1. Introduction
The use of biodegradable medical implants in orthopedics is growing significantly and is expected to advance robustly in the upcoming years [1]The primary reason for their popularity is their performance and advantages over permanent implants [2]. Rapid innovations in biodegradable implants and devices have opened up new opportunities and possibilities that can be achieved in the medical field, which was not possible like to promote desired biological responses and biomaterials can dynamically adapt their properties or functionalities based on specific triggers, such as changes in temperature, pH, or biochemical signals [3]. Biomaterial describes implants developed from synthetic materials that can temporarily replace and assist the performance of living tissues [4]. For medical purposes biomaterials are selected based on their mechanical properties [5], biocompatibility [6], molecular weight [7], biodegradability [8], and physiochemical properties [9]. They are classified as inert or autonomous, active, and receptive, based on their cellular and biological responses [10]. With the expansion of the world's population, surgical procedures—including dental implants, transplantation of bone (bone grafting), and cardiovascular and cerebral vascular biliary stents continue to increase [11,12]. Due to global aging and an overall rise in bone fractures, the need for medical implants is now elevated [13]. They are used in surgical applications like bone defect fillers, bone cement, fracture fixation plates, joint replacement, and artificial tendons [14]. Currently, titanium, cobalt-chromium, and stainless steel are the most commonly used implant material in orthopedic surgeries [15]. They possess high strength, and tremendous corrosion resistance that support healing [16,17].
However, these materials are not biodegradable, resulting in numerous adverse effects such as additional surgeries to remove the implants, growth restrictions, temperature sensitivity, and cross-contamination risks. Implants are required for medical treatments like bone substitution, dental surgeries, and bone-fixing but they have side effects [18]. Advancements in medical research resulted in the development of synthetic biodegradable polymer-based implants that eliminate the risk of multiple surgeries and improve healing time.
The last decade has witnessed a significant development in the advancement of innovative biomaterials that medical professionals use to improve the quality of existing treatments and life [19]. Recent developments in targeted/sustained drug delivery, regenerative medicine, and tissue engineering have encouraged the utilization of innovative biomaterials that possess biodegradation characteristics for advanced clinical applications [20].
Currently, bioresorbable materials consist of metallic and polymeric components. Polymeric materials including polydioxanone and poly (l-lactic acid) are found in stent applications of bile ducts [[21], [22], [23]]. Biliary stents have been made using a variety of biodegradable polymers with various biodegradation patterns. The selection of biodegradable polymers is based on the necessary characteristics (mechanical integrity, highly tensile, non-toxic, and regulation of degradation rate) [[23], [24], [25]]. Moreover, the chemical properties play a major role in their biocompatibility. Ideally, bioimplants are biocompatible to the human body, which makes them suitable for long-term physiological immersion. The most common metallic bio-absorbable metals include iron (Fe), Magnesium (Mg), and Zinc (Zn) [[26], [27], [28]]. These biomaterials corrode and degrade gradually in vivo after they serve their purpose in a medical performance like tissue healing, disease diagnosis, or assisting support without any harmful response in the host body [[29], [30], [31]]. Biodegradable metals demonstrate better performance and mechanical strength in bone implants and cardiovascular stents than polymeric materials [[32], [33], [34]]. The desired medical properties have opened up vast market opportunities for absorbable implant materials. Synthetic biodegradable polyesters have become the most competitive polymers for medical and orthopedic treatments and can also be produced with multiple characteristics and cost-effectively [[35], [36], [37]]. Based on recent research, the specific criteria and design constraints of biodegradable implants are shown in Table 1.
Table 1. Design criteria for biodegradable metallic implant devices in cardiovascular and orthopedic applications [[38], [39], [40]].
| Criteria | Orthopedic Internal fixation device | Cardiovascular stent |
|---|---|---|
| Mechanical properties |
Tensile strength>300 MPa Yield strength >230 MPa Elongation to failure >15–18 % Elastic modulus approx. to that of cortical bone (10–20 GPa) |
Tensile strength>300 MPa Yield strength >200 MPa Elongation to failure >15–18 % |
| Biocompatibility | Non-inflammatory, non-toxic, hypoallergenic. No particle retention or harmful release. Promotes osteoclast and osteoblast attachment. Prevent fibrous encapsulation | Non-toxic hypoallergenic. No retention of particles or harmful release. Avoid smooth muscle cell attachment and promote endothelial cell attachment |
| Corrosion Behavior |
Hydrogen Evolution<10 μLcm‾2.day‾1 Screws and plates (0.2–0.5 mm year‾1) |
Hydrogen Evolution <10 μLcm‾2.day‾1 Penetration rate <20 μm year‾1 |
| Mechanical integrity and resorption |
Osteotomy staples<3months Full absorption 1–2 years Screws and Plates <6months |
Mechanical integrity (UTS) 3–6 months Full absorption 1–2 years |
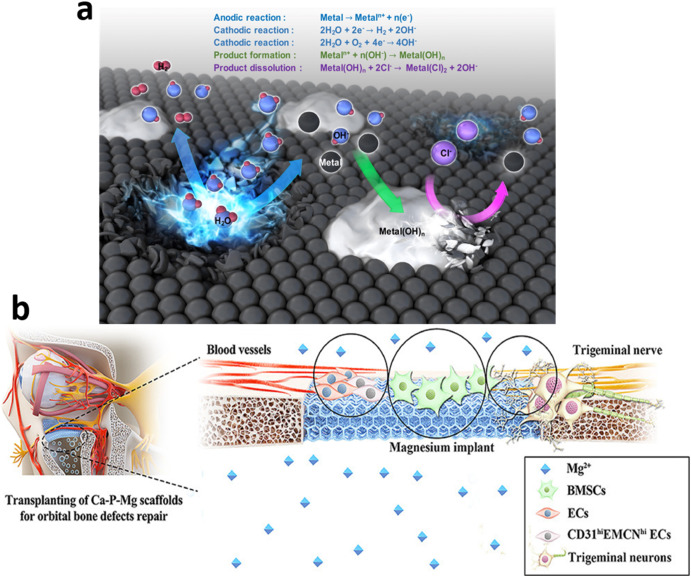
Based on these requirements, new challenges arise in the development of Biomaterials (BMs). The foremost concern is the degradation products that are released in the surrounding environment due to corrosion (Fig. 1(a)). These products can be toxic and result in further damage to the body. Thus, there is a need to optimize degraded products to guarantee biosafety. Additionally, the behavior of degrading BMs should be in line with the kinetics and evolution of the healing process [[41], [42], [43]]. In cardiovascular application, the healing process undergoes three stages: inflammation, granulation, and remodeling [25,44,45] (Fig. 1(b)). The healing process of fractured bones follows three stages: inflammation, repair, and remodeling.
 Fig. 1. (a) Metal Biodegradation process [46] (b) Schematic diagram of the trigeminal neurons in an orbital bone defect model, implanted with Ca–P-coated Mg–Zn-Gd scaffolds, and Trpv1 causes them to release the neuropeptide Calcitonin gene-related peptide (CGRP), which stimulates angiogenesis and osteogenesis response to accelerate the bone healing process [47].
Fig. 1. (a) Metal Biodegradation process [46] (b) Schematic diagram of the trigeminal neurons in an orbital bone defect model, implanted with Ca–P-coated Mg–Zn-Gd scaffolds, and Trpv1 causes them to release the neuropeptide Calcitonin gene-related peptide (CGRP), which stimulates angiogenesis and osteogenesis response to accelerate the bone healing process [47].The current paper aims to critically evaluate and review the recent advancement of biodegradable/bio-absorbable biomaterials for various medical applications, the current and possible medical applications of different bio-absorbable materials and their properties are discussed. New materials are demonstrating potential possibilities to be employed as biomedical applicationsbecause of their favorable chemical and mechanical properties Therefore, the possibility and potential of alloying new metals including Mo, Ti with Mg, Zn, and Fe is also discussed.
1.1. Novelty and mechanism
In this comprehensive review, our manuscript introduces several key innovations that advance the understanding and application of biodegradable metals in implantology. Notably, we shed light on recent breakthroughs in the development of novel alloys, such as Molybdenum-based materials, which exhibit remarkable mechanical properties previously unexplored in the context of biodegradable implants. Furthermore, we delve into cutting-edge surface modification and fabrication techniques, which play a pivotal role in optimizing the performance of biodegradable metallic implants. These innovations represent a significant departure from the conventional research landscape and offer promising avenues for addressing the limitations associated with traditional materials like Zinc and Iron. By elucidating these novel advancements, our manuscript provides valuable insights into the future of biodegradable implants and underscores their potential to revolutionize the field of biomedical applications.
To elucidate the underlying mechanisms of biodegradation and the performance of biodegradable metallic implants, this review extensively explores the complex interplay of electrochemical processes, physiological environments, and material properties [48]. The corrosion mechanism of these materials is intricately examined, highlighting the dynamic processes involved in their degradation over time. Moreover, we elucidate the crucial role of in vivo and in vitro studies in elucidating the response of biodegradable implants within the human body and controlled laboratory settings. These studies reveal the intricate interactions between the implant material, surrounding tissues, and the body's corrosive environment. By comprehensively addressing these mechanisms, our manuscript provides a holistic understanding of biodegradable metallic implants, serving as a foundation for future research endeavors and the development of implants with superior mechanical properties and controlled degradation rates.
2. Mg alloys and composites
Mg-based alloys are promising biomaterials for orthopedic applications [49,50]. Mg demonstrates the significant mapping of Young's modulus and anisotropicfeatures with human cortical bone. Additionally, Mg demonstrates high osteopromotive and biocompatible response while degradation [[51], [52], [53]] in physiological media. Mg found extensive usage in medical applications because of its increased availability. It was first reported to be used in 1878 when Edward C.H. successfully used Mg wires as blood vessel ligatures [51,52,54]. However, later several applications were tested, including utilizing Mg plates, sheets, and screws for arthroplasty, tubes, or connectors to treat nerve, intestinal, and vessel anastomoses and to treat cavernous hemangioma, respectively [53]. The results of these tests were less effective in terms of biocompatibility and degradation than expected. In later decades, concerns including post-operative subcutaneous gas cavities and premature loss of mechanical integrity were also identified [55,56].
In their study, Troitskii and Tsitrin [57] reported instances where fractures were successfully treated using Mg–Cd alloy incorporated into screws and plates. These Mg–Cd implants were found to promote the formation of robust calluses during fracture repair. Nonetheless, in certain cases, failures occurred as a result of the development of gas cysts or infections. It is noteworthy that no acute inflammatory reactions or abnormal changes in the serum magnesium levels were observed in any of the patients [49,58].
Mg alloys are proven to be better in terms of biocompatibility than bio-polymers, including poly l-lactic acid (PLLA), poly-ether ketone (PEEK), and polyglycolic acid (PGA). It also overshadows bio-ceramics like tricalcium phosphate (TCP) and hydroxyapatite (Hap) as these ceramics have low mechanical strength for bone implants [[59], [60], [61], [62]]. Mg alloys can be easily sterilized because of their surface quality, stiffness, and plasticity. Furthermore, the degraded by-products of Mg alloys and Mg implants, specifically Mg ions, are non-toxic and do not pose any health risks. Indeed, they exhibit distinctive osteopromotive characteristics [63]. The degraded Mg ions, facilitated by transporter proteins, elevate Osterix levels and effectively stimulate the bone regeneration process. However, the biomedical applicationsof this material are constrained by its rapid degradation, as its mechanical integrity tends to diminish almost before the surrounding tissues are fully healed [[64], [65], [66]].
The advancements in Mg-based studies (Fig. 2) suggest that earlier research was focused on controlling the degradation rate/corrosion of Mg alloys through ceramic coatings like hydroxyapatite (HA), Titanium dioxide (TiO2), and through alloying with inert elements [67]. Recent trends show that, apart from corrosion resistance now application of coatings is investigated to deliver diverse therapeutic responses. Apart from chemical coatings, various thermal (Laser cladding, laser shock peening, etc.) and mechanical techniques (SMAT, FSP) are gaining interest to tailor the grain size and texture of Mg alloys [68,69]. These techniques are effective in solving the long pending dilemma between strength-ductility-corrosion of Mg alloys by a single approach. Most recently, Mg has been established as an effective anti-malignant element. Additionally, The H2evolution during controlled degradation of Mg alloys also served as a novel antioxidant to combat inflammatory conditions after implantation [70,71].
 Fig. 2. A neural analysis of advancements in Mg studies to elicit biomedical applications.
Fig. 2. A neural analysis of advancements in Mg studies to elicit biomedical applications.2.1. Corrosion behavior of Mg alloys
Corrosion resistance, cytocompatibility, and hemocompatibility are critical for the biosafe performance of bioimplants during in-vivo conditions. Mg and Mg-based materials in orthopedic implants demonstrate a higher rate of corrosion than biosafety limits [72]. Especially if the corrosion medium contains chloride above 3 mM/L, the magnesium turns into magnesium chloride (MgCl2). It demonstrates high solubility in aqueous solution and facilitates further corrosion in the human body, which usually has more chloride traces [[73], [74], [75]]. The chemical processing involved in corrosion i.e. hydrogen evolution and chloride solubility, significantly impacts the in vivo performance of Mg devices. Hydrogen embrittlement raised from H2 entrapment in the corrosion cavities (Fig. 3), leading to premature failure of Mg implant even before the designed service span [76].
 Fig. 3. The fractography of a peak-aged Mg–4Zn sample subjected to straining in simulated body fluid (SBF). The image at low magnification (a) primarily reveals cleavage fractures, (b) surface cracks are evident on the lateral faces of the gauge region, indicating the occurrence of stress corrosion cracking. Furthermore, (c) depicts the development of grain boundary cracks stemming from microcracks, accompanied by highly cleaved surfaces. Lastly, (d) highlights a cleavage step with hexagonal-shaped surfaces, characteristic of transgranular stress corrosion cracking (SCC), a phenomenon typically observed in hexagonal close-packed (HCP) Mg alloy. The micrograph also reveals the presence of micro-pores associated with hydrogen embrittlement and provides evidence of micro-crack initiation within the high-magnification view of the fractographic sample [76].
Fig. 3. The fractography of a peak-aged Mg–4Zn sample subjected to straining in simulated body fluid (SBF). The image at low magnification (a) primarily reveals cleavage fractures, (b) surface cracks are evident on the lateral faces of the gauge region, indicating the occurrence of stress corrosion cracking. Furthermore, (c) depicts the development of grain boundary cracks stemming from microcracks, accompanied by highly cleaved surfaces. Lastly, (d) highlights a cleavage step with hexagonal-shaped surfaces, characteristic of transgranular stress corrosion cracking (SCC), a phenomenon typically observed in hexagonal close-packed (HCP) Mg alloy. The micrograph also reveals the presence of micro-pores associated with hydrogen embrittlement and provides evidence of micro-crack initiation within the high-magnification view of the fractographic sample [76].In contrast, a slower degradation rate of Mg alloys (less than 0.5 mm/year) facilitates bone healing more efficiently. Kirkland et al. [77]reported that most Mg alloys undergo localized corrosion due to galvanic coupling, a phenomenon that generates micro-galvanic cells and facilitates the degradation process. Mg alloys contain elements like Cu, Ni, and Al, having a less electronegative nature compared to Mg. Hence, they are prone to damaging the protective film Mg (OH)2 [77,78]. When the oxide film is compromised, it increases further corrosion due to the contact of the Mg matrix with the surrounding fluid. If not controlled, H2 release and metallic debris rise above the tolerance level, and implant fails permanently due to corrosion fatigue (CF) and stress corrosion cracking (SCC) [79,80] as shown in Fig. 3. This corrosion mechanism hinders the application of Mg for full-scale biomedical applications.
Mg alloys (WE43, AZ31, LAE442, and AZ29) were utilized by Witte et al. [81] to prepare rods. In vivo Mg alloy implants degraded at different rates. The degradation depended mainly on the alloy composition [82]. Among all the alloys, LAE442 demonstrated the slowest corrosion rate, and WE43 Mg showed Ca and P rich mineralization of apatite with prolonged immersion [83]. Apart from alloying, many surface modification techniques have emerged to improve the mechanical and corrosion response of Mg and Mg-based implants.
2.2. Designing Mg-based bioimplants
Recent advancements show significant improvements in the corrosion behavior and mechanical properties of Mg and Mg-based alloys through different surface treatment methods [84]. The summary of different surface modification treatments for Mg and its alloys for medical application is shown in Table 2. Micro arc oxidation (MAO) is one of the most effective and widely reported surface modification techniques [59,85,86]. Wang et al. [87] applied MAO treatment on Mg and observed changes in Young's modulus, Percentage (%) elongation, and ultimate tensile strength (UTS). The mechanism of MAO coating for corrosion resistance is explored by Gu et al. [47,82]. They suggested that the extent of corrosion damage depends on the uniformity and porosity of MAO coating and layer thickness. Some studies also focused on enhancing the hydrogen storage properties of Mg-based materials to counter embrittlement phenomena. Lu et al. [88] employed the plasma arc method to fabricate a core-shell Mg@Pt composite with a nanostructure. Additionally, the Rieke method was utilized to produce metal nanoparticles, allowing for the preparation of various metals such as Al, Mg, Ni, Co, and others. Suh et al. [89] adopted a data-driven approach based on machine learning to predict material properties and explore new materials. Their approach involved augmenting data by utilizing standard deviation and mean values. Fig. 4 illustrates two strategies for incorporating average and standard deviation into the data. The first method entails adding or subtracting the standard deviation for each input and output variable, effectively multiplying a single data point by twice the number of variables [90]. In this context, one data point can be expanded by a factor of 12 by iterating over a total of six variables, modifying the standard deviation of one variable while keeping the average value of the other variable constant.
Table 2. Summary of surface treatments for Mg and its Alloys.
| Techniques | Substrate | Surface Modification technique/method | Thickness | Structure of the main layer | Ref |
|---|---|---|---|---|---|
| Mechanical Method | AZ31B | Cryogenic machining, severe plastic deformation, and plastic burnishing | 1.5–3.4 μm | Nanocrystalline grain structure | [63,91] |
| Physical method |
AZ31 Mg-3Nd-0.2ZN-0.4Zr/Mg–Y-RE/AZ91 AZ91/WE43 |
Ion-based assisted deposition Titanium ion implantation, AI-O dual ion, and Oxygen Plasma Immersion Ion |
5∼10 μm | Zn and O | [91,92] |
| Electromechanical Treatment |
Mg–Zn–Ca Pure Mg/Mg–Ca Alloy/AZ91 |
Electrode-position Anodic Oxidation/MAO |
10∼20 μm <20 μm |
Hydroxyapatite Ca-defHA/FHA and brushite MgO/ |
[93,94] |
| Chemical Conversion Coatings |
pure Mg/Mg–Ca alloy/AZ3 LAE442/pure Mg/AZ31B |
Alkali thermal treatment Fluoride treatment |
100 μm 3∼200 μm |
Mg(OH)2/MgO MgF2 |
[63,91,92] |
| Sol-Gel | AZ31/Mg4Y/Mg/6Zn/AZ81/WE42 | Polymer and organic coating | 2∼70 μm | PCL/PLLA/PLGA/Silicane-epoxy coating® -TCP | [93,95] |
 Fig. 4. Proposed approach for data augmentation by machine learning approach for load bearing of Mg and its alloys to predict the tensile of implants modified with permission [89].
Fig. 4. Proposed approach for data augmentation by machine learning approach for load bearing of Mg and its alloys to predict the tensile of implants modified with permission [89].Various additive manufacturing (AM) methods are instrumental in the development of biomaterials due to their capacity to achieve complex material designs and intricate geometrical shapes. Recently, there has been a growing interest in employing AM processes for manufacturing magnesium (Mg) alloys, primarily to surmount the challenges associated with conventional manufacturing techniques [96,97]. The utilization of the latest 3D techniques for Mg production is inherently complicated due to the metal's uncontrolled oxidation and its highly reactive nature in its pure form. Moreover, the elevated surface energy of Mg raw materials, whether in wire, powder, or liquid form, renders them susceptible to oxygen-induced combustion when exposed to the atmosphere [59,98]. These factors collectively render the manufacturing of Mg alloys a formidable task, necessitating specialized equipment to establish a controlled environment and ensure safety during handling [99,100]. At present, the AM methods employed for Mg alloy production encompass powder bed fusion (PBF), selective laser melting (SLM), paste extrusion deposition, jetting, wire arc additive manufacturing (WAAM), and friction stir additive manufacturing. Recent research endeavors have utilized SLM and WAAM to craft Mg alloys. SLM, characterized by high laser energy, rapid cooling, and short processing durations, yields minimal heat-affected zones and well-defined grain structures conducive to structural integrity. PBF, on the other hand, stands out as the most extensively investigated method for fabricating Mg alloys [101]. This approach leverages low heat flux and offers the feasibility to achieve intricate internal and external geometries with densities reaching up to 96.13 %. While significant strides have been made in optimizing the performance of Mg-based biomaterials through treatments and alloying, there remains substantial work to be done to elevate Mg to the status of an ideal biodegradable material. Recent advancements in bioengineering and materials science have afforded researchers greater control over magnesium's mechanical properties and corrosion rates [3,102]. The heightened interest in biodegradable materials for bone implants and replacements has catalysed a resurgence in the utilization of magnesium alloys in cardiovascular and orthopedic applications.
2.3. In vivo and in vitro responses of Mg alloys
The primary limitation hindering the clinical application of magnesium (Mg) is its degradation behavior. Gu et al. [82] conducted a comprehensive study on the in vivo and in vitro performance of Mg–Sr alloy, demonstrating its remarkable attributes, including the highest ultimate tensile strength (203 MPa), finer microstructure (23 μm), superior osteogenic properties, and a slower degradation rate (0.37 mm/year). Zhao et al. [103] employed extrusion treatment on Mg–Sr alloys, yielding results characterized by favorable corrosion resistance, improved mechanical properties, and non-toxicity to cells. Their research also highlighted a slower degradation rate for Mg-0.5Sr alloy, uncovering the occurrence of Ni thrombosis during in vivo implantation. Additionally, the incorporation of zinc (Zn) and calcium (Ca) has been proven effective in enhancing the biocompatibility of Mg alloys [104]. Notably, the formation of calcium phosphate (Ca–P) during in vivo degradation contributes to improved bone mineralization. The addition of Zn also facilitates gene expression, promotes nervous system function, aids in protein synthesis, and exhibits anti-atherosclerosis properties. Fan et al. [105] prepared Mg alloys tailored for orthopedic implants, and the inclusion of Ca and Zn resulted in enhanced corrosion resistance in both in vivo and in vitro settings. Schulz et al. conducted a comprehensive investigation into the corrosion behavior of pure Mg, AZ91D, and AZ31 in various in vivo and in vitro environments. They implanted Mg/Mg alloys into mice to assess biocompatibility and in vivo weight loss [1,41]. Remarkably, they observed a lower biocorrosion rate in the in vivo subcutaneous environment compared to in vitro simulated conditions. Histological findings from their study indicated the biocompatibility of Mg/Mg alloys with the heart, liver, skin, lung, and kidney tissues of mice.
3. Zn and Zn based alloys and composites
Zinc (Zn) is another prominent biodegradable metal, prized for its high biocompatibility and abundance in the human body. Zn2+ ranks as the second most abundant element within the human body, primarily concentrated in bones and muscles [106]. Its mechanical and physical properties align well with those of human bones, surpassing stainless steel and titanium in compatibility. Remarkably, approximately 600 enzymes rely on Zn2+ for their proper functioning and structural integrity. However, it's important to note that while Zn is physiologically relevant, it is not typically regarded as a primary bioresorbable medical material [107]. There has been limited research on the potential medical applications of Zn, with fewer than 100 publications over the past decade, particularly in the context of cardiovascular and orthopedic applications. Furthermore, the perceived potential toxicity of many Zn alloyshas restricted their use in biomedical applications [108].
3.1. In vivo performance of Zn-based materials
Zinc (Zn) has emerged as a promising candidate for degradable metallic bioresorbable stents, and extensive studies have been undertaken to assess its bioactive effects, cytocompatibility, and corrosion behavior. Zn plays a vital role as a trace element in various biological reactions [109]. In several in-vivo tests, Zn has demonstrated good hemocompatibility [110], strong antiatherogenic properties [111], and non-cytotoxicity to endothelial cells. Pure Zn exhibits a corrosion rate between manganese (Mn) and iron (Fe) when exposed to Hank's Solution [2]. The in vivo degradation of Zn-based implants investigated by Törne et al. [112], revealed the formation of passivation films comprising inorganic components and biomolecules on Zn surfaces when immersed in plasma and whole blood, as compared to phosphate-buffered saline (PBS) and Ringer's solution evaluations, respectively. The mechanism behind the formation of this protective layer was also studied in whole blood using electromechanical measurements [113]. It's worth noting that the protein concentration in human blood serum (around 60–80 g L−1) significantly impacts implant corrosion behavior, and biodegradable Zn alloys exhibit different corrosion behavior in the presence of proteins [114].
Studies on the in vivo biocompatibility of Zn-based biomaterials for orthopedic applications are summarized in Table 3. Several studies have indicated that a high concentration of Zn ions released during degradation could lead to fibroblasts and cytotoxicity [[115], [116]]. Su et al. [111] reported the harmful effects of pure Zn on the differentiation and proliferation of MC3T3-E1 cells, and a similar effect was observed on MG-63 cells [117,118]. Krus et al. [114] suggested that while pure Zn can cause genotoxicity, alloying it with other metals, such as Mg (Zn–3Mg), reduces this risk in the human body. Kubasek et al. [119] found that Zn-0.8 Mg alloy did not result in any significant damage to U–2OS cells. Zn-0.05 Mg and Zn-0.5 al-xMg alloys were reported to positively impact cell proliferation. Furthermore, the degradation of Zn–Mg alloy stimulates osseointegration with the assistance of bone-like components like hydroxyapatite. Calcium (Ca) and strontium (Sr) were also reported to improve cytocompatibility [120].
Table 3. Surface modification approaches summary of Zn-based biomedical implants for orthopaedical applications.
| Substrate | Techniques | Main layer | Layer thickness | In vitro corrosion rate | In vitro biocompatibility | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Corrosion Medium | Electromechanical test | Cell line | Hemo compatibility | Cyto compatibility | |||||
| Pure Zn (99 %) | Collagen solution for 20 min at room temp. Dip coating in 1 mg/ml rat tail | Type 1 collagen | 2.4 μm | Hank's Solution | 0.088 ± 0.009 | Human endothelial cell (EA. hy926) |
Hemolysis rate was under 5 % Platelet activation on the surface |
Cell proliferation was improved Cell differentiation and proliferation were improved |
[3] |
| Pure Zn (99.9 %+%) | Immersion in M H3PO4 solution, pH 2–3 and 0.08MZn(NO3)2. | Zinc Phosphate | 4–6 μm | Hank's Solution | 0.005 ± 0.001 | Murine calvarial preosteoblast (MC3T3-E1) |
Good hemocompatibility was obtained in both hemolysis tests and platelet adhesion There were no signs of thrombogenicity |
Cell differentiation, proliferation, and adhesion were improved | [3,4] |
|
Zn–1Mg Zn–1Mg 0.5Ca |
Immersion in Dulbecco's Modified Eagle medium for 1 day under a humidified atmosphere with 5 % CO2 at 36 °C | Zn (OH)2 and ZnO | Murine mesenchymal stem cell (MSC) | Improved proliferation and adhesion | [5] | ||||
|
Atomic layer Deposition Magnetron sputtering |
zirconium (TDMAZ) as Zr precursor (heated to 110 °C), deionized water Target: high purity C, chamber pressure: |
ZrO2 Diamond-like carbon |
−100 μm | Hank's solution | 0.055 ± 0.015 | Murine osteoblast precursor cell (MC3T3-E1) | Improved cell proliferation and adhesion | ||
The summary of in vivo research in cardiovascular applications is depicted in Fig. 5. For instance, Yang et al. [121] implanted pure Zn stents in the abdominal aortas of rabbits and observed no severe inflammatory response, thrombosis formation, platelet aggregation, or intimal hyperplasia. The mechanical integrity of the stents remained intact. Zhou et al. [122] similarly reported the absence of thrombosis formation and inflammation when implanting Zn-0.8Cu alloy stents in coronary arteries for two years. Herelin et al. [123] implanted Zn–3Ag alloy stents in the iliofemoral arteries of juvenile pigs, resulting in excellent healing without vascular occlusion or thrombosis. Jin et al. [124] implanted Zn-xMg alloys in the abdominal aorta of rats, observing only mild inflammation and intimal activation. Zhao et al. [125] implanted Zn-0.1Li alloys in the abdominal aorta of rats and reported a mild inflammatory response. Bowen et al. [126] used Zn-xAl alloy strips in the abdominal aorta of rats, where they observed a moderate inflammatory response after implantation.
 Fig. 5. Mechanism of porous Zn–Mg-TCP scaffolds for in vivo osteogenesis (a) Schematic of in vivo osteogenesis and degradation products; (b) Microstructure of degradation products and new bone tissue development with corresponding EDS analysis [58].
Fig. 5. Mechanism of porous Zn–Mg-TCP scaffolds for in vivo osteogenesis (a) Schematic of in vivo osteogenesis and degradation products; (b) Microstructure of degradation products and new bone tissue development with corresponding EDS analysis [58].3.2. Surface modification for Zn and Zn-based materials
Researchers have utilized surface modification techniques to enhance the mechanical properties of Zn-based biomaterials [127]. These methods are considered effective as they modify the surface characteristics of Zn-based biomaterials without affecting the core matrix properties. The summary of various surface modification methods for Zn-based biomaterials in orthopedic applications is presented in Table 3. Although there are limited studies available on surface modification for Zn-based materials in orthopedic applications, chrome-based coatings have gained popularity as a method to improve corrosion resistance, adhesion, and wear reduction [128]. However, due to their toxic and carcinogenic nature, these coatings have been banned worldwide [129]. A commonly used chemical conversion treatment is Zn–P coating, which enhances surface characteristics such as wear resistance, corrosion resistance, and adhesion.
Zinc participates in the bundle of biological functions including gene expression, apoptosis regulation, signal transduction, and DNA metabolism. Zinc is also an efficient component in energy production and enzyme synthesis. They are reported to improve corrosion resistance by developing a protecting film on the surface of metal [130,131]. Fig. 6(a) explains the mechanism of surface modification (chemical etching, crimpling of balloon, inflation of pressure balloon burst) of the Zn–Cu stent two years implant in the coronary arty while Fig. 6(b) represents the electrophoretic deposition coating method to increase the properties of degradable Zn alloy implants.
 Fig. 6. Zn–Cu stent surface morphology after (a) chemical polishing; (b) crimping (c) inflated pressure; and (d) balloon-burst. The insert of (a) is the XRD pattern of the Zn–Cu stent [132]. Schematic diagram of γ-PGA-g-DA/Cu coatings on Zn alloy surface by electrophoretic deposition method [133].
Fig. 6. Zn–Cu stent surface morphology after (a) chemical polishing; (b) crimping (c) inflated pressure; and (d) balloon-burst. The insert of (a) is the XRD pattern of the Zn–Cu stent [132]. Schematic diagram of γ-PGA-g-DA/Cu coatings on Zn alloy surface by electrophoretic deposition method [133].4. Fe-based alloys and composites
Iron has been widely employed as an engineering material due to its numerous advantages, including exceptional machinability, outstanding mechanical properties, and cost-effectiveness [134]. These characteristics make iron-based materials an excellent choice for the development of biodegradable materialsfor medical applications. The literature suggests that the use of Fe-based materials primarily falls into three categories: bone implant materials for surgical procedures [52], intravascular stents, and scaffolds in bone tissue engineering [135]. The utilization of non-degradable metal implants, such as plates and screws [7], in bone surgeries has been associated with adverse effects, including toxicity [136]. The removal of such implants can lead to issues like pain or defects at the implantation site [8]. Fe-based metallic implantsaddress these shortcomings as they possess mechanical properties similar to stainless steel, meeting the requirements for bone repair [137,138]. Another crucial application of Fe-based materials is in scaffolds that provide favorable conditions for tissue repair. Thanks to their remarkable biodegradability and mechanical integrity, iron-based porous scaffolds are primarily employed for the repair of hard tissues like bones. Scaffolds made from iron-based materials overcome the limitations of non-degradable polymer scaffolds [139,140].
4.1. In vivo and in vitro responses of Fe alloys
Iron (Fe) has exhibited excellent biocompatibility in various in vitro studies. When pure electroformed Fe was used in cardiovascular stents, it was found not to limit the metabolic activity of rat smooth muscle cells (SMCs) and prevent cell proliferation [141]. Chen et al. [142] also reported no cytotoxicity of pure industrial-grade Fe and suggested that it displayed excellent hemocompatibility and biocompatibility. Lin et al. [143] examined the in vitro cytotoxicity of nitride iron stents and found that the availability of oxygen and the extraction medium were crucial during incubation and extraction. Extracts with a high iron concentration showed no cytotoxicity to L929 cells. However, the biocompatibility of Fe alloys was observed to be influenced by alloying elements [117,118]. Research on alloying cells suggested that the alloy concentration in the medium significantly impacted the inhibition effect. Studies also investigated multicomponent Fe alloys. Schinhammer et al. [144] studied the cytocompatibility of Fe–Mn–C (-Pd) alloy with human umbilical vein endothelial cells (HUVECs) and found that metabolic activity and cell viability were highly dependent on the concentration of the extract medium. Cell viability was also closely related to the corrosion rates of Fe alloys [145], with high corrosion leading to low cell viability [10]. Orinak et al. [146,147] studied porous Fe-based scaffolds in vitro static cell cultures and reported significant changes in morphology, improved blood compatibility of scaffolds, and cell viability. However, fibroblasts died after 24 h, suggesting that cytotoxicity evaluation should be conducted in a dynamic environment to prevent the accumulation of excessive corrosion byproducts. Fe/bioceramics were found to improve cell viability [148,149].
Surface modification has also played a crucial role in enhancing the biocompatibility of Fe-based materials, a primary criterion for selecting biomaterials. They must demonstrate slow corrosion and biocompatibility [11]. Table 4 below summarizes the results of in vivo tests of Fe-based materials. The first in vivo testing of iron stents was conducted by Peuster et al. [150], who implanted pure Fe stents in the descending aorta of white rabbits. During the follow-up period, no thromboembolic complications or side effects were observed. Furthermore, no prominent systemic toxicity, inflammatory response, or neointimal proliferation was observed (Fig. 7). Subsequently, the same stents were implanted in the aorta of pigs and monitored for over a year, revealing no Fe-related organ toxicity or Fe overload. No local toxicity was observed due to corrosion products. In another short study, Fe stents were implanted in the coronary arteries of juvenile pigs for 28 days to assess efficacy and safety [12]. No thrombosis or particle embolization was noted, and histomorphometry exhibited no fibrin deposition or excessive inflammation.
Table 4. Summary of in vivo responses of Fe-based alloys.
| Implants | Results | Ref |
|---|---|---|
| Fe Stent |
Implantation in Mini Pigs: No local or systemic toxicity Implantation in White Rabbit: No thromboembolic complications or adverse events Implantation in domestic juvenile pigs: No stent particle embolization or thrombosis; no traces of excess inflammation or fibrin deposition No adverse events or adverse effects on local tissues Slight luminal harm and relative stenosis post 12 months implantation Mild hyperplasia, absence of any sort of inflammation or other adverse reactions |
[140] [144] [141] [145] [146] [147] |
| Fe-wire | The implant resulted in the formation of neutrophils, macrophages, and multinucleated giant cells. The Fe-wire covered with granulation tissue; dilation of blood vessels | [117] |
| Fe–21Mn (–0.7C)– 1Pd (cylindrical pins) | The implant was well integrated in all animals and showed no signs of inflammation or toxicity in animals | [143] |
| Fe– (0.5–6.9) Mn (cylindrical plate) | No signs of infection or inflammation | [148] |
| 1.5Fe–Mn–Si (small piece) | Demonstrate excellent biocompatibility. No adverse reactions observed | [149] |
| Fe–HA/BCP/TCP (small slice) | No negative reaction observed for up to 70 days | [150] |