see Table 6.
| HBA | HBD | HBA:HBD(molar ratio) | Refractive index 298 K | Reference |
|---|---|---|---|---|
| ChCl | urea | 1:2 | 1.504 (303 K) | (Shah & Mjalli, 2014) |
| ChCl | glycerol | 1:2 | 1.87 | (Leron et al., 2012) |
| ChCl | EG | 1:2 | 1.468 | (Leron et al., 2012) |
| ChCl | Phyenylacetic acid | 1:2 | 1.526 | (Abbott et al., 2004) |
| ChCl | Citric acid | 1:2 | 1.502 | (Abbott et al., 2004) |
| DEAC | EG, glycerol | 1:1 | 1.4677 (EG), 1.4856 (glycerol) | (Siongco et al., 2013) |
| Butylammonium bromide | glycerol | 1:2 | 1.92 | (Chen et al., 2017) |
| Propylammonium bromide | glycerol | 1:2 | 1.495 | (Chen et al., 2017) |
| Ethylammonium bromie | glycerol | 1:2 | 1.497 | (Chen et al., 2017) |
Bibliography.
Abbott, A. P., Boothby, D., Capper, G., Davies, D. L., & Rasheed, R. K. (2004). Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. Journal of the American Chemical Society, 126(29), 9142–9147. https://doi.org/10.1021/ja048266j.
Chen, Z., Ludwig, M., Warr, G. G., & Atkin, R. (2017). Effect of cation alkyl chain length on surface forces and physical properties in deep eutectic solvents. Journal of Colloid and Interface Science, 494, 373–379. https://doi.org/10.1016/j.jcis.2017.01.109.
Hansen, B. B., Spittle, S., Chen, B., Poe, D., Zhang, Y., Klein, J. M., Horton, A., Adhikari, L., Zelovich, T., Doherty, B. W., Gurkan, B., Maginn, E. J., Ragauskas, A., Dadmun, M., Zawodzinski, T. A., Baker, G. A., Tuckerman, M. E., Savinell, R. F., & Sangoro, J. R. (2021). Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chemical Reviews, 121(3), 1232–1285. https://doi.org/10.1021/acs.chemrev.0c00385.
Leron, R. B., Soriano, A. N., & Li, M.-H. (2012). Densities and refractive indices of the deep eutectic solvents (choline chloride + ethylene glycol or glycerol) and their aqueous mixtures at the temperature ranging from 298.15 to 333.15 K. Journal of the Taiwan Institute of Chemical Engineers, 43(4), 551–557. https://doi.org/10.1016/j.jtice.2012.01.007.
Omar, K. A., & Sadeghi, R. (2022). Physicochemical properties of deep eutectic solvents: A review. Journal of Molecular Liquids, 360, 119524. https://doi.org/10.1016/j.molliq.2022.119524.
Shah, D., & Mjalli, F. S. (2014). Effect of water on the thermo-physical properties of Reline: An experimental and molecular simulation based approach. Phys. Chem. Chem. Phys., 16(43), 23900–23907. https://doi.org/10.1039/C4CP02600D.
Siongco, K. R., Leron, R. B., & Li, M.-H. (2013). Densities, refractive indices, and viscosities of N,N-diethylethanol ammonium chloride–glycerol or –ethylene glycol deep eutectic solvents and their aqueous solutions. The Journal of Chemical Thermodynamics, 65, 65–72. https://doi.org/10.1016/j.jct.2013.05.041.
Refractive indexes are used to calculate molar refraction using the Lorentz-Lorenz equation.(3)where RD is the molar refraction (cm3mol−1), M is the molar mass (g/mol), ρ density (g cm−3) y nD refractive index.
Molar refraction shows the molecular interaction between HBA and HBD of DESs components. The refractive index depends on the interaction between HBA and HBD, a decrease in the hydrogen bond interaction between the DESs component decrease the refractive index value. Therefore, the refractive index is inversely proportional to temperature, an increase in temperature, as indicated above, decreased hydrogen bond interactions between DESs components leading to decreased DESs viscosity due to decreased refractive index (Leron et al., 2012). The size of molecules influences the refractive index; those with a larger size have a higher refractive index. Hong-Zhen et al. (SU et al., 2015) found that DESs obtained from the same HBA (tetrabutiamonium chloride, TBAC) and different HBD as phenylacetic acid (PAA) in a 1:2 M ratio had the highest refractive index compared to the 1:2 M ratio of TBAC with propionic acid (PA). Both HBDs differ in a methyl group from a phenyl group bound to the carboxylic acid structure. Another parameter that influences the refractive index is the length of the alkyl chain, the refractive index of the DESs increases according to the following series:
butyl ammonium bromide < propyl ammonium bromide < ethyl ammonium bromide < choline chloride, as shown in Table 6.
The refractive index has been used to correlate the polarizability of liquids and assess the accuracy of ab initio calculations, as well as to understand how organic molecules associate with each other in binary alcohol mixtures(Seki et al., 2012). Kucan et al. (Zagajski Kučan & Rogošić, 2019) studied the refractive index of ChCl and glycerol of different molar proportions (1:1.5; 1:2; 1:3) as a function of temperature, found that the eutectic ratio had the highest refractive index in a temperature range of 288–328 K.
5.6. Surface tension
Surface tension is an average of the energy needed to increase a material surface and is related to a material tendency to have a smaller surface (Hansen et al., 2021). DESs surface tension is considered a physical property that demonstrates the impact of molecular structure on the intensity of interaction between the hydrogen bond acceptor and the donor in the DES mixture. Studies of this physical property are more limited compared to other physical properties. Relative surface tension values generally vary between 35 and 75 mN m−1 a 298 K (García et al., 2015)(Ibrahim et al., 2019). Surface tension is highly dependent on the intermolecular interaction between the HBA and the HBD forming the DESs mixture. The surface tension depends of the temperature, the molar ratio, the nature of the HBDAs/HBDs and the length of the alkyl cation chain.
Gajardo-Parra et al. (Gajardo-Parra et al., 2019) measured the surface tension of three DES based on ChCl with levulinic acid, phenol and ethylene glycol. The surface tension of ethaline measured was 45,66 mN m−1 to 298 K and 101,3 kPa it was lower than that of pure ethylene glycol, 48,90 mN m−1. This same trend was observed for the other ChCl:HBA combinations. The decrease in surface tension from pure HBD to DESs formation was attributed to the addition of ChCl. Surface tension measurements show that ChCl acts as a surfactant and decreases cohesive forces (which affects the H-junction) on the surface of DESs ethaline.
The increase in the alkyl chain length decreases surface tension (Omar & Sadeghi, 2020b)(Omar & Sadeghi, 2020a)(Marcus, 2019). An increase in organic salt decreases the network of hydrogen bonds decreasing the surface tension of DESs (Y. Chen, Chen, et al., 2019). Abbot et al. (Abbott, Harris, et al., 2011) concluded that surface tension in ChCl systems: Gly for different salt concentrations presents a linear correlation with temperature, as shown in Fig. 6. These same authors in another paper determined that the surface tension of DESs ChCl:U (1:2) is greater than for ChCl:EG (1:2) due to the strong interactions of hydrogen bonds (Abbott, Barron, et al., 2011). Surface tension is affected by temperature and decreases linearly with it (García et al., 2015)(Lapeña et al., 2019)(Nunes et al., 2019). The effect of surface tension is shown in Table 7.
 Fig. 6
Fig. 6| HBA | HBD |
HBA:HBD (molar ratio) |
Surface tension (Nm m−1) at 298 K | Reference |
|---|---|---|---|---|
| ChCl | Urea | 1:2 | 64.14 | (Lapeña et al., 2020) |
| ChCl | Glycerol | 1:2 | 58 | (AlOmar et al., 2016) |
| ChCl | EG | 1:2 | 52 | (Klein et al., 2020) |
| ChCl | Lactic Acid | 1:2 | 47.4 | (Y. Chen et al., 2019) |
| ChCl | Lactic Acid | 1:4 | 44.4 | (Y. Chen et al., 2019) |
| ChCl | Phenylacetic acid | 1:2 | 57.96 | (Abbott et al., 2004) |
| ChCl | 1,4-Butanediol | 1:3 | 47.17 | (Wazeer et al., 2018) |
| MTPB, BTPC, TBAB | EG | 51 (TBAB), 65 (BTPC), 67 (MTPB) | (Ibrahim et al., 2019) | |
| MTPB, BTPB, ATPB, TBAB | Glycerol | 37 (TBAB) − 54 | (AlOmar et al., 2016) | |
| ChCl | MEA, DEA, MDEA | 32 (MDEA) − 48 (MEA | (Adeyemi et al., 2018) | |
| Butylammonium bromide | Glycerol | 1:2 | 44.9 | (Z. Chen et al., 2017) |
| Propylammonium bromide | Glycerol | 1:2 | 51.7 | (Z. Chen et al., 2017) |
| Ethylammonium bromide | Glycerol | 1:2 | 57.6 | (Z. Chen et al., 2017) |
Bibliography.
Abbott, A. P., Boothby, D., Capper, G., Davies, D. L., & Rasheed, R. K. (2004). Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. Journal of the American Chemical Society, 126(29), 9142–9147. https://doi.org/10.1021/ja048266j.
Adeyemi, I., Abu-Zahra, M. R. M., & AlNashef, I. M. (2018). Physicochemical properties of alkanolamine-choline chloride deep eutectic solvents: Measurements, group contribution and artificial intelligence prediction techniques. Journal of Molecular Liquids, 256, 581–590. https://doi.org/10.1016/j.molliq.2018.02.085.
AlOmar, M. K., Hayyan, M., Alsaadi, M. A., Akib, S., Hayyan, A., & Hashim, M. A. (2016). Glycerol-based deep eutectic solvents: Physical properties. Journal of Molecular Liquids, 215, 98–103. https://doi.org/10.1016/j.molliq.2015.11.032.
Chen, Y., Chen, W., Fu, L., Yang, Y., Wang, Y., Hu, X., Wang, F., & Mu, T. (2019). Surface Tension of 50 Deep Eutectic Solvents: Effect of Hydrogen-Bonding Donors, Hydrogen-Bonding Acceptors, Other Solvents, and Temperature. Industrial & Engineering Chemistry Research, 58(28), 12741–12750. https://doi.org/10.1021/acs.iecr.9b00867.
Chen, Z., Ludwig, M., Warr, G. G., & Atkin, R. (2017). Effect of cation alkyl chain length on surface forces and physical properties in deep eutectic solvents. Journal of Colloid and Interface Science, 494, 373–379. https://doi.org/10.1016/j.jcis.2017.01.109.
Hansen, B. B., Spittle, S., Chen, B., Poe, D., Zhang, Y., Klein, J. M., Horton, A., Adhikari, L., Zelovich, T., Doherty, B. W., Gurkan, B., Maginn, E. J., Ragauskas, A., Dadmun, M., Zawodzinski, T. A., Baker, G. A., Tuckerman, M. E., Savinell, R. F., & Sangoro, J. R. (2021). Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chemical Reviews, 121(3), 1232–1285. https://doi.org/10.1021/acs.chemrev.0c00385.
Ibrahim, R. K., Hayyan, M., AlSaadi, M. A., Ibrahim, S., Hayyan, A., & Hashim, M. A. (2019). Physical properties of ethylene glycol-based deep eutectic solvents. Journal of Molecular Liquids, 276, 794–800. https://doi.org/10.1016/j.molliq.2018.12.032.
Klein, J. M., Squire, H., Dean, W., & Gurkan, B. E. (2020). From Salt in Solution to Solely Ions: Solvation of Methyl Viologen in Deep Eutectic Solvents and Ionic Liquids. The Journal of Physical Chemistry B, 124(29), 6348–6357. https://doi.org/10.1021/acs.jpcb.0c03296.
Lapeña, D., Bergua, F., Lomba, L., Giner, B., & Lafuente, C. (2020). A comprehensive study of the thermophysical properties of reline and hydrated reline. Journal of Molecular Liquids, 303, 112679. https://doi.org/10.1016/j.molliq.2020.112679.
Omar, K. A., & Sadeghi, R. (2022). Physicochemical properties of deep eutectic solvents: A review. Journal of Molecular Liquids, 360, 119524. https://doi.org/10.1016/j.molliq.2022.119524.
Wazeer, I., Hayyan, M., & Hadj-Kali, M. K. (2018). Deep eutectic solvents: designer fluids for chemical processes. Journal of Chemical Technology & Biotechnology, 93(4), 945–958. https://doi.org/10.1002/jctb.5491.
DESs with high viscosity also have high surface tension. Sugar-based DESs such as choline chloride:D-glucose and choline chloride: D-fructose have higher surface tension values, reflecting their extensive network of hydrogen bonds (Hayyan et al., 2013)(Hayyan et al., 2012).
5.7. Polarity
Polarity or polarizability reflects the overall solvation capacity of solvents it can affect the solubility of metals in hydrometallurgy methods. DESs can be used as substitutes for conventional organic solvents for organic reactions, extraction of natural products and metallurgy, therefore the determination of DESs polarity is an essential parameter. However, despite their importance, the number of publications based on the polarity of DESs are limited. Different parameters can be used to express polarity, the most frequent are permittivity (dielectric constant) and spectral parameters (Shaibuna et al., 2022).
Most DESs are polar and their polarity can be calculated by solvatochromic parameters, where hypochromic (blue) or bathochromic (red) displacement of UV–Vis bands is considered for solvatochromic negative dyes, such as Reichardt dye, or positive solvatochromic dyes, such as Nile red, depending on the polarity of the solvent (El Achkar et al., 2021) (Dimroth et al., 1963)(Reichardt, 1994). The most used scales are the polarity of Dimroth Reichardt 30 on a scale of ET (30) (Reichardt, 1994) which is defined as the electronic transition energy of a probe dye in kcal/mol at normal temperature and pressure according to the expression:(4)
The dye betaine 30 is the most commonly used to measure the polarity of DESs. However, this can be replaced by betaine 33, the value of ET(33) is calculated as the value of ET(30). The dye betaine 30 is unstable to DESs such as ethaline, glyceline, maline and reline. (Pandey et al., 2014), for these DESs betaine should be used. Table 8 shows the polarity parameters for different DESs. The polarity of ethaline and glyceline is not affected by temperature change except reline due to probe instability (Valvi et al., 2017)(Kadyan et al., 2016). The interaction between HBAs and HBDs leads to the formation of the DES system and the increase in polarity, as shown in Table 8. Glycerol polarity increased after the formation of the DESS mixture.
| HBA | HBD | HBA:HBD(molar ratio) | ET(30) Kcal mol−1 | ET(33) Kcal mol−1 | Reference |
|---|---|---|---|---|---|
| ChCl | Glycerol | 1:1 | 58.49 | – | (Zhang et al., 2012) |
| ChCl | Glycerol | 1:1.5 | 58.21 | – | (Zhang et al., 2012) |
| ChCl | Glycerol | 1:2 | 58.28 | – | (Zhang et al., 2012) |
| ChCl | Glycerol | 1:3 | 57.96 | – | (Zhang et al., 2012) |
| ChCl | Glycerol | 1:2 | 58 | 66.4 | (Pandey et al., 2014) |
| ChCl | Urea | 1:2 | 57 | 65.4 | (Pandey et al., 2014) |
| ChCl | Ethylene glycol | 1:2 | 57.3 | 65.7 | (Pandey et al., 2014) |
| – | Glycerol | – | 57 | 66.3 | (Pandey et al., 2014) |
Bibliography.
Omar, K. A., & Sadeghi, R. (2022). Physicochemical properties of deep eutectic solvents: A review. Journal of Molecular Liquids, 360, 119524. https://doi.org/10.1016/j.molliq.2022.119524.
Pandey, A., Rai, R., Pal, M., & Pandey, S. (2014). How polar are choline chloride-based deep eutectic solvents? Phys. Chem. Chem. Phys., 16(4), 1559–1568. https://doi.org/10.1039/C3CP53456A.
Zhang, Q., De Oliveira Vigier, K., Royer, S., & Jérôme, F. (2012). Deep eutectic solvents: syntheses, properties and applications. Chemical Society Reviews, 41(21), 7108. https://doi.org/10.1039/c2cs35178a.
The polarity of DES from different proportions of ChCl:Gly was determined by Abbott et al. (Abbott, Harris, et al., 2011), results showed a linear increase of polarity with ChCl concentration. Pandey et al. (Pandey et al., 2014) studied common DESs such as reline, ethanol, glyceline, and maline (a combination of ChCl (HBA) and urea, 1,2-ethanol, glycerol, and malonic acid (HBD) in ratio 1:2, respectively) using several optical spectroscopic probes, They concluded that high polarity was significantly influenced by the nature of HBD, the ChCl:Gly(1:2) having the highest polarity value.
DESs are highly soluble in water, methanol, ethanol, etc., and insoluble in aprotic solvents (e.g., toluene, hexane, ethyl acetate and acetonitrile). This indicates that solvents capable of forming strong hydrogen bonds with the chloride ion tend to be miscible (Naser et al., 2013). In 2003, Abbott et al. (Abbott et al., 2003) studied the solvation properties of ChCl-urea DESs at 50 C. Compounds such as amino acids, aromatic acids, inorganic salts and poorly soluble salts in water (e.g., AgCl) are very soluble in DESs. DESs can dissolve several metal oxides due to the high anion concentration of these liquids. Compared to most molecular solvents, DESs has unusual solvent properties. They are similar to IL and solvation properties are strongly influenced by the strength of hydrogen bonds. HBDs have a significant effect on the determination of the physical properties of DESs (Shaibuna et al., 2022).
5.8. Acidity and basicity
The acidity and basicity of DESs are considered one of the most important properties that will give them applications in various industrial fields, in addition this parameter can influence the selection of pipe materials, Reaction tanks etc. in an industrial process. This is another critical parameter in the selection of DES for application in hydrometallurgy processes, mainly in leaching methods (W. Chen, Jiang, et al., 2019).
Hydrogen ions and the pH have an important role in hydrometallurgy processes. The term pH refers to the concentration of hydrogen ions in aqueous solution. This is calculated to the negative log of the hydrogen ion concentration. The term “aqueous solution” means pure water or water with a small quantity of substances dissolved in it. The values of pH in different media are related through the Gibbs free energy of the proton exchange between solvents. However, even at the theoretical level, a valid comparability of pH values in different media has been impossible. The pH is indicative of the acidic or basic conditions of water. However, pH is not equivalent to acidity or alkalinity. The alkalinity and acidity are defined as the capacity of an aqueous solution to resist a change in the pH. Alkalinity and acidity are measured by determining the amount of a solution of acid or base, as appropriate, of known concentration that is required to completely neutralize the acidity or alkalinity of the aqueous solution (Jančíková et al., 2022).
DESs can be defined as a system formed from a mixture of Lewis or BrØnsted acids and bases. Therefore, the acidity and basicity of the HBA and HBD govern the pH of the DESs system. This acidity and basicity depend on the nature of pure constituents (HBA and HBD) and their molar ratio.
The BrØnsted acidity/basicity of nonaqueous solvent is evaluated by Hammett acidity function or by the pH measurement (Shaibuna et al., 2022). For a basic solution, the Hammett function measures the tendency of a solution to capture protons (Q. Zhang et al., 2012). The Hammett function is defined:(5)where pK(HI) is the thermodynamic ionization constant of the indicator in water, [I-] and [HI] represent the molar concentrations of anionic and neutral forms of the indicator, respectively. Higher values of H_indicates strong basicity medium. The H_value for the DES ChCl: Urea (1:2) was 10.86 using as indicator 4-nitrobenziylcyanide, this parameter indicate that this DES has weakly basic nature (Li et al., 2008). It is also important to indicate that the content in water affect the H_value, when the system contain 1–3% of water, the H_value decrease from 10.77 to 10.65 due to a partial solvation of basic sites (Q. Zhang et al., 2012).
The Hammett acidity function is restricted to BrØnsted acidic DES and it is not used to DESs containing both BrØnsted and Lewis acid sites. The acidity measurements of this types of mixtures have done by FT-IR spectroscopy using as basic molecule pyridine. The methods are based in the vibration bands in the range of 1400–1700 cm−1, the vibration band at 1450 cm−1 indicates the Py-Lewis complex (band formed by the interaction of pyridine with Lewis acid sites) and the band at 540 cm−1 indicate the interaction between pyridine with BrØnsted acid sites (Zhou et al., 2022) (Shaibuna et al., 2022).
The chemical nature of the HBDs has a strong effect on the acid or basic strength of the DESs. Different studies have been carried out evaluating the effect of HBDs on the pH of DESs, showing that an increase in the molar ratio of fructose caused an increase in the pH of DESs (Guo et al., 2013). Abbot et al. (Abbott et al., 2018) verify that the addition of chloride ions to the ChCl: Glycerol mixture reduces the acidity of the DESs and a change in the pH of the mixture to basic is observed.
DESs have a highly acidic pH, such as ChCl:Ac. Citrus and ChCl: Ac. Malonic and basic DESs such as ChCl:Urea, ClCh:Triethanolamine and potassium carbonate:Glycerol as shown in Table 9 (Mjalli et al., 2014)(Cui et al., 2017).
| HBA | HBD |
HBA:HBD (molar ratio) |
pH at 298 K | Reference |
|---|---|---|---|---|
| ChCl | urea | 1:2 | 10.7 (303 K) | (Shah & Mjalli, 2014) |
| ChCl | Glicerol | 1:2 | 4.47 | (Skulcova et al., 2018) |
| ChCl | EG | 1:2 | 4.38 | (Skulcova et al., 2018) |
| ChCl | Malonic acid | 1:1 | 1.28 | (Skulcova et al., 2018) |
| ChCl | Malonic acid | 1:1 | 1.61 | (Skulcova et al., 2018) |
| ChCl | oxalic, lactic, citric, glycolic, malenic acid | 1:1, 1:5 (lactic), 1:3 (glycolic) | 1.22 (oxalic) − 1.73 (citric), −0.3(malenic) | (Skulcova et al., 2018) |
| ChCl | MEA, DEA, MDEA | 1:6 | 11.04 (MDEA) − 12.8 (MEA) | (Skulcova et al., 2018) |
| Betaine | Lactic acid | 1:2 | 2.45 | (Skulcova et al., 2018) |
| Lactic acid | alanine, glyceline | 9:1 (alanine), 2:1 (glyceline) | 2.15 (alanine), 2.74 (glyceline) | (Skulcova et al., 2018) |
| ChCl | Fructose | 1:1 | 6.1 | (Hayyan et al., 2012) |
| ChCl | Fructose | 1:1.5 | 6.8 | (Hayyan et al., 2012) |
| ChCl | Fructose | 1:2 | 6.9 | (Hayyan et al., 2012) |
| ChCl | Fructose | 1:1.25 | 7.1 | (Hayyan et al., 2012) |
| Chcl | Triethanolamine | 1:2 | 10.66 | (Bahadori et al., 2013) |
| Potassium carbonate | Glycerol | 1:5 | 13.24 | (Naser et al., 2013) |
| Benzyltriphenylphosphonium Chloride | Glycerol | 1:5 | 7.06 | (Kareem et al., 2010) |
| Benzyltriphenylphosphonium Chloride | Ethylene Glycol | 1:3 | 5.74 (328 K) | (Kareem et al., 2010) |
Bibliography.
Bahadori, L., Chakrabarti, M. H., Mjalli, F. S., AlNashef, I. M., Manan, N. S. A., & Hashim, M. A. (2013). Physicochemical properties of ammonium-based deep eutectic solvents and their electrochemical evaluation using organometallicreference redox systems. Electrochimica Acta, 113, 205–211. https://doi.org/10.1016/j.electacta.2013.09.102.
Hansen, B. B., Spittle, S., Chen, B., Poe, D., Zhang, Y., Klein, J. M., Horton, A., Adhikari, L., Zelovich, T., Doherty, B. W., Gurkan, B., Maginn, E. J., Ragauskas, A., Dadmun, M., Zawodzinski, T. A., Baker, G. A., Tuckerman, M. E., Savinell, R. F., & Sangoro, J. R. (2021). Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chemical Reviews, 121(3), 1232–1285. https://doi.org/10.1021/acs.chemrev.0c00385.
Hayyan, A., Mjalli, F. S., AlNashef, I. M., Al-Wahaibi, T., Al-Wahaibi, Y. M., & Hashim, M. A. (2012). Fruit sugar-based deep eutectic solvents and their physical properties. Thermochimica Acta, 541, 70–75. https://doi.org/10.1016/j.tca.2012.04.030.
Kareem, M. A., Mjalli, F. S., Hashim, M. A., & AlNashef, I. M. (2010). Phosphonium-Based Ionic Liquids Analogues and Their Physical Properties. Journal of Chemical & Engineering Data, 55(11), 4632–4637. https://doi.org/10.1021/je100104v.
Naser, J., Mjalli, F., Jibril, B., Al-Hatmi, S., & Gano, Z. (2013). Potassium Carbonate as a Salt for Deep Eutectic Solvents. International Journal of Chemical Engineering and Applications, 114–118. https://doi.org/10.7763/IJCEA.2013.V4.275.
Omar, K. A., & Sadeghi, R. (2022). Physicochemical properties of deep eutectic solvents: A review. Journal of Molecular Liquids, 360, 119524. https://doi.org/10.1016/j.molliq.2022.119524.
Shah, D., & Mjalli, F. S. (2014). Effect of water on the thermo-physical properties of Reline: An experimental and molecular simulation based approach. Phys. Chem. Chem. Phys., 16(43), 23900–23907. https://doi.org/10.1039/C4CP02600D.
Skulcova, A., Russ, A., Jablonsky, M., & Sima, J. (2018). The pH behavior of seventeen deep eutectic solvents. BioResources, 13(3), 5042–5051. https://doi.org/10.15376/biores.13.3.5042–5051.
Other factors affecting the pH of DESs are temperature, an increase in temperature decreases linearly the pH of DES(Skulcova et al., 2018), this decrease depends on donors of hydrogen bonds, such as alcohol-based DESs, where the pH slowly decreases with increasing temperature. However, the DES obtained from carboxylic acids the pH decreases abruptly with increasing temperature.
Hayyan et al. (Hayyan et al., 2013) studied the pH for different molar compositions of ChCl with D glucose DESs, the result indicated a pH value of 7 between 298 and 318 K, these authors observed a slight linear dependence of the pH with the temperature increase at 358 K. A similar study conducted by Skulcova et al. (Skulcova et al., 2018) analysed this behaviour and observed a similar behaviour, a decrease in pH to increase in temperature.
5.9. Hydrophilicity and hydrophobicity of DESs
Most DESs are hydrophilic, although there are DESs that are hydrophobic, immiscible in water. Hydrophobic DESs are widely used for the extraction of solutes by creating biphasic systems with hydrophilic compounds such as water. The hydrophobicity of the DESs depend on the chemical nature of the eutectic mixture, namely the HBAs and HBDs. For example, long hydrophobic alkyl chains of HBAs lead to hydrophobic DES due to steric impediment that prevents the salt of the core from being charged with water.
Van Osch et al. (van Osch et al., 2015) studied the first hydrophobic DESs obtained from highly hydrophobic compounds such as decanoic acid and quaternary ammonium salts or fatty acids as acceptors of hydrogen bonds, see Table 10. Studies have been carried out for the extraction of non-polar compounds using different hydrophobic DESs (Florindo, Branco, et al., 2017) (Hizaddin et al., 2016)(Sharma et al., 2013). Hydrophobic DESs are characterized by low densities and moderate viscosity at room temperature.
| HBA | HBD |
HBA:HBD (molar ratio) |
Ρ (g cm 3) | Ƞ (cps) | Reference |
|---|---|---|---|---|---|
| Tetrabutylammonium chloride | Decanoic acid | 1:2 | 0.916 | 265.26 | (van Osch et al., 2015) |
| Tetraheptylammonium chloride | Decanoic acid | 1:2 | 0.890 | 172.87 | (van Osch et al., 2015) |
| Tetraoctylammonium chloride | Decanoic acid | 1:2 | 0.888 | 472.58 | (van Osch et al., 2015) |
| Tetraethylammonium iodide | 1-Nonanol | 1:8 | 0.857 | 14.76 | (Omar & Sadeghi, 2022c) |
| Tetrapropylammonium bromide | 1-Nonanol | 1:8 | 0.894 | 136.82 | (Omar & Sadeghi, 2022a) |
| Tetrabutylammonium bromide | Glycerol | 1:4 | 0.895 | 43.65 | (Omar & Sadeghi, 2021) |
Bibliography.
Omar, K. A., & Sadeghi, R. (2021). Novel Nonanol-Based deep eutectic solvents: Thermophysical properties and their applications in Liquid-Liquid extraction and amino acid detection. Journal of Molecular Liquids, 336, 116359. https://doi.org/10.1016/j.molliq.2021.116359.
Omar, K. A., & Sadeghi, R. (2022a). Hydrophobic deep eutectic solvents: thermo-physical characteristics and their application in liquid–liquid extraction. Journal of the Iranian Chemical Society, 19(8), 3529–3537. https://doi.org/10.1007/s13738-022–02547-2.
Omar, K. A., & Sadeghi, R. (2022b). Physicochemical properties of deep eutectic solvents: A review. Journal of Molecular Liquids, 360, 119524. https://doi.org/10.1016/j.molliq.2022.119524.
Omar, K. A., & Sadeghi, R. (2022c). Hydrophobic deep eutectic solvents: thermo-physical characteristics and their application in liquid–liquid extraction. Journal of the Iranian Chemical Society, 19(8), 3529–3537. https://doi.org/10.1007/s13738-022–02547-2.
van Osch, D. J. G. P., Zubeir, L. F., van den Bruinhorst, A., Rocha, M. A. A., & Kroon, M. C. (2015). Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chemistry, 17(9), 4518–4521. https://doi.org/10.1039/C5GC01451D.
The size of the anion influences the physical properties of hydrophobic DESs a larger size of the anion leads to a greater viscosity of DES, the same effect is observed with longer HBA alchemical chains. Increased temperature reduces density and viscosity of hydrophobic DESs (Rocha et al., 2013).
According to the definition of hydrophobic DES is all DES insoluble in water and can be formed by both hydrophilic and hydrophobic compounds, such as a DES obtained from a water-soluble quaternary ammonium salt such as HBA and an apolar compound such as fatty acids or alcohols such as HBD. However, studies carried out by Shishov et al. (Shishov et al., 2020) considered that this type of DESS would fall into the category of quasi-hydrophobes or pseudo-hydrophobes, since the soluble parts of DESS, such as quaternary ammonium or short-chain carboxylic acids, Upon contact with water, these water-soluble components are dissolved and therefore the mixture of DESs is disintegrated.
It should be noted that the synthesis of hydrophobic DES can be limited by the lack of availability of its constituents in an easy and inexpensive way.
5.10. Effect of water on DESs physicochemical properties
DES are obtained from the formation of hydrogen bonds with their constituents, making most DES hygroscopic mixtures, so the presence of water makes their uptake inevitable. (Florindo et al., 2014)(Du et al., 2016) and makes difficult to dry out completely.
Water presents duality properties as acceptor and donor of hydrogen bonds. Being able to interact with the HBD or HBA of the DES and break the hydrogen bonding interactions between the organic salt and the hydrogen bond donor by forming multiple hydrogen bonds (Shah & Mjalli, 2014).
Therefore, knowing the effect of water on DES before and after hydrometallurgical processes is fundamental to know their reuse capacity due to the effect that water has on their properties. The traces of water can be considered as impurities in the DES, there is an infinity of works in which water is added intentionally in order to adjust the properties of the DES. However, it must be taken into account that the presence of water not only affects the physicochemical properties, but can also endanger the integrity of the DES. (El Achkar et al., 2019).
The adsorption capacity of water from the air during 8 h of DES obtained from Choline Chloride as HBA and urea, methyl urea, ethylene glycol, glycerol, glucose, xylitol and oxalic acid as HBD was studied by Chen et al. (Y. Chen, Yu, et al., 2019). Research has shown that glutamic acid-based DESs have a higher water adsorption capacity than other DESs. They described the effect of water adsorption according to the hydroxyl, methyl, and chain length groups. The results indicated that the presence of methyl group has a lower absorption capacity for water molecules and that HBD with a high number of hydroxyl groups have a higher water absorption capacity. Furthermore, an increase in the chain length of carboxylic acid-DES increases water absorption. HBAs and HBDs for DESs obtained from choline chloride as HBA and from diethylene glycol triethylene glycol and poly(ethylene glycol) 200 show strong hydrogen interactions, which gradually decrease as water is added, preserving to a certain extent the super molecular structure (Gabriele et al., 2019).
Hydrophobic DESs, such as that obtained from tetrabutylammonium chloride and decanoic acid, contain minor amounts of water, however, these amounts significantly influence the physical properties of DESs by switching from binary to tertiary DES systems. (Kivelä et al., 2022).
The effect of water on the physicochemical properties of DESs, melting point, density, viscosity, etc., is described.
An increase in the water content of DESs mixtures lowers the melting point. (Omar & Sadeghi, 2022). Choline Chloride: Urea absorbs up to 20% by weight of water, Meng et al. (Meng et al., 2016) studied the effect on the melting point of adding up to 10% by weight, the results showed a linear decrease in the melting point, falling from 303 K for the dry DESs to 288 K when the DESs contains 5% by weight. of water. The research of Smith et al. (P. J. Smith et al., 2019) are in agreement with previous results, these authors followed the variation of the melting point of DESs over a full range of water content, reaching a minimum melting point of 225 K for a mole fraction of water of 0.67, from which a linear increase in the melting point is observed. Due to the behaviour of DESs the authors propose the formation of a ternary DES ChCl:Urea:water 1:2:6.
Therefore, the observed differences in melting points may be due to the amounts of water absorbed by the DESs.
An increase in the water content in the DESs mixture decreases the density and viscosity, due to the decrease in hydrogen bonds between the HBA and the HBD of the DESs, as well as an increase in ionic mobility. Van Osch et al. (Gutiérrez et al., 2009) studied the effect of water on the viscosity of various hydrophobic DESs, the water content varied between 200 and 1000 ppm and observed a slight decrease in viscosity with increasing water content. The inverse relationship between viscosity and ionic conductivity leads to an increase in the conductivity of DESs. Viscosity and conductivity are 13 times lower and 10 times higher for reline (ChCl:Urea 1:2) hydrated (Du et al., 2016).
Agieienko et al. (Agieienko & Buchner, 2019) observed a slight decrease in density for the DES ChCl:urea of 0.14% for a 0.008 water mass fraction and 22% for a 0.005 fraction. Shah et al. (Shah & Mjalli, 2014) studied the effect of water on the properties of the reline, a change in the melting point, density and viscosity of the DES was observed. A 10% by weight of water decreases the viscosity by 80% and increases 3 times the conductivity compared to dry DESs.
The linear decrease in viscosity and density in the presence of water is also observed for natural DESs. The conductivity of five natural ternary DESs from choline chloride, organic acids, sugars and water increases to peak values 10 to 100 times higher than pure DES upon addition of 60–80 wt% in water, from these values the conductivity decreases (Dai et al., 2015). For DESs obtained from choline chloride and different glycols such as HBD the viscosity value is reduced by half with the addition of 7–10% by weight of water, and the conductivity increases by 6–15 times when incorporating 60% by weight of water, and then decreases. Ionic dissociation of deep eutectic solvents causes the initial increase in conductivity and the effect of dilution of electrolytes to higher water contents causes the conductivity to decrease. Polarity studies carried out by Gabriele et al. (Gabriele et al., 2019) for these DES showed a linear increase in polarity with increasing water content.
The study of the surface tension of DL-methanol:octanoic acid showed a decrease in surface tension with increasing water content up to a value of 4000 ppm of water, a value above which the surface tension increaseS (Nunes et al., 2019). The same behaviour was observed for choline chloride:malonic acid DESs (Sanchez-Fernandez et al., 2017).
The addition of water increases the dipolarity/polarizability and decreases the basicity of hydrogen bonds for choline chloride:urea, choline chloride:glycerol, and choline chloride:ethylene glycol DES. The fluorescent probes show more intense hydrogen bonding interactions between added water and choline chloride:glycerol DES and choline chloride:ethylene glycol DES compared to urea DES. These differences may be due to structural differences between the HBDs, as well as the interstitial arrangement of water molecules within the choline chloride:urea (Pandey & Pandey, 2014).
Water can be used as an environmentally friendly co-solvent to improve the physical properties of DES for use in various applications (Shaibuna et al., 2022).
Therefore, a fundamental parameter that needs to be controlled is the water content of the DES, given that the proportion of water can significantly modify its properties and this have an influence in the metal recovery methods (Omar & Sadeghi, 2023). So, when the DESs are used the content of water have to be mentioned to the aim to understand different in the DES properties.
5.11. Solubility
DES and their mixture are getting increasing attentions as green solvents alternatives to conventional aqueous and organic solvent because their unique properties, such as high solubilization ability. Due to the flexibility in their numerous combinations of constituents (HBA-HBD) DESs can exhibit high selectivity towards metals of interest, which makes them suitable for use in metal extraction processes.
The ability to accept or donate electrons or protons to form hydrogen bonds gives them these excellent solvation properties (Abbott, Frisch, et al., 2011). They are a suitable medium for the solubilisation of a wide variety of substances, including metal salts. Currently, DESs are among the non-aqueous solvents that have advanced various metal leaching processes by addressing major challenges (Tran et al., 2019) (Dupont & Binnemans, 2015).
In 2003 Abbot et al. demonstrated the ability of DESs for the solution of metal oxides, the solubility of CuO in ChCl:urea at 323 K was 0.12 mol/L (E. L. Smith et al., 2014). he nature of the DESs influences the solubility of the different compounds, Fe3O4 is more soluble in ChCl:oxalic acid (1:1) while it is 20 times less soluble in the DES ChCl:phenylpropionic acid (1:2). On the contrary, CuO is more soluble in DES ChCl:phenylpropionic acid (1:2) than in ChCl:Oxalic acid (1:1). These differences in the solubility of the different metal oxides in DESs allow for the selective recovery of metals (Q. Zhang et al., 2012).
The solubilisation ability of ChCl:urea when dissolving ZnO was evaluated according to the complexation ability of both components, mass spectrometryshowed that a cluster of m/Z signals 174, 176 and 178 with an isotopic pattern consistent with the formation of the [ZnClOurea] anion, indicating the coordinating role of urea in the dissolution process. This phenomenon was also observed for CuO and CuCl2 (Q. Zhang et al., 2012) Table 11
| Metal oxide | Malonic acid | Urea | Ethylene glycol |
|---|---|---|---|
| TiO2 | 4 | 0.5 | 0.8 |
| V2O3 | 365 | 148 | 142 |
| V2O5 | 5809 | 4593 | 131 |
| Cr2O3 | 4 | 3 | 2 |
| CrO3 | 6415 | 10,840 | 7 |
| MnO | 6816 | 0 | 12 |
| Mn2O3 | 5380 | 0 | 7.5 |
| MnO2 | 114 | 0.6 | 0.6 |
| FeO | 5010 | 0.3 | 2 |
| Fe2O3 | 376 | 0 | 0.7 |
| Fe3O4 | 2314 | 6.7 | 15 |
| CoO | 3626 | 13.6 | 16 |
| Co3O4 | 5992 | 30 | 18.5 |
| NiO | 151 | 5 | 9 |
| Cu2O | 18,377 | 219 | 394 |
| CuO | 14,008 | 4.8 | 4.6 |
| ZnO | 16,217 | 1894 | 369 |
Despite the advantages and characteristics of DES, the application of these solvents in metal leaching processes on a pilot or industrial scale is still very limited. (Han et al., 2023). This literature review covers the use of DES as leaching and extraction agents in metallurgical processes.
6. Use of DESs in hydrometallurgy methods
The increasing need of metals due to the technological development and the energy transition implies the search of improved metals recovery methods from low-grade ores and industrial wastes and by-products. Focused on more eco-friendly methods. DESs have emerged as a greener alternative to traditional solvents. A review on the use of DESs as leaching and liquid–liquid extraction agent for the recovery of metals from waste and industrial by products are showed below.
6.1. Leaching of metals with deep eutectic solvents
Metal leaching is a process that consists of treating a powdered material with acid or basic solutions to dissolve the valuable metals, whereas some impurities remain insoluble. Different processes can be used to recovered the metals in solution (solvent extraction (Martı́n & Alguacil, 1998)(F. J. Alguacil et al., 2019), extraction with liquid membranes (F. J. Alguacil & Martín, 2003)(F. J. Alguacil et al., 2003)(García-Díaz et al., 2017), adsorption (Martín et al., 2005)(F. Alguacil et al., 2018)(García-Díaz et al., 2018), precipitation, cementation (López et al., 2003), etc.). In 2003, Abbot et al. (Abbott et al., 2003) published a first communication on new types of solvents, compounds of choline chloride (ChCl) and urea, liquid at room temperature and with interesting properties as solvents. Subsequently, Abbot et al. (Abbott, Boothby, et al., 2004) published the synthesis of deep eutectic solvents made of ChCl as a hydrogen bond acceptor (HBA) and of various carboxylic acids as hydrogen bond donors (HBD) (1 ChCl:1 Oxalic acid, 1 ChCl:1 Malonic acid and 1 ChCl:2 Phenilpropionic acid), that show good properties for dissolving metal oxides (ZnO, CuO and Fe3O4), with potential application for metal extraction. In another work, the same group Abbot et al. (Abbott, Capper, Davies, et al., 2006) confirmed that the solubility of each metal oxide (TiO2, V2O5, Cr2O3, MnO, Fe2O3, ZnO, Cu2O, etc.) depends on the DES used, which could be used to selectively leach the metals. In Table 12 is shown that higher solubility is observed with the more ionic oxides such as ZnO in an analogous manner to that expected for aqueous acidic solutions. More covalent oxides such as TiO2 exhibit negligible solubility.
| DESs | Solubility (mol/L) | ||
|---|---|---|---|
| CuO | Fe3O4 | ZnO | |
| ChCl:Malonic acid | 0.246 | 0.071 | 0.554 |
| ChCl:oxalic acid | 0.071 | 0.341 | 0.491 |
| ChCl:phenylpropionic acid | 0.473 | 0.014 | >0.491 |
The electric arc furnace process of steel production generates large amounts of dust loaded with toxic metals (Zn, Pb, Cd, etc.) present as oxides. Abbot et al. (Abbott et al., 2009) demonstrated the possibility to leach these metals from the dust with DESs of 1 ChCl: 0.5 Urea + 2 Ethylene glycol. The extraction of Zn (60–70%) was efficient and its precipitation was as zinc chloride it was feasible by adding ammonia to the DESs. The extraction of Pb was lower.
Zürner et al. (Zürner & Frisch, 2019) recovered valuable metals (Fe, Zn, Pb, Cu, In and Sn) from zinc flue dust using an choline chloride and oxalic acid based deep eutectics solvent. The leaching rates obtained were higher than 80% for all metals studied after 48 h of reaction at 323 K.
Jenkin et al. (Jenkin et al., 2016) used DESs for the leaching of metals contained in minerals (sulfides and tellurides) as Au, Ag, Bi and Te. The authors showed that selective dissolution could be obtained by electrolysis of the DES. Dissolution rates were similar (t = 5–15 min, T = 318–323 K) to the cyanide processes currently applied.
Riaño et al. (Riaño et al., 2017) studied choline chloride and lactic acid based DESs (1 ChCl:2 Lactic acid) for the leaching of rare earths (Nd, Dy, Pr and Gd) and other metals (Fe, B and Co) from NdFeB magnets. High leaching rates (80%) were obtained after 24 h of leaching at 343 K. The high solubilities of the oxides in the DES can be explained by the reaction of the protons in the lactic acid with the oxides to form water and the coordinating abilities of lactic acid and choline chloride helping dissolution, according to the following equation:
Nd2O3 + 6CH3OHCOOH ↔ 2(NdCH3OHCOO)3 + 3H2O Equation 6.
The formation of a complex between Nd and deprotonated lactic acid allow the solubilization of the metal from the oxide. Thus, the use of acidic DES is favorable for metal leaching (Cui et al., 2017).
Entezari-Zarandi et al.(Entezari-Zarandi & Larachi, 2019) used DESs of choline chloride and urea or malonic acid as hydrogen bond donors for separation of heavy and light rare earths (REs) due to differences in solubility of each metal in the DESs used. Heavy REs are more soluble in DES than light REs.
DESs also have application for leaching of metals from discarded electrical and electronic equipment. Tran et al. (Tran et al., 2019) published that a ChCl:Ethylene glycol DES can extract metals (Li and Co) from spent lithium-ion batteries. High leaching rates were obtained (99.3%) for both metals (t ≥ 24 h, T > 323 K). The DES colour turned blue after leaching, which was attributed to the formation of the CoCl4- anion. The leaching of metals seems to occur by coordination with the chloride from ChCl as hydrogen bond acceptor, and this seems to promote the leaching. Despite the high percentage of extraction obtained, the leaching kinetics is slow (t ≥ 24 h) and high temperatures (T > 323 K) are needed. Leaching the same material with nitric acid only takes 2 h and lower temperatures (338 K) obtaining similar leaching yields (Lee & Rhee, 2002).
Chen et al. (Y. Chen et al., 2020) studied different DESs (PEG/thiourea (2:1), p-toluenesulfonic acid/water/ChCl (1:1:1) and ChCl/ethylene glycol (1:2)) to dissolve lithium-ion batteries cathode LiCoO2 at different temperatures. The authors also use slow leaching kinetics (t = 24 h) and high temperatures (298–433 K). Results showed that the Co concentrations in PEG200/thiourea (2:1) at 393 and 433 K for 24 h were 3480.02 and 8440.14 ppm, respectively, which is much higher than the Co concentration at 353 K for 24 h (2121.14 ppm), showing the increase in mass transfer with increasing temperature. This solubility of LiCoO2in PEG200/thiourea (2:1) at 353 K is about 2 (1283.11 ppm) and 35 times (61.03 ppm) that in p-toluenesulfonic acid/water/ChCl (1:1:1) and ChCl/ethylene glycol (1:2), respectively, while keeping other conditions identical. At room temperature (298 K), the Co concentration in PEG200/thiourea (2:1) could reach 33.73 ppm at 353 K for 24 h. The recovery of Co from the DESs-LiCoO2 mixture could be realized by electrodepositing or extraction. The same authors Chen et al. (Y. Chen et al., 2021) later used Polyethylene Glycol-Based Deep Eutectic Solvents (PEG:PSA (1:1)) at low temperatures to dissolve lithium-ion batteries cathode LiCoO2 with leaching efficiency of 99.5% at 373 K and 70.4% at 353 K, which is much higher than previous reports by DESs in the same condition. This work provides novel DESs for the green and efficient dissolution of lithium cobalt oxide at a relatively mild condition.
Topçu et al. (Topçu et al., 2021) studied a treatment of copper converter slag with a deep eutectic solvent (1 ChCl: 2 Urea) as a leaching agent for recovery copper and zinc. The leaching conditions were T = 298–368 K and long reaction times (t = 2–72 h). The optimized conditions (T = 368 K and t = 48 h) for the leaching process gave a extraction of 89.9% Cu and 65.3% Zn. The total copper recovery was determined as 63% after the iron cementation as metallic copper form. The Zn is recovered by electrowining.
Yu et al. (H. Yu et al., 2022) used choline chloride and glycerol based DESs (1 ChCl:1.5 Glycol) to recovery the cobalt from lithium cobalt oxide (LiCoO2). The leaching efficiency of Co increased rapidly with the increase in the reaction temperature. Nearly 93% of Co can be leached from lithium cobalt oxide after a leaching treatment at 573 K for 10 h. The authors shown leaching efficiency data of other studies in Table 13. A higher leaching efficiency is seen than the previously reported routes by using other chemical configurations of DES. In addition the DES ChCl:Ethylene glycol presents a slower kinetics (24 h) (Tran et al., 2019). Leaching using DESs results in higher leaching efficiency. The temperature and time required are much high compared to leaching using minerals acids (sulfuric acid) (He et al., 2017).
| Reaction system | Reaction conditions | Leaching efficiency (%) | Reference |
|---|---|---|---|
| H2SO4 (1 M) | 313 K, 1 h | 40 | (He et al., 2017) |
| DES (ChCl:Ethylene glycol) | 468 K, 24 h | 69.14 | (Tran et al., 2019) |
| DES (ChCl:Urea) | 433 K, 2 h | 60 | (Wang et al., 2021) |
| DES (ChCl:Ethylene glycol) | 333 K, 4 h | 99 | (Coleman, 1966) |
| DES (ChCl:Glycol) | 473 K, 10 h | 92.7 | (Yu et al., 2022) |
The temperature and time required are lower using leaching agents such citric or oxalic acid. Peeters et al. (Peeters et al., 2020) used DESs made of ChCl and citric acid (2 ChCl: 1 citric acid) diluted with 35 wt% water to leach spent lithium-ion batteries. When 35% water is added, leaching may accelerate due to lower viscosity of the lixiviant. The optimized leaching conditions were 1 h of leaching at 313 K with a solid-to-liquid ratio of 20, which ensured to leach>98% Co (II) of the LiCoO2. In this study the copper was the most effective reducing agent for cobalt (III), thus requiring no additional reducing reagents. Cobalt in the DES phase was found to exist predominantly in the form of chloro-cobalt complexes due to the interaction with the chloride anion from coline chloride. The use of DESs allowed avoid the release of chlorine gas, which is a major issue when using hydrochloric acid as leaching reactive in the traditional process. The authors also studied the extraction and stripping stages.
This process consisted of a Cu(I/II) extraction step with the extractant LIX 984, followed by selective extraction of Co(II) with the extractant Aliquat 336. Both metals were stripped from the loaded organic phases by oxalic acid. The recovery yield of cobalt was 81%, as a 99.9% pure oxalate precipitate.
Thompson et al. (Thompson et al., 2022) studied choline chloride oxalic acid and based DESs (ChCl:Oxalic acid) to extract Co (II), Mn (II), Li (II) and Ni (II). The authors were able to extract with a stirring time of 2 h, temperature 353 K and a solid–liquid ratio of 15 g/L, achieving the separation of nickel (<10% extraction) under the same experimental conditions (Fig. 7). In the Fig. 8 shows a diagram of the process.
 Fig. 7
Fig. 7 Fig. 8
Fig. 8Yurramendi et al. (Yurramendi et al., 2021) used DESs made of choline chloride, lactic acid and citric acid (33% ChCl:53% Lactic acid + 13% Citric acid) to recover valuable metals from lithium ion batteries (LIBs). Leaching with studied DES at different temperatures, solid/liquid ratio = 1/50 and addition of water DES:Water = 50:8 (Due to viscosity limitations, a certain amount of water dosage (20%) was needed to ease ion mobility and to improve the leaching yield), achieved an extraction yield of >94% for the cobalt (Table 14). The viability of leaching most of the Co present in a black mass residue from LIBs (>99%) in <3 h and at low working temperature (328 K) was demonstrated by using diluted DES mixtures composed by mild organic compounds.
| Tleaching (K) | Coleached (%) | tleaching (h) |
|---|---|---|
| 328 | 94 | 7.5 |
| 343 | >99 | 4.8 |
| 358 | >99 | 3.81 |
Tian et al. (Tian et al., 2022) studied a multi-functional DES based on lactic acid (LA) and guanidine hydrochloride (GHC) to extract cobalt and lithium ions from LiCoO2. Due to the strong acidity (protons) and abundant chlorine coordinating ions of LA/GHC, the solubility of LiCoO2 in LA/GHC could reach as high as 19.9 mg/g (stirred at 353 K for 24 h). Cl- ions in guanidine hydrochloride have a stronger coordination ability than those in choline chloride (the typical hydrogen bond acceptor in DESs), which could be attributed to the delocalized π bond in guanidine, making Cl- ions easier to coordinate with metals.
Schiavi et al. (Schiavi et al., 2021) used a novel solvometallurgical process (Fig. 9) with a DES of choline chloride and ethylene glycol (1 ChCl: 2 Ethylene glycol) for the selective recovery of Co from the electrode powder of end-of-life lithium ion batteries (LIBs). Solvometallurgy involves process which uses non-aqueous solvents. These non-aqueous solvents can be molecular organic solvents, ionic liquids, deep-eutectic solvents (DESs), but also the inorganic solvents such as liquefied ammonia, concentrated sulfuric acid, or super-critical carbon dioxide. However, the use of non-aqueous solvents do not imply anhydrous conditions, but rather a low water content, no >50 vol% (Binnemans & Jones, 2017). In the Fig. 10. can be observed this DES reached around 90% Co and 10% Ni extraction at 453 K and 24 h. Co was recovered as cobalt oxalate which was employed to produce lithium cobalt oxide cathode material. The deep eutectic solvent was recycled and reused as feed to the process, with similar results to unused DES.
 Fig. 9
Fig. 9 Fig. 10
Fig. 10Peeters et al. (Peeters et al., 2022) studied choline chloride and ethylene glycol/urea based DESs (1 ChCl:2 EG and 1 ChCl:2 U) to for cobalt recovery from lithium-ion battery cathode materials. 1 ChCl:2 EG was used as a lixiviant at 453 K, for up to 24 h to leach cobalt. The authors showed that the DES 1 ChCl:2 EG is not thermally stable at these elevated temperatures (453 K) and that toxic decomposition products are even formed, such as trimethylamine or 2-chloroethanol. They also utilized el DES 1 ChCl:2 U to leach lithium cobalt oxide (LiCoO2) at 453 K during 18 h. Unfortunately, an extremely viscous DES leachate was obtained after leaching. The high viscosity of the 1 ChCl:2 U leachate maked it difficult to upscale the recovery process.
Roldán-Ruiz et al. (Roldán-Ruiz et al., 2020) utilized choline chloride and p–Toluene sulfonic acid (PTSA) based DESs (ChCl:PTSA) to cathode recycling of Li batteries. The authors studied the Co and Li recovery from spent LIBs using DESs (PTSA:H2O:ChCl; 1 PTSA: 2 H2O: 1ChCl and 1 PTSA: 3 H2O: 1 ChCl) at temperatures as low as 363 K, at times of reaction of 15 min. The leaching efficient of Co was 97/100/88% with DESs of molar ratio 1:1:1/1:2:1/1:3:1 respectively. The results for leaching of Li were 91/100/85% with DESs of molar ratio 1:1:1/1:2:1/1:3:1 respectively. Co recovery from the PTSA:ChCl-based DESs solutions was accomplished using either Na2CO3 or (NH4)2CO3 to precipitate a salt suitable for the preparation of new LIBs (Co(CO3)0.5(OH)·0·.11H2O) that, after calcination, resulted in its complete transformation into Co3O4. Co recovery efficiencies from spent LIBs was of up to 94% when considering the whole process. In this work, it is worth noting the fast kinetics that leaching presents, since the authors used 15 min for the process.
Luo et al. (Luo et al., 2022) carried out the efficient dissolution of the cathode materials of spent Ni-Co-Mn lithium batteries using deep eutectic solvents. DES was formed from betaine hydrochloride and ethylene glycol (1 Betaine hydrochloride:5 Ethylene glycol), The leaching rates of Ni, Co, Mn, and Li all reached 99% under the conditions of T = 413 K, t = 10 min. Compared with previous work, this study was able to accomplish efficient leaching in an very short time. The ionized hydrogen in the solvent reacted with the oxygen of lithium batteries, thereby destroying the crystal structure. Subsequently, the Cl−in B-DES complexed with transition metal ions, which led to lithium batteries dissolution. Further extraction of metal ions from the leachate can be achieved by electrodeposition or extraction.
Recently, other authors (Suriyanarayanan et al., 2023) have also extracted Co from spent lithium-ion batteries using a nonionic deep eutectic solvent (ni-DES) composed of N-methylurea and acetamide under relatively mild conditions (heating at 453 K for 24 h). Cobalt could be recovered with an extraction efficiency of > 97%. Recovered extracted cobalt was used to fabricate new fully functional new lithium-ion batteries from which the cobalt could again be retrieved for subsequent reuse.
Ma et al. (Ma et al., 2023) studied the cobalt and lithium recovery from spent lithium-ion batteries using a microwave-assisted deep eutectic solvents to shorten the extraction time and decrease the leaching temperatureFig. 10. It was found that 99.05% of Li could be selectively leached and 99.21% of Co could be precipitated as cobalt oxalate at 373 K in 10 min simultaneously. The purity of cobalt oxalate precipitated was 95.36% without any purification procedures. It formed a new polymer compound (Co2+[(Ch+)x(Cl−)y(HC2O4−)z]) during the microwave-assisted leaching process. Fin ally, the authors showed that the DES for the recovery of ithium-ion batteries could be cycled four times with favorable selectivity.
Zhu et al. (Zhu et al., 2023) studied a microwave-enhanced approach to shorten the leaching time in the urea/lactic acid: choline chloride: ethylene glycol DES system for recovery of valuable metals from spent lithium-ion batteries. The synthesized DESs were ChCl: Ethylene glycol, ChCl: Ethylene glycol:Lactic acid and ChCl: Ethylene glycol:Urea. 90% of Li and Co could be fast leached at 4 min and 160 W in the lactic acid/urea: choline chloride: ethylene glycol DES system. The sequences of Li and Co leaching ability at the same conditions were ChCl: Ethylene glycol:Lactic acid DES > ChCl: Ethylene glycol:Urea DES > ChCl:Ethylene glycol DES. That means that acidity and reducibility are helpful in this leaching process. The ultra-fast leaching in this process was achieved because of the electric field of the microwave-induced dipole momentof active components in the DESs system.
Wang et al. (M. Wang et al., 2023) utilized choline chloride and formic acidbased DESs (ChCl:FA) for extraction of critical metals (Li, Ni, Co, and Mn) from cathode materials (LiFePO4:Li(NiCoMn)1/3O2 mass ratio of 1:1) under mild conditions (353 K, 2 h) with a solid − liquid mass ratio of 1:200. The leaching performance of critical metals could be further enhanced by mechanochemical processing (600 rpm, 30 min) because of particle size reduction, grain refinement, and internal energy storage. The system can simultaneously solve the recycling challenges of spent Ni−Co−Mn and LiFePO4 batteries. Compared with the high temperature (973 K) of pyrometallurgy, the energy consumption of mechanochemical processing combined with DESs would be significantly lower. Compared with hydrometallurgy, the leaching time of critical metals by ChCl–FA could be shortened and rendered applicable for mixed cathode materials. Although the environmental impact of DES system needs to be further quantified, it has undoubtedly suggested considerable potential for future applications.
The analysis of these works shows that time and temperature of leaching with DESs are critical parameters for comparison with the values used in the leaching with mineral acids. The use of DESs formed by citric, oxalic or lactic acid could be an alternative to reduce the temperature and time of leaching con DESs. In these processes considering the physicochemical properties of the mixture is also important since diffusion mechanisms usually govern the mass transfer. In view of the two works done by Ma et al.(Ma et al., 2023) and Zhu et al. (Zhu et al., 2023) it can be considered that using microwaves for the leaching process, the process time is considerably reduced, obtaining similar results in the percentage of extraction of metals contained in Li-ion batteries. Another challenge is the extraction or recovery of the metals from the DES after the leaching. This is a subject currently rarely addressed by authors who have studied metal leaching processes with DESs, so it would be important to optimize the process with these solvents. Another point of interest is to study the corrosivity and long-term behaviour of DESs to determined to allow their application.
6.2. Liquid-liquid extraction of metals with hydrophobic deep eutectic solvents
Most of the above DESs are hydrophilic, which limits their use for metal extraction using L-L extraction. Some authors have studied the use of hydrophobic DESs (HDESs) that can replace conventional solvents used in dissolvent extraction. The first authors who described the ability of HDESs to extract metals from aqueous solutions were Tereshatov et al.(Tereshatov et al., 2016) in 2016. These authors extracted In from hydrochloric media into tetraheptylammonium cloride based HDESs containing carboxylic acids (decanoic acid, oleic acid and ibuprofen) as hydrogen bond donor. The metal extraction was similar to what was obtained with quaternary amines extracting molecules.
The metal extraction with these HDESs is likely to occur thanks to carboxylic acid, according to the follow equation:(7)(Mn+)aq + n(RCOO-)aq ↔ (M(RCOO)n)DES
The carboxylic acids allowed the extraction of cationic species. Since this mechanism requires the deprotonation of the carboxylic acid, the pH of the aqueous phase should be lowly acidic.
The authors of this work shown a lauric acid y DL-menthol based DES that allowed extract indium in both hydrochloric and oxalic acid media.
Ruggeri et al.(Ruggeri et al., 2019) used decanoic acid and tetrabutylammonium chloride (TBACL) HDESs (1 TBACL: 2 Decanoic acid) with 2.4% w/w H2O to extract Cr (VI) from aqueous media. Extraction of Cr was higher than 99%. Other metals as Cu (II) and Ni (II) led to the jellification of the HDES.
Van Osch et al.(van Osch et al., 2016) developed HDESs to remove transition (Co, Ni, Zn, Cu) and alkali (Na, K and Li) metal ions from non-buffered water. The HDESs used were prepared with lidocaine and decanoic acid 1:2, 1:3 and 1:4 in molar ratios. The extraction was dependent on components of HDESs the molar ratio. The transition metals were extracted with high efficient > 99% due to the good affinity of carboxylic acids for these metals, while alkali metal ions showed low distribution coefficients. The metal extraction occurred via the exchange of protonated lidocaine with the extracted metal, therefore, the aqueous phase would contain a high level of organic molecules. The authors demonstrated that regenerating the HDES used for extraction using sodium oxalate (0.1 M Na2C2O4) was feasible (Table 15), but only if the HDES contains an initially high concentration of decanoic acid (molar ratio: lidocaine:decanoic acid = 1:3 and 1:4). Higher molar ratios (hydrogen bond accept: hydrogen bond donor) facilitate low viscosity and low water solubility. Molar ratio ≥ 1:3 facilitates the metal extraction process.
|
Lidoc.:Decanoic acid (1:2) |
Lidoc.:Decanoic acid (1:3) |
Lidoc.:Decanoic acid (1:4) |
||
|---|---|---|---|---|
| Extraction | Co2+ | > 0.996 | > 0.996 | 0.983 |
| Stripping | Co2+ | 0.848 | 0.867 | 0.899 |
| Extraction after regeneration | Co2+ | 0.706 | 0.994 | 0.995 |
Ola et al. (Ola & Matsumoto, 2019) used the same HDES (lidocaine:decanoic acid in molar ratio 1:2) for the separation of Fe and Mn from aqueous solutions. Depending on the pH, the extraction could occur by exchange cationic with lidocaine or ion pair formation with decanoic acid. Fe (III) was extracted to pH = 1.0–2.0 by formation of ion pair formation with decanoic acid. At > pH the extraction mechanism cannot be evaluated due to the precipitation of the aqueous phase. Mn (II) was extracted to pH < 2.2 and > 3.5 by formation of ion pair formation with decanoic acid and to pH = 2.2–3.5 by exchange del Mn (II) with lidocaine.
Fig. 11 shows that the concentration of DES needed to reach the completely separation of Fe (III) was lower than that for Mn (II). In the case of Fe (III), the separation was complete at the DES concentration of about 25 g/L, whereas the complete separation of Mn (II) was reached at the DES concentration of about 300 g/L. The authors obtained a high extractability of iron (100% for 10 mmol/L of iron) and a high separability from manganese (separation factors > 100).
 Fig. 11
Fig. 11This method was very selective when was applied to the separation of Fe (III) from Mn (II), because the separation of Fe (III) was complete at the DES concentration of about 25 g/L whereas the complete separation of Mn (II) was reached at the DES concentration of about 300 g/L. Extraction of Fe was 100% (for an iron concentration = 100 mmol/L) and the separation factor Fe-Mn was high (SFFe/Mn). h (SFFe/Mn).
Three new DHESs based on thymol + TOPO (trioctylphosphine oxide), TOPO + capric acid and hydrocinnamic acid + capric acid were investigated as extracting media for the recovery and separation of platinum group and transition metals in HCl media (Schaeffer et al., 2020). Systems based on TOPO showed good selectivity for Pt4+, Pd2+ and Fe3+ ions in the range of conditions studied. The same authors (Schaeffer et al., 2018) used natural HDESs made from terpenes for the extraction of Cu2+, Fe3+ and Zn2+, using decanoic acid combined with thymol or menthol. Its extraction highly depends on the initial concentration of the metal: [Cu]initial < 0.01 mol/L - E (Cu) > 90%, [Cu]initial = 0.02 mol/L - E (Cu) < 50%.
Yueyue et al. (Shi et al., 2020) used trioctylmethylammonium chloride and alkyl chain parabens HDESs to separate Cr (VI) from water samples. The extraction efficiency of HDES for Cr (VI) was>90%. After being placed in air for 3 months, the chemical stability, hydro-phobicity and extraction performance of the HDES did not change.
DHESs based on tetra butyl ammonium chloride (TBAC) and oleic acid (TBAC: oleic acid) (W. Chen et al., 2021) were investigated for selective lithium recovery from the mother liquor obtained during the process of Li2CO3 production. The authors used different molar ratios to carry out the tests (TBAC:Oleic acid, 1 TBAC:2 Oleic acid and 1 TBAC:3 Oleic acid). In the Fig. 12a it can be observed that when the organic phase is pure HDES, the extraction efficiency of Li+ is 19.1% without ammonia in aqueous phase. It increased to 23.2% when ammonia is added into the aqueous phase. When TBP is used as organic phase, the extraction efficiency is relatively low and almost unchanged, regardless of whether ammonia is added to the aqueous phase or not. However, when the mixture phase of HDES and TBP is used as the organic phase, the extraction efficiency increased significantly from 11.8% to 45% with the addition of ammonia in the aqueous phase. The results show that ammonia and HDES played a crucial role in Li+ extraction, suggesting the synergism of HDES, TBP and ammonia.
 Fig. 12
Fig. 12Fig. 12b shows the influence of the DES concentration on the extraction performance. As the concentration of HDES increases, the extraction efficiency increases. Li recovery was 45.0%, 52.4% and 63.1% (TBAC/OA, TBAC/2OA and TBAC/3OA respectively). With the improvement of extraction performance, the content of TBAC cation (TBA+) in aqueous phase also increases. The Li extraction process occurred by a cation exchange mechanism between Li+ and TBA+, which improves the extraction efficiency of metal ions. Stripping of lithium was carried out with solutions of hydrochloric acid 0.5–2.5 mol/L, being the optimal concentration used 1 mol/L HCl. Stripping was 96.8% Li. Herein, HDESs have great potential to replace traditional organic solvents and ionic liquid in liquid–liquid extraction.
Hahada et al.(Hanada & Goto, 2021) prepared HDESs for Li recovery from a model brine solution containing a high concentration of alkali metals. The recovery of Li with the HDES (1 trioctylphosphine oxide (TOPO):2 thenoyltrifluoroacetone (HTTA)) was 95.7% (T = 298 K, t = 1 h, A/O ratio = 2/1). Furthermore, stripping of Li from the metal-loaded HDES phase was achieved using 1 M HCl and 89% of the Li could be recovered in a two-stage stripping procedure.
Milevskii et al.(Milevskii et al., 2022) studied a process based on Aliquat 336 and L-menthol to form a HDES with for the extraction of Li (I), Co (II), Ni (II), Mn (II) and Fe (III), from aqueous solutions in hydrochloric acid medium. The authors studied different molar ratios for the experimental tests (3 Aliquat 336:1 L-menthol, 3 Aliquat 336:2 L-menthol, 3 Aliquat 336: 3 L-menthol and 3 Aliquat 336: 7 L-menthol), being 3 Aliquat 336: 7 L-menthol the most convenient molar ratio. The extraction process carried out in different stages. In a first stage the authors recovered 99% of Fe (III) (with 1 mol/L HCl in solution and O/A ratio = 1/5). In a second stage they recovered 99% of Mn (II) (with 3 mol/L HCl in solution and O/A ratio = 1/5) using 20 extraction stages. Co (II) recovery was 99% (with 5 mol/L LiCl in solution and O/A ratio = 1/5) using 3 extraction stages. The stripping of each metal was studied until two cycles, 1 mol/L NaH2PO4 and 0.5 mol/L H3PO4 were used as stripping agents for Fe (III), 0.01 mol/L HCl was used for Co (II) and H2O for Mn (II). In all cases, 99% stripping efficiency was obtained. The Ni (II) was separated via precipitation, leading to a concentrated hydrochloric solution of Li (I).
Lastly, Kozhevnikova et al. (Kozhevnikova et al., 2022) used Aliquat 336, Di-(2-ethylhexyl)phosphoric acid (D2EHPA) and menthol based HDESs for the extraction of metal ions (Co (II), Cu (II), Ni (II), Fe (III), Al (III) and Li (I)) from a real hydrochloric acid solution after leaching the cathodes of three different types of Li-ion batteries (LIB 1, 2 and 3). The optimal HCl leaching conditions chosen were 2 M HCl, T = 353 K, t = 6 h and S/L ratio = 1/25. The eutectic mixtures used were Aliquat 336: menthol (HDES 1) and D2EHPA: menthol (HDES 2). In LIB 1 the authors extracted Cu (II) and Co (II) with HDES 1 and Al (III) with HDES 2, obtaining a rafinate of Li (I). In LIB 2 they extracted Fe (III) and Co (II) with HDES 1 and Al (III) with HDES 2 leading to a raffinate of Ni (II) and Li (I). The Ni (II) was separated via precipitation. In LIB 3 they extracted Fe (III) and Al (III) with HDES 2 obtaining a raffinate of Li (I). The extraction rate of valuable individual elements was above 98%.
The metals (Cu, Co, Al and Fe) stripping of LIB 1 and 2 was realized with water from the HDES phase. Al (III) and Fe (III) of LIB 3 were re-extracted with a HCl solution.
The studies of liquid–liquid extraction de metals applying DESs as solvent media are recent in the literature, and still lacking information about the process. The structure and the nature of the HBA and the HBD have impact on the ability to extract the metals using liquid–liquid extraction (Tereshatov et al. 2016), more recently, different authors have used TOPO-based DESs, and carboxylics acids as HBDs to produce DESs and achieve this separation (Hanada et al, 2021) (Shaeffer at al., 2018) (Phelps et al., 2018) (Gilmore et al., 2018). The properties of DESs for liquid–liquid extraction of metals are still limited by their intrinsic properties, particularly their high viscosity. In these processes extraction of metals the cation exchange mechanism leads to the contamination of the aqueous phase and should be avoided. The pH is related to precipitation and metal complex formation, which depends on the nature of the metal studied; therefore, the impact of this parameter must be addressed in the extraction processes. Another challenge is the recycling and reuse of DESs after extraction.
6.3. Electrochemical recovery of metals with deep eutectic solvents
In 2013, Mendes et al. (Mendes et al., 2013) used 1 ChCl:1.5 EG:0.5 Malonic acid DES to show that solvents made with complex agents as ethylene glycol/malonic acid increase the electrical conductivity of the solvents when compared to the standard DES (pure hydrogen bond donor). The metals were dissolved in the DES solution for 48 h at 353 K. It was demonstrated the use of DES to dissolve large amount of minerals (Ag, Al, Co, Cr, Cu, Ni, Pb, Zn, Au, etc).
Poll et al. (Poll et al., 2016) used etilenglycol and choline chloride based DESs (1 ChCl:2 Ethylene glycol) to recycling of Pb from hybrid perovskites of solar panels using the electrodeposition technique. 99.8% of Pb was removed from the DES with 120 h of electrodeposition, using a potential of −0.9 V vs. Ag. The same technique was applied to the extraction of Zn from electric arc furnace dust (Bakkar & Neubert, 2019). The DES used was 1.5 ChCl:1.5 Urea:0.5 EG. The arc furnace dust was dissolved in the DES, then the electrochemical behaviour of Zn was established using cyclic voltammetry. 38% of Zn was removed from the DES with 48 h of electrodeposition, using a potential of −1.2 a −1.5 V vs. Ag. The authors showed that the process is limited by the diffusion of Zn ions in the DES. Bakkar et al. (Bakkar, 2014) show that about 60% of Zn and 39% of Pb could be dissolved from the dust when stirred for 48 h in the 1 ChCl:2 Urea DES at 60 °C.
Almeida et al. (Almeida et al., 2020) studied the recovery of W and As from secondary mine resources with a malonic acid + oxalic acid and choline chloride based DES (1 ChCl:2 Malonic acid:1 Oxalic acid). 82% of As and 77% of W were recovered by electrodialysis using a current of 100 mA for 4 days.
Wang et al. (S. Wang et al., 2020) used DESs made of ChCl and urea (1 ChCl:2 urea) for recycling of spent Li-ion batteries (LIBs), using the cyclic voltammetry technique to determine the reducing power of DESs. The Li and Co extraction efficiency was around 95% at a temperature of 453 K and duration of 12 h. Kinetic essays kinetic tests showed that the solution diffusion and electron diffusion through the DES controlled the metals extraction. The time needed for leaching was long and the temperature high compared to leaching using minerals acid. The cobalt was recycled as a cubic cobalt oxide spinel (Co3O4) using H2C2O4 and NaOH precipitants using a dilution-precipitation-calcination process.
Perera et al. (Perera et al., 2023) studied the cobalt electrochemical recovery using DESs compounded of ethylene glycol (EG) (67 M %, 82 M % or 100 M %) and choline chloride (ChCl). The electrochemical results show that increasing the amount of EG together with a small concentration of sulfate anions, in conjunction with Cl- anions (two different cobalt sources, CoCl2·6H2O and CoSO4·7H2O, were studied), in the solution mixture favours the reduction of Co2+. This improved electrochemistry seems to be related to changes of Co2+speciation easing the reduction process. The nature of the Co salt had a significant impact on the recovery efficiency, morphology, and purity of the Co electrodeposit.
The recovery of metals through of electrochemical techniques with DESs could be a via promising, but in some cases the efficiency was not good (Abbott et al., 2009) and sometimes the reaction times requires to recover the metals are long. The water content and decomposition of the DEs during the application of a current lead to a decrease in current efficiency, which appears to be a clear limitation and deserves further investigation. The use of low viscosity DESs will also be a priority to favor mass transfer kinetics.
7. Conclusions
DESs are emerging as an environmentally friendly alternative for the recovery of metals from industrial waste or by products. DESs are obtained from the mixture of two or three substances with a given composition where the melting points of each individual components are higher than the mixture. Synthesis methods are simple and their components are cheap and natural. The physical properties of DESs, such as density, viscosity, surface tension and conductivity are essential to determine their applications on the hydrometallurgy methods. The physico-chemical characteristics of DESs depend among other factors such as the nature of the hydrogen bond acceptor and donor components, which form the eutectic mixture. Other important parameter is the water content; it could be modifying the physico-chemical properties of the DESs. So, it is possible to adjust their physico-chemical properties according to their application in the recovery of metals by hydrometallurgy methods.
Although many of the physico-chemical properties of DESs have been studied, as this review shows, there are still gaps in the feasibility of their use in hydrometallurgical methods. Hydrometallurgical studies using DESs as leaching or extraction agents need to further investigate the properties and influence of the different stages of metal recovery processes. So, a wide space for the research in the area of measurement, prediction and correlation of physical properties of the DES with their behaviour in the area of recovery metal is still unfilled.
For example, one fundamental parameter that needs to be controlled is the water content of the DES, given that the proportion of water can significantly modify its properties and this have an influence in the metal recovery methods. So, when the DESs are used the content of water have to be mentioned to the aim to understand different in the DES properties.
Despite different studies have made significant progress toward the utilization of DESs in metal leaching and extraction, much work remains in process development, optimization, and scale-up. The possibility of achieving higher metal extraction efficiencies with minimal production of waste materials via non-aqueous solvent approaches needs to be determined.
One advantage of DESs comes from the ability to selectively leach metals from waste and by-products by the different metal solubilities in the DES. However, there is still a long way to go to reach the replacement of mineral acids by DESs. The main problem comes from the large leaching times, which takes several hours. So, to achieve a pilot or industrial scale application the leaching time have to be shorter. Other disadvantages are the higher leaching temperatures. One alternative to minimize these negative effects would be the use of DESs formed by oxalic, citric or lactic acid.
Studies of metal leaching with DESs microwave-assisted to improve the kinetics of the process have recently appeared. The authors report results similar to those achieved without the use of microwave but with much lower reaction times (on the order of minutes).Another challenge is the extraction or recovery of the metals from the DES after the leaching. This is a subject currently rarely addressed by authors who have studied metal leaching processes with DESs, so it would be important to optimize the process with these solvents. Another point of interest is to study the corrosivity and long-term behaviour of DESs to determined to allow their application.
The properties of deep eutectic solvents for metal extraction are still limited by their intrinsic properties, in particular their high viscosity, but there is still room for the design of new DESs that could improve the environmental aspects of solvent extraction. The cation exchange mechanism leads to contamination of the aqueous phase and should be avoided. The high water solubility of DESs is also problematic. To avoid this disadvantage the addition of salts in the aqueous phase modifies the solubility of ionic liquids and could be investigated in the case of DESs. Hydrophobic DES are other alternative however, their use can be limited by the lack of availability of its constituents in an easy and inexpensive way. Compared to ionic liquids, DESs appear to have lower toxicity. Some potential problems have to be investigated to allow the industrial application of DESs, such as its long-term behaviour and its corrosivity. It should also be considered that the introduction of the metal could modify the structure of the DESs and, consequently, have an effect on its properties and potential reuse. The pH is related to precipitation and metal complex formation, which depends on the nature of the metal studied; therefore, the impact of this parameter must be addressed in the extraction processes. Another challenge is the recycling and reuse of DESs after extraction, because these two topics are not considered by many authors in their research in the field of the DESs.
DESs allow the electrochemical recovery of numerous metals, but the slowness of the process must be considered, which is limiting its feasibility and more research is needed to improve the electrochemical recovery of metals from DESs. The water content and decomposition of the DEs during the application of a current lead to a decrease in current efficiency, which appears to be a clear limitation and deserves further investigation. The use of low viscosity DESs will also be a priority to favor mass transfer kinetics.
On the basis of the limitations and advantages of the application of DESs in the processes of metal extraction, it would be necessary to further study the kinetics and extraction mechanisms and carried out a design of continuous processes with real or industrial solutions and scale-up the laboratory processes to pilot or industrial scale.
CRediT authorship contribution statement
M.I. Martín: Writing – original draft, Writing – review & editing. I. García-Díaz:Writing – original draft, Writing – review & editing. F.A. López: Writing – review & editing, Project administration, Funding acquisition, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to the Interdisciplinary Platform for Sustainable Plastics towards a Circular Economy (SusPlast-CSIC) for the financial support Dr. M.I. Martín through project (n° 20219PT055) and to the Ministry of Science and Innovation for the contract PTA2020-018866-I of Dr. I. García-Díaz. This work is part of the BATERURGIA project (Misiones CDTI Programme Ref. MIG-20221014) and has received Next Generation EU funding (Recovery, Transformation and Resilience Plan).
Data availability
Data will be made available on request.
Further reading
-
Abbott et al., 2004c
A.P. Abbott, G. Capper, D.L. Davies, R.K. Rasheed
Ionic liquid analogues formed from hydrated metal salts
Chem. A Eur. J., 10 (15) (2004), pp. 3769-3774, 10.1002/chem.200400127
-
Abbott et al., 2006b
A.P. Abbott, G. Capper, S. Gray
Design of improved deep eutectic solvents using hole theory
ChemPhysChem, 7 (4) (2006), pp. 803-806, 10.1002/cphc.200500489
-
Abbott et al., 2011a
A.P. Abbott, J.C. Barron, G. Frisch, S. Gurman, K.S. Ryder, A. Fernando Silva
Double layer effects on metal nucleation in deep eutectic solvents
PCCP, 13 (21) (2011), p. 10224, 10.1039/c0cp02244f
-
Abbott et al., 2011c
A.P. Abbott, R.C. Harris, K.S. Ryder, C. D’Agostino, L.F. Gladden, M.D. Mantle
Glycerol eutectics as sustainable solvent systems
Green Chem., 13 (1) (2011), pp. 82-90, 10.1039/C0GC00395F
-
Chen et al., 2019a
W. Chen, J. Jiang, X. Lan, X. Zhao, H. Mou, T. Mu
A strategy for the dissolution and separation of rare earth oxides by novel Brønsted acidic deep eutectic solvents
Green Chem., 21 (17) (2019), pp. 4748-4756, 10.1039/C9GC00944B
-
Chen et al., 2019b
Y. Chen, W. Chen, L. Fu, Y. Yang, Y. Wang, X. Hu, F. Wang, T. Mu
Surface tension of 50 deep eutectic solvents: effect of hydrogen-bonding donors, hydrogen-bonding acceptors, other solvents, and temperature
Ind. Eng. Chem. Res., 58 (28) (2019), pp. 12741-12750, 10.1021/acs.iecr.9b00867
-
Chen et al., 2019c
Y. Chen, D. Yu, W. Chen, L. Fu, T. Mu
Water absorption by deep eutectic solvents
PCCP, 21 (5) (2019), pp. 2601-2610, 10.1039/C8CP07383J
-
Chen et al., 2021a
W. Chen, X. Li, L. Chen, G. Zhou, Q. Lu, Y. Huang, Y. Chao, W. Zhu
Tailoring hydrophobic deep eutectic solvent for selective lithium recovery from the mother liquor of Li2CO3
Chem. Eng. J., 420 (2021), Article 127648, 10.1016/j.cej.2020.127648
-
Chen et al., 2021b
Y. Chen, Y. Wang, Y. Bai, Y. Duan, B. Zhang, C. Liu, X. Sun, M. Feng, T. Mu
Significant improvement in dissolving lithium-ion battery cathodes using novel deep eutectic solvents at low temperature
ACS Sustain. Chem. Eng., 9 (38) (2021), pp. 12940-12948, 10.1021/acssuschemeng.1c04220
-
Florindo et al., 2017a
C. Florindo, L.C. Branco, I.M. Marrucho
Development of hydrophobic deep eutectic solvents for extraction of pesticides from aqueous environments
Fluid Phase Equilib., 448 (2017), pp. 135-142, 10.1016/j.fluid.2017.04.002
-
Florindo et al., 2017b
C. Florindo, M.M. Oliveira, L.C. Branco, I.M. Marrucho
Carbohydrates-based deep eutectic solvents: Thermophysical properties and rice straw dissolution
J. Mol. Liq., 247 (2017), pp. 441-447, 10.1016/j.molliq.2017.09.026
© 2023 The Author(s). Published by Elsevier Ltd.