Introduction
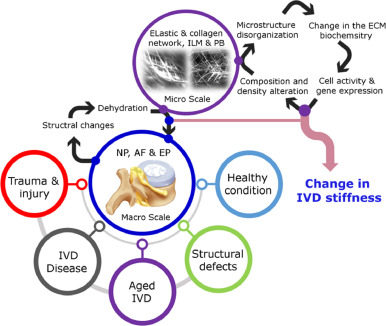
The intervertebral discs (IVD) are fibrocartilaginous joints that locate between adjacent vertebrae in the spinal column. By occupying approximately 25% of the spinal column's height, IVDs enable vertebral motions and serve as a shock-absorbing system [1]. From a multiscale anatomical point of view, an IVD is composed of Nucleus Pulposus (NP) at the center and surrounding Annulus Fibrosus (AF) which are situated between cartilaginous endplates (EP) that interface with the vertebral bodies [2]. The NP is a soft, gel-like material at the center of the IVD and the AF is arranged in a concentric lamellar structure with a higher stiffness compared to the NP [3, 4]. The AF and NP mainly consist of collagen type I and II, respectively. At the interface of the NP and the AF, a region which is known as the transition zone, a transient structural and compositional gradient is often observed. At the sub-tissue level, parallel collagen bundles are organized at alternating angles (from ± 25⁰ to ±40⁰) in adjacent lamellae of the AF [5]. The adjacent lamellae and collagen bundles in the AF are connected by a well-organized and continuous elastic network, creating regions (interlamellar matrix (ILM) and partition boundaries (PB)) with a high density of elastic fibers [6], [7], [8]. The AF elastic network transforms into a honeycomb structure at the IVD transition zone and develops a radially oriented elastic network within the NP [9, 10]. The IVD extracellular matrix (ECM), which largely contains proteoglycans, interacts with collagen and elastic fibers assembly and develops an osmotic environment essential for water retention and IVD resilience [11].
The IVD's intricate hierarchical structure at the multi-scale (from mm to μm), including quantity, type, and organization of the components, is pivotal to the IVD's physiological function and controls the mechanical properties [12], [13], [14], [15]. It is believed that the mechanical strength of the IVD is contributed to collagen fibers while elastic fibers regulate the elastic properties and are responsible for returning the IVD to its original shape after deformation [16], [17], [18], [19], [20]. In addition, the biochemical characteristics, composition, and structural organization of the ECM at the micro and nano level play a crucial role in regulating the biological activities of cells (i.e. cell phenotype and differentiation as well as gene expression) [21], [22], [23], [24]. Among different mechanical properties, the stiffness of the IVD which is manifested by the deformation resistance in response to an applied force is an effective toolbox for diagnosis in pathology [25], [26], [27]. Largely affected by the structural properties, composition, and biochemical characteristics, the IVD stiffness is closely related to function and health status [28], [29], [30]. Therefore, the quantification of IVD stiffness may show the IVD pathologic and degeneration states and is often indicative of age [31], [32], [33] (Fig. 1).
 Fig. 1
Fig. 1The stiffness of the IVD is measured by standard mechanical testing which involves force–displacement characterization. The IVD is exposed to a compressive or tensile force while deformation (displacement) is measured [34], [35], [36], [37], [38]. The average slope for the force–displacement curve indicates the stiffness of the region of interest. Providing bulk stiffness and being invasive are the major clinically non-relevant drawbacks of this approach. Other drawbacks are difficulties in performing mechanical tests at the micro and nano levels and the use of intact tissue which restricts the evaluation of disease-related changes in microstructure to local changes in IVD stiffness. There are other techniques such as rheology and atomic force microscopy which allow IVD stiffness measurement across length scales; however, the abovementioned limitations still exist which restricts the application in clinical practice [39], [40], [41], [42], [43]. Standard imaging techniques such as Magnetic Resonance Imaging (MRI) and Computed Tomography (CT scan) have also been used to indirectly assess the IVD mechanical properties based on the measurement of relaxation time and attenuation coefficient, respectively [44], [45], [46], [47], [48], [49]. The narrow range of values affects the accuracy of measurements and is considered the main disadvantage of these imaging modalities. In this regard, elastography, which is a mechano-imaging technique, provides non-invasive approaches to quantify several IVD mechanical properties such as shear modulus, stiffness, and strain including their spatial distributions; hence, is the preferred option [50, 51]. One of the commonly used elastography techniques in clinical practice is ultrasound-based methods due to their ease of use and relatively low costs [52], [53], [54], [55]. These approaches have shown promising results in the assessment of the liver to identify fibrosis tissue and the application is growing for other soft tissues such as breast, brain, kidney, lymph, and prostate [56], [57], [58], [59], [60], [61], [62]. However, ultrasound imaging is not a conventional imaging technique for some organs including IVD. Because this imaging technique can't provide structural information and identify IVD abnormalities as accurately as MRI does. Limitations to further broader ultrasound utilization have been the uncertainty in teaching the imaging modality and the lack of reproducible protocols for ultrasound routine and interpretation. These drawbacks have led to a large body of inconsistent evidence in diverse settings of spine pathology. MRI is more clinically popular for IVD imaging, and therefore, Magnetic Resonance Elastography (MRE) is more likely to provide a quantitative understanding of IVD tissue microstructural integrity and degeneration through the measurement of IVD stiffness.
Apart from IVD, different clinical settings use the MRE concept to identify abnormal stiffness of various soft tissues; eg liver, breast, brain, kidney, etc., for early detection of fibrosis and cancer [63], [64], [65], [66], [67], [68]. Since at the same structural level the stiffness likely varies between healthy and diseased soft tissues, the measurement of stiffness can be used for disease detection. Of particular importance, characterization of the tissue stiffness may contribute to the detection and diagnosis of degenerative diseases [69], [70], [71], [72]. MRE, as a novel quantitative method to measure the stiffness of soft tissue non-destructively, has been used as a diagnostic marker for tissue pathology [73], [74], [75], [76], [77], [78], [79], [80] (Table 1). As such, the non-invasive assessment of mechanical properties of soft tissues using MRE techniques has attracted substantial attention.
The degeneration of IVD is associated with the development of low back pain, which is the most frequently reported musculoskeletal disorder in adults. Despite the huge economic, clinical, and social burdens of low back pain, the molecular mechanisms underpinning tissue stiffening and degeneration are not well understood. In addition, a reliable clinical approach for the quantification of the mechanical properties of human IVD to detect early degeneration or identify the pathophysiology beneath that is currently lacking. In addition, a significantly lower number of MRE studies have been performed to quantify the mechanical properties of IVD compared to other tissues. Motivated by the current gaps and potential clinical competence of MRE to distinguish IVD pathologies, the current paper aims to review the application of MRE in the study of IVD biomechanics. The current study identifies the basic elements of MRE, including hardware design and software development, that have been used for the mechanical characterization of IVD to explore the feasibility and potential diagnostic value of the technique for future clinical applications in vivo.
Review methodology
A systematic search through Web of Science Core and PubMed was conducted including English-written and published in peer-reviewed journals. The keyword search terms used were “Magnetic Resonance Elastography” AND “intervertebral disc” covering a period from 2015–2021. Performing a search using the “Magnetic Resonance Elastography” keyword found 5711 and 1754 published papers in PubMed and Web of Science Core, respectively. 16 papers were found when the search was refined using the combination of two keywords. The bibliographies of the 16 papers were used to identify additional relevant papers that did not appear in the keyword search. Apparently, less than 0.3% of total MRE publications are relevant to the IVD indicating the importance of the current study.
Magnetic resonance elastography (MRE)
MRE is a quantitative elastographic imaging technique based on using an MRI scan to detect tissue strain and calculate tissue stiffness under the application of dynamic or static mechanical forces. MRI provides precise control of magnetic field gradients at different imaging planes to identify deep tissue structures in both healthy and injured states. In addition, the mechanical excitation of tissue using custom-made force pulse sequences during MRI imaging will allow direct measurements of tissue displacement and wave velocity to measure tissue elasticity. The stiffness (shear modulus) of tissue allows clinicians to identify tissue pathology based on differences of mechanical properties often observed between healthy and diseased tissues. MRE often involves three steps; tissue excitation, MRE imaging, and inversion (Fig. 2). The first stage is tissue mechanical excitation where the tissue of interest is exposed to mechanical motions (often cyclic) using different external mechanical sources. Image acquisition, the second stage, involves an MRE imaging sequence which is applied to image the tissue displacements. The last stage is image processing which encompasses the conversion of tissue displacement data into a stiffness map using mathematical algorithms (i.e. inversion).
 Fig. 2
Fig. 2The excitation stage involves the use of different external sources to generate mechanical waves for tissue vibration or deformation. As a result of mechanical deformation, protons in the target tissue oscillate and develop a measurable spin displacement using the MRE technique. Piezoelectric actuators, rotating eccentric masses, rotary and linear electromechanical motors, electromechanical voice coils, pneumatic systems, and air compressors have been widely used in MRE for both preclinical research and clinical applications [81, 82] (Fig. 3).
 Fig. 3
Fig. 3In addition, internal sources such as heart rate and blood flow pulses in vessels have been examined in few studies to excite tissue. External sources of mechanical waves can be categorized as active and passive entities to minimize electrical and magnetic interferences with the MRI system. The active parts are often sited outside the MRI suite, while the passive actuators are placed inside the MRI bore. To achieve appropriate waveforms and suitable amplitudes, proper mechanical coupling with the imaged tissue is crucial and may affect the quality of data acquired. In most commercial MRE systems that utilize pneumatic generators, the driver system is placed outside the MRI suite to generate compressed air oscillating through flexible tubes to activate passive actuators located in the MRI bore [83]. The use of MRI-compatible material is not necessary to make passive actuators, making them cost-effective compared to the active ones. In addition to the active and passive actuators, some studies have utilized electromagnetic coils to excite tissue through the MRI main field. For the electromagnetic coil, the motion displacement (Fig. 3) is usually perpendicular to the coil [84, 85] and the number of turns in a coil and the current intensity passing through can tune the amplitude of the tissue excitation.
These methods of excitations are often used to generate time-harmonic plate, needle, and concentric shear waves (often < 200 Hz) or a continuous static compressive wave within the tissue of interest (Fig. 3) to reach a steady-state of deformation essential to optimize the signal-to-noise ratio. The measurement of tissue displacement followed by the application of a continuous static compressive force is the simplest approach and is based on the assumption that lower displacement often occurs in stiffer tissues. However, this approach is not fully reliable due to the creep and stress relaxation properties of the tissue generating artifact in measurement, and in this regard, dynamic approaches are most accurate. In the dynamic approaches, the tissue of interest can be excited at low (≈ 1 Hz) or high (20–200 Hz) frequencies using a mechanical wave. The application of mechanical forces at low frequency leads to the development of almost constant tissue stress in each imaging cycle [86, 87]. High frequencies are favorable because they generate shorter wavelengths and will enable to generate robust stiffness maps. A summary of mechanical excitation frequencies and amplitudes that have been used in MRE in vivo is shown in Table 2.
Table 2. Frequently used MRE mechanical excitation frequencies and amplitudes to measure the stiffness of different tissues in vivo.
Another important factor is the amplitude of tissue displacement. Larger tissue displacement (> 10 μm) is likely to increase the accuracy of the measurement and is an acceptable strategy when lower tissue excitation frequency is considered. However, this strategy may induce patient discomfort and more importantly lead to less reliable data due to tissue's non-linear behavior with large displacement.
Recording tissue displacement using a phase-contrast MRI imaging pulse sequence is the second step in an MRE workflow. A key challenge here is to optimize the displacement to noise ratio that is associated with the signal-to-noise ratio and the magnitude of phase offset per unit displacement of tissue. The optimization challenge involves finding a proper balance between resolution and duration of data acquisition or presenting an efficient motion encoding scheme [88]. In this regard, different pulse sequences can be used for image/data acquisition strategies including gradient-echo (GRE), spin-echo (EPI), and balanced steady-state free precession (bSSFP) techniques. GRE and EPI (mostly preferred because of a shorter scan time) are the most common sequences that are frequently used in different clinical applications. The use of oscillating motion‐sensitizing gradients enables displacement encoding in the phase of the acquired data. This will allow harmonizing the tissue excitation and image capture at one frequency, which is the most critical challenge in the data acquisition strategy affecting the time of data acquisition.
Some studies have suggested fractional encoding, which involves encoding of tissue displacement with single-cycle gradient waveforms and allows harmonizing at a lower frequency than that of the excitation, to reduce the echo time. The MRI field strength is another parameter that affects tissue displacement recording with higher field strength (> 3 T) allowing image/data quality enhancement compared to the lower field strength (i.e. 1.5 T). It is important to note that while the impact of field strength on T2 relaxation time is almost negligible, T1 relaxation time and T2* are significantly longer for 3T compared to 1.5 T magnetic field strength. This leads to a longer data acquisition time adversely impacting patient comfort and may affect the final resolution of the acquired data/images.
Inversion algorithms to estimate tissue mechanical properties from wave data are the last step in an MRE workflow [89], [90], [91]. To recover tissue mechanical properties or form a relevant image from the acquired MRE data, different inversion algorithms have been developed based on different assumptions [83, 92]. The linear mechanical behavior is one assumption, which is acceptable under small deformation. However, it is important to notice where on the stress-strain curve the tissue is during excitation and MRE data acquisition, since tissue may experience pre-conditioning for a specific physiological reason. The contribution of tissue isotropy assumption to MRE data is usually eliminated by using a spatial high-pass filter [93]. The assumption of local homogeneity in numerous inversion algorithms reduces process time and makes it simple, however, artifacts specifically for tissue boundaries are induced. These inversion algorithms use the Helmholtz equation to calculate tissue shear modulus and therefore a subtle sensitivity to noise is observed. To reduce noise sensitivity data smoothing, regularization of the inversion (sparsity-based or soft prior regularization), decoupling compressional and shear waveforms and the use of directional filters are often essential [94], [95], [96].
Magnetic resonance elastography and IVD biomechanics
With excellent capability in providing high spatial resolution and unique contrast between IVD regions, MRI is a non-invasive imaging technology that is frequently used by spine surgeons to diagnose IVD disease. MRI is one of the most frequently used modalities in IVD pathology such as structural abnormalities, degeneration, and herniation. The use of MRI multiple transmitted radiofrequency pulses to measure tissue relaxation times (i.e. T1 and T2) and identify their correlation with the IVD biomechanical properties have been the focus of several studies. These studies have been motivated by the presence of a subtle relationship between the biochemical and mechanical properties of the IVD. So far, MRE has been used in a limited number of studies to characterize the mechanical properties of the intact IVD, and to the best of our knowledge displacement-encoded-MRI technology is lacking in the IVD clinical practices. As such, most studies have used different ex-vivo animal models to measure the IVD biomechanical properties using MRE technology.
The internal deformation (2D) and strains in the human IVDs (# 7; L1/2 and L2/3) were measured using MRI (3T) before and during the application of 1 kN compression. This was the first study that presented a non-invasive technique to identify the strain fields and displacement of the intact IVDs using MRI [97]. Tissue mechanical excitation was performed using an MRI-compatible device consisting of a hydraulic cylinder that was pressurized by a nitrogen source. The study used a high-resolution T2-weighted turbo spin-echo sequence to collect midsagittal MRI images. 2D Lagrangian strains were calculated using Vic2D software to ultimately measure the axial displacement of endplates, IVD height, and the radial displacement of the AF at the mid-IVD height. The results of the study showed an average IVD height loss of 0.4 ± 0.2 mm, which was corresponded to 4.4% ±1.3% axial compressive strain. In addition, 0.36 ± 0.10 mm average radial displacement for the outer AF was reported. This study examined the feasibility of employing MRE to develop IVD strain maps (radial, axial and absolute shear) for different regions of the IVD (outer and inner as well as anterior and posterior AF, and the NP) and IVD health condition (moderate-to-severe degeneration) [97].
The mechanical deformation of degenerated rabbit IVDs (L3/4 and L4/5, #8 each), produced by annular puncture, was studied using MRI (400 MHz, 9.4 T). Both control and degenerated IVDs were bathed in phosphate-buffered saline and exposed to a cyclic 30N compressive load every 3 s (1.5 s recovery) using an MRI-compatible device [98]. Both displacement (normalized by IVD height) and strain (Green-Lagrange) patterns in the IVD were measured after 200 cycles of loading using fast-spin echo scans and a computer-controlled electro-pneumatic interface. This study revealed a transverse displacement for the NP, towards the surrounding AF, under cyclic compression. In addition, a heterogeneous strain pattern for both control and degenerated IVDs was reported while the average strain was not significantly different between control and degenerated IVDs. Since animals were killed four weeks after needle puncture, treated IVDs were more likely at their early stage of degeneration revealing similar strains for healthy and moderately degenerated IVDs. It was also stated that the use of data smoothing during image post-processing to estimate strain fields might cause detail loss, affecting MRE results. To minimize the error, utilizing a higher signal-to-noise ratio was a better option allowing the use of less destructive filtering of the displacement fields. This study revealed that using displacement encoding with stimulated echoes and a fast-spin echo is not efficient due to long imaging time (> 9 h), making the technique impractical for clinical applications [99].
In another study, the feasibility of using MRE to detect shear wave propagation in the IVD was investigated. L3/4 IVDs from baboons (#2) were harvested and exposed to mechanical shear vibration (1–1.5 kHz) using a piezoelectric driver (Fig. 4a). A full-body MRI scanner (1.5 T) with a spin echo-based MRE sequence was used to acquire displacement data, and the MRE wave images bandpass filtered using a local frequency estimation (LFE) inversion algorithm. The propagation of the shear wave at different frequencies was observed (Fig. 4b) and the corresponding nucleus stiffness, with an increasing trend with frequency (79 ± 15, 201 ± 32 and 515 ± 175 kPa at 1000, 1200, and 1500 Hz, respectively), was reported in this research [100].
 Fig. 4
Fig. 4The results of this study suggested that IVD excitation at low frequencies provides sufficient shear wave information to estimate reliable stiffness for the NP and it was not possible to measure the mechanical properties of the AF. The NP plays a critical role in the initiation and progress of the IVD degeneration process. IVD degeneration often starts at the NP and its swelling stress and average modulus are lower at the degeneration state compared to the healthy IVDs. Therefore, the author suggested the feasibility of their approach as a biomarker to identify the stage of IVD degeneration; however, the proposed method requires significant development to be used in vivo.
In another study, MRE (7 T) was used to measure the shear modulus of the NP of the human IVD (L4/5, #30) and to understand its correlation with the degeneration stage (normal, mild, severe). An electromechanical actuator composed of a solenoid coil, a pivoted shaft, and an outer acrylic cylinder was used to induce mechanical vibration to the IVD tissue (Fig. 5a). A 3D gradient echo pulse sequence was used in the study to include a motion-sensitizing gradient block, with a similar frequency to the actuator. First-order spatial derivatives of the displacement were developed and used as an inversion algorithm to calculate the shear modulus. The approach enabled visualizing displacement fields for both healthy and degenerated IVD (Fig. 5b) and revealed a significantly lower T2 relaxation time (Fig. 5c) and shear modulus (Fig. 5d) for the degenerated IVDs (mild and severe = 135 and 71 kPa, respectively) compared to the healthy ones (661 kPa), where the shear modulus was significantly correlated with T2 (r2= 0.3). In addition, this study identified a shear deformation of less than 1.0 between degenerated and normal IVDs, based on the displacement fields.
 Fig. 5
Fig. 5The main conclusion of the study was the feasibility of using MRE and the IVD NP shear modulus as a biomarker to differentiate between normal, mild, and severe degeneration. While the study presented an effective clinical tool to identify the mechanical integrity of the IVD, the lack of the inversion method to model the NP as a finite medium with biphasic characteristics was mentioned as the limitation, and therefore the relative displacements between the solid and liquid phase of the NP were not established [101].
The feasibility of multifrequency MRE of the lumbar spine to measure the NP mechanical properties was performed ex-vivo (bovine #2) and in-vivo using 16 human volunteers (IVD# = 32; L3/4 and L4/5; 25–52 years old with no history of IVD herniation and back pain). This study employed a posterior wooden plate transducer that was connected to a loudspeaker and positioned in the lumbar region of volunteers (Fig. 6a). The loudspeaker was excited by an external audio amplifier to generate five different frequencies (50 to 70 Hz; step = 5). Volunteers’ bodyweight assured appropriate contact between the lumbar regions and the actuator. The magnitude (G*) and the phase angle (φ) of the complex shear modulus were recovered by multifrequency dual elasto visco (MEDV) inversion (Fig. 6b, 6c). The results of this study for a single volunteer (investigated 7 times) and pooled data (16 volunteers) showed a significant decrease for G* as degeneration advanced, while φ remained unchanged (Fig. 6b and 6c). In addition, this study found approximately 11% diurnal changes in the mechanical parameters. This research also found that ex vivo experimental setup including probe geometry, the position of the sample, preload condition, and frequency might impact the MRE results. The study also indicated that the employed method was not suitable to assess the mechanical properties of the AF region of the IVD due to the low intensity of the MRE map. The MRE approach in this study revealed that group means values of the magnitude (G*) and the phase angle (φ) of the complex shear modulus were 5.46 ± 1.14 kPa and 1.194 ± 0.137 for L3/4 and 6.71 ± 1.52 kPa and 1.157 ± 0.201 for L4/5 levels, respectively. A significant difference in the magnitude of the shear modulus was reported between L3/4 and L4/5 levels [102].
 Fig. 6
Fig. 6The AF region of the IVD is less hydrated and stiffer compared to the NP and therefore MRE of the IVD is often restricted to the NP region. One study employed a high-frequency needle MRE (4.7 T) to characterize the shear modulus of both the NP and AF regions of bovine IVDs (# 6). IVDs were embedded in silicon to minimize dehydration, prevent proteoglycan loss, and preserve the mechanical integrity of the IVD. Silicon stiffness was set between 50 and 100 kPa to minimize wave reflection at tissue boundaries. This study utilized an amplified piezoelectric actuator to vibrate a needle (1000–1800 Hz; step = 200) and excite tissue (Fig. 7a). The needle was inserted into the center of the region of interest with a maximum displacement of 60 μm, approximately. MRE data were collected using a spin-echo sequence with sinusoidal motion encoding gradients and noises were reduced by using a Gaussian filter. A temporal fast Fourier transformation was conducted to extract the motion components at the excitation frequency. The results of this study revealed a lower displacement amplitude for the outer AF compared to the NP of the IVD (Fig. 7b). This study also observed a lower shear modulus of the NP (56 ± 0.4 kPa @ 1200 Hz) compared to the AF (85 ± 2 kPa @ 1200 Hz), while the shear modulus was increased with excitation frequency for both regions (Fig. 7c). Conventional mechanical characterizations of the IVD have identified that the AF was ten times stiffer than the NP. Consistent with that finding, the results of this study indicated stiffer AF compared to the NP by a factor of two. The suggested technique was invasive and therefore less effective for in vivo measurements. It is likely to adopt the technique and assess the mechanical property of the IVD in vivo through needle insertion; however, the risk of permanent injury is a major drawback [103].
 Fig. 7
Fig. 7In another study, the displacement and strain patterns in adjacent cervical IVDs (#10 healthy volunteers) were measured in vivo using a dualMRI (3T) approach. IVD excitation was conducted through cyclic flexion-extension motions (10⁰) that were generated by an MRI-compatible pneumatic device (Fig. 8a). The study combined a displacement encoding with stimulated echoes pulse sequence during flexion, with a single-shot fast spin-echo sequence during extension, to perform displacement-encoded MRI. The results of this study revealed that displacement was significantly correlated with the position of the cervical IVDs (Fig. 8b), while significant level-dependent differences were not found for principle (Ep1 and Ep2) and maximum shear (Esm) strains (Fig. 8c). In addition, no significant correlation between maximum strain values and relaxometry factors (T1 and T1ρ) was reported in this study, indicating the inability of each to be served as a substitute for the other. However, the collection of relaxometry (T2 and T1ρ) and dual MRI (strain and displacement) data in the same imaging session would allow comparing relaxometry-based structural with dual MRI-based functional measures. This study found that higher maximum shear strain occurred in the posterior compared to the anterior region of the IVD. In addition, this study revealed that the C5/6 cervical IVD demonstrated a higher average shear strain with maximum Ep1 and minimum Esm, while the adjacent C4/5 presented the 10% absolute Esm and minimum Ep1. This relative difference for strain patterns in healthy IVDs might explain why C5/6 is more susceptible to damage during rapid flexion [104].
 Fig. 8
Fig. 8The impact of magnetic field intensity (3T to 9.4T) on the lumbar IVD displacement patterns was the focus of another study using the human spine (# 3). The tissue was excited by a cyclic axial compression force at 445 N in the superior-to-inferior direction and displacements were encoded with stimulated echoes and true fast imaging under steady-state precession sequence. The results of the study showed similar patterns for tissue displacement in x (dx) and y (dy) directions measured at 9.4 T to those measured at 3.0 T (Fig. 9). The results of linear regression in this study revealed agreement between tissue displacement in the x-direction (dx) on the 9.4T and on the 3.0T (R2 = 0.66–0.85), and a similar outcome was observed for tissue displacement in the y-direction (dy) (R2 = 0.39–0.82). The spatial heterogeneities of the main and gradient magnetic fields as well as biological variation between IVDs were considered responsible for the large degree of displacements (R2 = 0.39–0.85) observed [105].
 Fig. 9
Fig. 9The internal 3D strain fields in the human IVDs (# 9, L4/5) were determined using MRE (7T) technique and a custom-made loading system to apply 50 N preload and 5%, 10%, and 15% compressive strains. Image processing and registration were validated with three different assessments of IVD volume, AF lamellar structure, and axial strains. The identification of strain fields, at various directions (axial, radial, and circumferential) and in different regions of the IVD including anterior, anterior–lateral, posterior, posterior–lateral, and lateral was the novel findings of this study. The results of the study showed approximately similar strain patterns for all levels of applied strains (5–15%) across all nine IVDs while negative axial strains were found predominant in all regions except for near the IVD-endplate boundaries. In addition, this study reported negligible circumferential strain near the endplates and had high positive values in the posterior and lateral regions of the IVD. A decrease in radial strain was also reported for the inner AF (positive values) towards the outer AF (negligible at the outermost layers of the AF). Important technical improvements in this study were 1) the fabrication of a custom-built loading device that allowed long relaxation times while maintaining compressive forces and hydration during MRE and 2) the development of an advanced image registration technique that was validated using IVD volume and lamellar structure of the AF. In addition, the study provided strong discussion to link the variation in magnitude of strains (mainly axial and circumferential) with IVD geometry [106].
A cross-sectional MRE (3T) study for lumbar IVDs was performed using 47 volunteers (#230 IVDs with an age range of 20–71 years; without low back pain at the time of the study) to identify the reproducibility of the MRE-derived shear stiffness measurements. Lumbar IVDs (supine position) were excited using a passive pneumatic driver (80 Hz) that was positioned between the iliac crests and L3/4 IVD. The degeneration grade of the IVDs (Pfirrmann) was determined and the stiffness of the NP and AF regions were evaluated using principal frequency analysis. The results of this study revealed that shear stiffness was not significantly different between afternoon and morning measurements for all levels of the lumbar IVDs indicating high technical repeatability for both the NP and AF regions with R = 0.92 and 0.83, respectively. The study also found that MRE shear stiffness was weakly correlated with age (R ≤ 0.32) and significantly increased with IVD degeneration [107]. Table 3 shows key findings for the mechanical characterization of IVD using the MRE technique.
Table 3. IVD MRE experiment setting and key findings.
| Sample | Tissue excitation | Main outcomes | Ref |
|---|---|---|---|
| Human lumbar IVD (in vitro) | Hydraulic cylinder (constant 1 kN compression) | The feasibility of employing MRE to develop IVD strain maps for different regions of the IVD (outer and inner as well as anterior and posterior AF, and the NP) and IVD health condition (moderate-to-severe degeneration) was examined. | [88] |
| rabbit IVD(in vitro) | Cyclic 30N compressive load every 3 s for 200 cycles | Transverse displacement for the NP, towards the surrounding AF, under cyclic compression, was observed.The average strain was not significantly different between healthy and degenerated IVDs. | [89, 90] |
| Baboon IVD(in vitro) | Mechanical shear vibration (1–1.5 kHz) using a piezoelectric driver | IVD excitation at low frequencies provides sufficient shear wave information to estimate reliable stiffness for the NP but the AF. | [91] |
| Human lumbar IVD (in vitro) | Electromechanical actuator (multi-frequency) | This study identified a shear deformation of less than 1.0⁰ between degenerated and normal IVDs. Stiffness was significantly lower for degenerated compared to health IVDs. | [92] |
| Human lumbar IVD (ex vivo) | Sound wave (loudspeaker) at 50–70 Hz | The technique was suitable to characterize NP only. This study found approximately 11% diurnal changes in the IVD mechanical parameters. | [93] |
| Bovine IVD (ex vivo) | Piezoelectric actuator (1- 1.8 kHz) | Lower displacement amplitude and shear modulus were observed for the outer AF compared to the NP of the IVD. | [94] |
| Human cervical IVD (in vivo) | A pneumatic device with cyclic flexion-extension motions (10⁰) | This study found that higher maximum shear strain occurred in the posterior compared to the anterior region of the IVD. | [95] |
| Human lumbar IVD (ex vivo) | Cyclic compression (445 N) | Similar lumbar IVD displacement patterns were observed for low (3T) and high (9.4T) magnetic field intensities. | [96] |
| Human lumbar IVD (ex vivo) | Mechanical actuator (displacement control) | This study found a correlation between the variation in magnitude of strains (mainly axial and circumferential) and IVD geometry. | [97] |
| Human lumbar IVD (ex vivo) | Pneumatic device (80 Hz) | Shear stiffness was not significantly different between afternoon and morning measurements for all levels of the lumbar IVDs. Also, MRE shear stiffness was weakly correlated with age and significantly increased with IVD degeneration. | [98] |
Future prospective
The current state of the elastography methods allows for non-invasive biomechanical characterization of the IVD in the spine to readily measure internal displacements, detect strain field distributions and calculate tissue stiffness. Advanced MRE techniques can identify the IVD mechanical properties that may lead to the detection of IVD degeneration or anatomic abnormalities and result in the development of new treatment techniques to pause degeneration progress or propose IVD regeneration. In addition, the availability of an MRI facility in almost every medical center is another advantage that makes MRE highly accessible as a potential biomarker for IVD pathology. Even though the clinical application of MRE to diagnose IVD problems is still in an early stage and more challenges and clinical considerations have to be addressed before this technology can benefit patients with low back pain and IVD problems.
Current MRE technologies utilize different actuators (including pneumatic, rigid mechanical connectors, electromagnetic, and piezoelectric) to excite IVD tissue. Pneumatic actuators and rigid mechanical connectors are relatively inexpensive; however, the compressibility of air and rigidity of mechanical connectors can affect precise control. Electromagnetic drivers are an option that provides precise control even at higher frequencies (i.e. 20 kHz). The use of an electromagnetic actuator induces a separate magnetic field that may affect the main magnetic field of the MRI and increases the risk of missile effect. Piezoelectric actuators employ electrical potential to induce shape changes with more accuracy, precise motion, and quick response to exciting IVD tissue during MRE. The drawback here is the small displacement of the actuator which may not be sufficient enough to induce and propagate proper shear waves. Moreover, piezoelectric actuators often require a higher voltage to deform which may risk patient safety.
Newer areas to develop methods for IVD excitation may include the use of laser light, wearable actuators based on electromagnetic coils or ultrasound transducers, and air-jet to produce shear waves [108], [109], [110], [111]. Advanced actuator designs to generate shear waves during MRE will be attractive when several factors are considered. Firstly, they should be user-friendly and easy to install to save time. MRI is an expensive process that is extensively used for a variety of clinical situations. Another requirement is simplicity, making MRE easy to operate while reducing maintenance costs. At the same time, it would be ideal to introduce a tissue exciting module for multiple purposes to cover different clinical applications. In this regard, the capability of the module to create different sample-specific frequencies will be crucial to achieving optimum tissue excitation.
In terms of acquisition strategies, a number of pulse sequences have been used for different MRE applications. Amongst them, the use of gradient-recalled echo and spin-echo sequences are most common in clinical practices. Table 4 shows the most frequently used MRE sequences for IVD assessment.
Table 4. The most frequently used MRE sequences and acquisition parameters for the characterization of the IVD mechanical properties.
| IVD sample | Test condition | sequence | Other acquisition parameters | Reference | |
|---|---|---|---|---|---|
| human | Ex-vivo | spin-echo | TR1 = 3000 milliseconds, TE2 = 113 milliseconds, 10 averages, total scan time 12.5 minutes | [97] | |
| Rabbit | Ex-vivo | A custom displacement‐sensitive MRI pulse sequence incorporating displacement encoding (DENSE) and fast spin-echo (FSE). | tenc = 1.0 ms, Gde =100 mT/m for DENSE.TR = 3000 ms, TE = 6.05 ms, number of echoes per excitation = 16, in‐plane spatial resolution = 100 × 100 μm2, image matrix size = 256 × 256 pixels2, number of averages = 4, and slice thickness = 1.0 mm for FSE. | [99] | |
| Baboon | Ex-vivo | Spin-echo | 30 total cycles of 1000 Hz motion-encoding gradients (2.4 G/cm), motion sensitivity = 12.9 μm/π, offsets = 4, 290/50-ms TR/TE, 8-cm FOV3, one 8-mm slice, 256 × 96 matrix, 1 NEX | [100] | |
| Human | Ex-vivo | Modified three‐dimensional gradient echo | The amplitude of the gradients= 23 mT/m with rising time of 150 μs, number of cycles = 12, TE = 16 ms | [101] | |
| Bovine | Ex-vivo & In-vivo | modified spin‐echo echo planar imaging (EPI) | TR= 1960 ms, TE= 68 ms, FoV = 256 × 208 mm2, matrix size: 128 × 104 (yielding an isotropic pixel, motion‐encoding gradient (MEG) frequency= 80 Hz, fractional encoding = 23, MEG amplitude: 30 mT/m, scan time < 8 min | [102] | |
| Bovine | Ex-vivo | Spin-echo sequence with sinusoidal motion encoding gradients | magnetic field gradient amplitude = 15 G/cm, TE = 23 to 26 ms, number of averages = 2, voxel size = 1 mm isotropic | [103] | |
| Human (Cervical) | In-vivo | phase-contrast-based displacement encoding | FoV: 270 × 270 mm2, Matrix: 256 × 128 pixels2, Slice Thickness: 4 mm, Views Per Segment: 64, TR = 1.2 s, Number of Slices: 26 | [104] | |
| Human | Ex-vivo | displacement encoding with stimulated echoes (DENSE) and a true fast imaging under steady-state precession (TrueFISP) sequence for 9.4 T scanner & single-shot fast spin echo (SSFSE) for 3T scanner | (TE) = 1.6 ms, FoV = 64 mm × 64 mm, spatial resolution = 234 μm × 234 μm, and slice thickness = 2 mm for 9.4 T scanner & TE = 62 ms, FOV = 180 mm × 180 mm, spatial resolution = 703 μm × 703 μm, slice thickness = 3 mm for 3T scanner | [105] | |
| Human | Ex-vivo | 3D turbo spin-echo | TR/TE = 3000/34 ms, matrix = 256 × 256 × 32, turbo factor = 7, fat suppression, Scan time = 2.8 h | [106] | |
| Human | In-vivo | gradient-recalled-echo | TR/TE = 37.5/18.55 ms; FoV = 300 × 300 mm; acquisition matrix, 256 × 64 reconstructed to 256 × 256; flip angle, 25°; section thickness, 5 mm or 2.5 mm; MR elastographic phase offsets = 4 | [107] | |
| 1 - TR: Repetition Time, 2 - TE: Time to Echo, 3 – FoV: Field of View | |||||