Highlights
-
•
Thermophilic bacteria growing >50 °C provide advantages for industrial production.
-
•
Parageobacillus thermoglucosidasius is a promising thermophilic platform organism.
-
•
It has a versatile metabolism, such as partial capacities to use hemicellulose.
-
•
A rapidly increasing number of plasmid and genome-engineering tools is available.
-
•
Demonstrated tools and examples show potential to widen substrate and product range.
Abstract
Parageobacillus thermoglucosidasius is a thermophilic and facultatively anaerobic microbe, which is emerging as one of the most promising thermophilic model organisms for metabolic engineering. The use of thermophilic microorganisms for industrial bioprocesses provides the advantages of increased reaction rates and reduced cooling costs for bioreactors compared to their mesophilic counterparts. Moreover, it enables starch or lignocellulose degradation and fermentation to occur at the same temperature in a Simultaneous Saccharification and Fermentation (SSF) or Consolidated Bioprocessing (CBP) approach. Its natural hemicellulolytic capabilities and its ability to convert CO to metabolic energy make P. thermoglucosidasius a potentially attractive host for bio-based processes. It can effectively degrade hemicellulose due to a number of hydrolytic enzymes, carbohydrate transporters, and regulatory elements coded from a genomic cluster named Hemicellulose Utilization (HUS) locus. The growing availability of effective genetic engineering tools in P. thermoglucosidasius further starts to open up its potential as a versatile thermophilic cell factory. A number of strain engineering examples showcasing the potential of P. thermoglucosidasius as a microbial chassis for the production of bulk and fine chemicals are presented along with current research bottlenecks. Ultimately, this review provides a holistic overview of the distinct metabolic characteristics of P. thermoglucosidasius and discusses research focused on expanding the native metabolic boundaries for the development of industrially relevant strains.
Keywords
Parageobacillus thermoglucosidasius
Metabolic engineering
Alternative feedstocks
Microbial cell factories
Hemicellulose utilization
Thermophilic organism
1. Introduction
Bio-based processes have a central role in the bioeconomy offering significant advantages in terms of environmental impact as opposed to conventional petrochemical-based production practices (Antranikian and Streit, 2022; Ewing et al., 2022). Currently, most of the bioprocesses for bulk chemical production are categorized as first-generation because the primary feedstocks for bioproduction consist of sugarcane syrup or corn starch (Mohr and Raman, 2013). Whilst the substitution of petrochemical-based production with first-generation bioprocesses represents a positive move towards accomplishing reduced CO2 emissions, the exploration of alternative methodologies such as the use of second-generation non-edible feedstocks or waste streams has been pointed out as a more sustainable option (Liao et al., 2016; Hassan et al., 2019). The valorization of waste feedstocks is considered to be a sustainable and value-adding practice; however, the implementation of such processes can be hindered by, for example, the availability of an appropriate microbial host for the bioconversion. As previously discussed by others (Blombach et al., 2022), specific attributes such as the ease of handling, the genetic accessibility, the innate ability to synthesize particular products, and the metabolic versatility are likely to render one microbe more advantageous when compared to another.
Biotechnological mesophilic model organisms such as Escherichia coli (Pontrelli et al., 2018), Saccharomyces cerevisiae (Lian et al., 2018), Bacillus subtilis (Gu et al., 2018), Pichia pastoris (recently renamed Komagataella pfaffi) (Peña et al., 2018), and Corynebacterium glutamicum (Becker et al., 2018) possess most of the abovementioned favorable characteristics and have been successfully implemented in industrial bioprocesses, as discussed by Calero and Nikel (2019). On the other hand, thermophilic microorganisms have gained ground in scientific and industrial research as they thrive at elevated temperatures, benefitting from increased reaction rates associated with higher temperatures and ameliorating expenses incurred on bioreactors’ cooling. Moreover, bioprocesses at high temperatures, which are based on thermophilic organisms benefit from an increased substrate solubility as well as reduced viscosity, which may lead to increased productivities. Apart from being thermophilic, the capacity to use complex feedstocks like lignocellulose (a polysaccharide found in plant cell walls) is a beneficial trait for microbes in the bio-based industries. Lignocellulose, which is abundant as a waste by-product from agricultural and logging activities (Arevalo-Gallegos et al., 2017; Sankaran et al., 2021), holds significant potential for biotechnological applications. The bioconversion of lignocellulosic material relies on enzymatic conversion of the polysaccharides into smaller sugars, a step that can be accelerated by the increased reaction rates that thermophilic enzymes display at their optimal temperature of function (Turner et al., 2007). Hence, thermophilic enzymes and thermophilic cell factories have been proposed for use in lignocellulose fermentation (Turner et al., 2007; Svetlitchnyi et al., 2013). Another notable characteristic of thermophiles is that they are a source of thermostable enzymes, which typically exhibit high solvent tolerance and resilience toward harsh conditions (Sharma et al., 2019). This in turn makes these enzymes a more favorable choice for applications in lignocellulose conversion, starch conversion, and paper/textile manufacturing when compared to enzymes of mesophilic origin (Rigoldi et al., 2018).
In recent years, biotechnological research has focused on the isolation and metabolic engineering of thermophiles that exhibit optimal growth at moderately high temperatures ranging from 50 °C to 80 °C (Escuder-Rodríguez et al., 2018). However, their genetic modification is hampered by a multitude of challenges, including in some cases, the limited availability of selection markers, a paucity of shuttle plasmid vectors for genetic engineering, and a lack of effective transformation methods (Le and Sun, 2022). On the other hand, there are certain thermophilic species belonging to bacilli, clostridia, and pyrococci that are genetically accessible and demonstrate proficiencies in the catabolism of complex substrates, rendering them more desirable than other species. Among these microorganisms, Parageobacillus thermoglucosidasius a Gram-positive, endospore-forming, moderately thermophilic bacterium with an optimal growth temperature of 60 °C (Yang et al., 2021) has emerged as an attractive thermophilic microbial platform for production of chemicals (Hussein et al., 2015). P. thermoglucosidasius was also previously named Bacillus thermoglucosidasius, Geobacillus thermoglucosidasius and Geobacillus thermoglucosidans (Najar and Thakur, 2020). This species was first isolated from soil (Suzuki et al., 1983), but it has also been found in other areas on earth such as marine sediments or the dessert due to atmospheric transport and its extreme longevity in the form of spores (Zeigler, 2014). The genomes of P. thermoglucosidasius strains, of which seven have been completely sequenced (NCIMB 11955T, DSM 2542T, TM242, 23.1, BH4-1, C56-YS93, and TNO-09.020), have sizes ranging from 3.7 to 4.0 Mb, can contain one or two large plasmids, have a GC content of about 44%, and possess 3.800 to 4100 genes of which about 3600–3850 encode proteins. Furthermore, P. thermoglucosidasiusexhibits a versatile metabolism as being capable of utilizing both C5 and C6 sugars (Liang, 2021; Bidart et al., 2023), is also capable of degrading the hemicellulose part of lignocellulose (Ibenegbu and Leak, 2022) and can utilize CO to generate energy (Aliyu et al., 2021a). This metabolic versatility of P. thermoglucosidasius and its ability to grow in moderately high temperatures both under aerobic and anaerobic conditions are traits with industrial relevance, which arguably showcase the high potential of the species to be implemented in bio-based processes.
During fermentation, P. thermoglucosidasius generates acetate, lactate, formate, succinate, and ethanol depending on the fermentation conditions (Liang, 2021). For example, under aerobic conditions acetate accumulates at higher yields than other compounds, while under microaerobic conditions lactate and ethanol dominate in the fermentation mixture (Liang et al., 2021). Additionally, some P. thermoglucosidasius strains display CO conversion to H2, which is a unique feature for a facultatively anaerobic microbe (Aliyu et al., 2021b). Recently, a genome-scale metabolic model was developed for P. thermoglucosidasiusrevealing the minimal requirements for growth, thus enabling for first time the growth of the species in true minimal media (Mol et al., 2021). All in all, P. thermoglucosidasius has been increasingly garnering attention as a thermophilic model microorganism, as it is genetically accessible, can be cultivated in minimal media, displays hemicellulolytic activity and can grow on both C5 and C6 sugars contained in lignocellulosic feedstocks. Several strains of P. thermoglucosidasiushave been isolated from different sources. The type strain DSM 2542 has been one of the most used to overproduce several bulk or fine chemicals (Section 4). Other strains, such as the C56-YS93 display increased hemicellulose conversion capabilities (Section 3.1).
Here, we showcase the potential of P. thermoglucosidasius as a microbial chassis for use in bio-based processes. First, we briefly review several molecular biology tools specifically designed for use in P. thermoglucosidasius. Next, we focus on the substrate utilization capabilities, including lignocellulose valorization and CO-mediated hydrogen production. Last, we provide an overview of the biosynthesis of diverse products demonstrated in P. thermoglucosidasius strains.
2. Molecular tools effective for host engineering in P. thermoglucosidasius
Research on fundamental aspects as well as host engineering of P. thermoglucosidasius has been possible due to multiple molecular tools tailor-made for use in this species (Kananavičiūtė and Čitavičius, 2015; Drejer et al., 2018). Examples of inducible promoters are listed in Table 1. The PtetR system is composed of a tetracycline repressor (TetR) and a tet promoter. The TetR repressor is thermostable at temperatures up to 60 °C blocking the expression of a gene of interest controlled by the tet promoter. The system is induced either by adding the inducer anhydrotetracyline or by increasing the temperature above 65 °C at which the TetR repressor becomes inactive and the promoter is activated (Mol, 2022). Additionally, a variety of constitutive promoters has been developed for use in P. thermoglucosidasius (Table 1). Reeve et al. constructed a library of different strength promoters based on the strong PRpls promoter, which shows constitutive expression in both E. coli and P. thermoglucosidasius (Reeve et al., 2016). In the same study, the authors developed a ribosome binding site (RBS) library; thus increasing the variability of available transcription and translation regulatory elements in P. thermoglucosidasius and also constructed a modular plasmid set (pGxxx), which included several reporter proteins, antibiotic resistance markers as well as Gram positive and Gram negative replicons (Table 2). Similarly, the pMTL6000 plasmid series showcases a comparable concept of modular vectors (Sheng et al., 2017; Madika et al., 2022). pMTL6000 vectors encompass a wide selection of Gram-positive replicons (Table 2). They also contain one new antibiotic marker (aad9) conferring resistance to spectinomycin for use in Gram-positive bacteria, seven Gram-positive replicons, four Gram-negative replicons (for cloning in E. coli), and two reporter genes. Whilst disparities can be identified between the two modular systems, both of them were designed to facilitate ease of rearrangement via classic restriction-ligation cloning as the interchangeable parts are flanked by unique restriction enzyme sites. Arguably, the development of modular plasmid systems has increased the available options beyond the pNW33n (Hussein et al., 2015) or pUCG18 (Taylor et al., 2008), which used to be the traditional vectors of choice in many P. thermoglucosidasius studies. Indisputably, the development of modular plasmid vectors are forecasted to accelerate the metabolic engineering of P. thermoglucosidasius.
| Name | Type | Reference |
|---|---|---|
| Pβglu | Cellobiose-inducible promoter | Bartosiak-Jentys et al. (2013) |
| PxylA | Xylose-inducible promoter | Pogrebnyakov et al. (2017) |
| TetR-Ptet | Tetracycline-inducible promoter | Mol (2022) |
| Pldh | Aeration-sensitive promoter | Bartosiak-Jentys et al. (2012) |
| PRpls library | Constitutive promoters | Reeve et al. (2016) |
| PUp2n38 | Constitutive promoter | Bartosiak-Jentys et al. (2013) |
| eCGP123 | Fluorescent reporter protein | Lau et al. (2021) |
| β-glucosidase | Reporter enzyme | Bacon et al. (2017) |
| PheB | Reporter enzyme | Bartosiak-Jentys et al. (2012) |
| mCherrya | Fluorescent reporter protein | Reeve et al. (2016) |
| sfGFP | Fluorescent reporter protein | Reeve et al. (2016) |
| sfGFP(N39D/A179A)b | Fluorescent reporter protein | Frenzel et al. (2018) |
| CasIB | Endogenous CasIB | Yang et al. (2023) |
| GeoCas9 | Cas9 protein | Harrington et al. (2017) |
| stCas9c | Cas9 protein | Horvath et al., 2008 |
| ThermoCas9 | Cas9 protein | Mougiakos et al. (2017) |
- a
-
limited thermostability, functional until ∼50 °C.
- b
-
thermostable variant of sfGFP, active at 60 °C.
- c
-
limited thermostability; used at 52 °C.
| Name | Parageobacillus Replicon (type of replication – copy number)a | Gram + ve antibiotic resistance marker | E. colireplicon | Reference |
|---|---|---|---|---|
| pUCG3.8 | pBST1 (θ) | kan | ColE1 | Bartosiak-Jentys et al. (2013) |
| pNW33N | pTHT15 (RC) | cam | ColE1 | Drejer et al. (2018) |
| pG1C, pG1K | pBST1 (θ – 160) | cam, kan | ColE1 | Reeve et al. (2016) |
| pG2K | pUB110 (RC -115) | kan | ColE1 | Reeve et al. (2016) |
| pMTL61xxx | pUB110.1 (RC - 130), | kan, kanHT, aad9 | ColE1, p15a | Sheng et al. (2017); Madika et al. (2022) |
| pMTL62xxx | pUB110.2 (99) | kan, kanHT, aad9 | ColE1, p15a | Madika et al. (2022) |
| pMTL63xxx | pUB110.3 (37) | kan, kanHT, aad9 | ColE1, p15a | Madika et al. (2022) |
| pMTL64xxx | pNCI001 (52) | kan, kanHT, aad9 | ColE1, p15a | Madika et al. (2022) |
| pMTL65xxx | pNCI002 (100) | kan, kanHT, aad9 | ColE1, p15a | Madika et al. (2022) |
| pMTL66xxx | pBST1 (168) | kan, kanHT, aad9 | ColE1, p15a | Madika et al. (2022) |
| pMTL67xxx | pGEOTH02 (297) | kan, kanHT, aad9 | ColE1, p15a | Madika et al. (2022) |
- a
-
RC: rolling circle replicon; θ: theta replicon; reported copy number per chromosome.
Heterologous plasmid based expression is pivotal in investigating the potential of heterologous enzyme and protein production during the initial stages of host engineering, however, for later industrial strain development, expression from native genomic elements is preferable as it is more stable and does not rely on the use of antibiotics. Stable expression in P. thermoglucosidasius can be achieved by integrating new genetic elements in the native plasmid pNCI001 instead of into the chromosome (Lau et al., 2021). To prove this, the authors employed Cas9-mediated integration of the fluorescent reporter gene eCGP123 into a pseudogene of pNCI001 and downstream of the pyrE genomic locus, using stCas93 from Streptococcus thermophilus. The protein expression levels in the case of integration in the native pNCI001 plasmid were significantly lower than those observed when using a heterologous high-copy number pMTL plasmid, yet higher compared to the ones observed in the case of native chromosomal integration. Millgaard et al. recently developed an integrative shuttle sfGFP-based vector, which accelerates the process of generating marker less genomic modifications (Millgaard et al., 2023). In this study, an improved thermostable sfGFP (Frenzel et al., 2018) was flanked by homology arms and integrated into the genome during a first recombination event allowing for the correct selection of recombinant clones, which fluoresce under the blue light. The sfGFP can then recombine out again during a second recombination event, which results in a theoretical fraction of 50% mutants and 50% wild-type revertants in the colonies that underwent second recombination and lost fluorescence. A PCR-based screening can be done to identify mutants. Studies of this nature along with other counter selection systems such as the X-glu Bgl system (Bacon et al., 2017), and the development of effective thermostable genome editing CRISPR/Cas9 systems like the stCas9 (Horvath et al., 2008), the GeoCas9 (Harrington et al., 2017), and the ThermoCas9 (Mougiakos et al., 2017) can further increase the efficiency of genome engineering in this species (Liang et al., 2022b; Bidart et al., 2023). Lastly, the development of sporulation-deficient strains, exemplified by the case of the company TMO RENEWABLES LTD (Atkinson et al., 2011) and the studies on sporulation deficiency by Millgaard et al. (2023) paves the way for the implementation of P. thermoglucosidasius in the context of a biorefinery, where it is imperative to secure safety and minimize contamination risk.
Recently, a P. thermoglucosidasius native type-IB CRISPR/Cas system has been used for genome editing and multiplexed transcription repression. This tool was further employed to identify and downregulate genomic targets which increased transformation efficiency of P. thermoglucosidasius. Based on this, the authors developed a method to produce highly competent cells (108 CFU/μg DNA; Yang et al., 2023). Arguably, the recently expanded toolbox and additional genetic engineering tools, which will likely be developed further are anticipated to enable more extensive modifications of the metabolic capabilities of P. thermoglucosidasius.
3. The native metabolism and feedstocks of P. thermoglucosidasius: from hemicellulose utilization to CO-mediated hydrogen production
P. thermoglucosidasius can assimilate monomeric pentose (C5) and hexose (C6) sugars, as well as oligomeric sugars and other organic compounds. Strikingly, this bacterium is also able to convert carbon monoxide (CO) into energy. The metabolic capabilities of P. thermoglucosidasius involving the native hemicellulose and CO catabolism are presented in this section through a series of examples.
3.1. Valorization of waste biomass streams
3.1.1. Starch conversion
The realization of biorefineries using carbon-rich waste streams to produce biochemicals and biofuels via microbial fermentation holds the potential to drive growth in the global bioeconomy. P. thermoglucosidasius gained interest as a cell factory because it can break down and catabolize several waste streams, such as starch-containing food waste streams. For instance, the P. thermoglucosidasius KCTC33548 strain was shown to be capable of converting potato peel waste biomass (PWB) to hydrogen (Singhvi et al., 2021). Despite being promising, PWB conversion titers were rather low reaching a range between 2 g H2 per L of fermentation medium. PWB contains mainly potato starch, cellulose, and pectin. Nevertheless, only the starch fraction was efficiently catabolized by the cells pointing out that more work needs to be done to transform this strain into an efficient PWB degrader. In another study, 94–96% of the total sugars in waste bread (WB) were converted to ethanol by the natural starch-degrading P. thermoglucosidasius TM333 (Ibenegbu and Leak, 2022) reaching a titer of 14.24 g/L. The achieved yield was significantly higher than the one observed when the conventional ethanologenic S. cerevisiae was used as it converted only 26.8% of the total sugars in WB to ethanol (3.74 g/L). While previous WB fermentation studies relied on the external addition of α-amylase, glucoamylase, and protease (Pietrzak and Kawa-Rygielska, 2014), this time complete WB degradation, as well as higher ethanol yield, was achieved by only adding crude α-amylase in the fermentation mix. In summary, in both examples, P. thermoglucosidasius demonstrated its capability to utilize starch-containing waste streams.
3.1.2. Hemicellulose and cellulose conversion
Lignocellulose has a more complex biochemical structure than starch and is considered as a potential renewable feedstock for biorefineries. Lignocellulose in the form of agricultural or logging residues is the most abundant biopolymer on earth (Smith, 2019) and contains a vast amount of sugars, yet most of these residues are currently being burnt to generate low-value, thermal energy (Anwar et al., 2014). One of the main reasons obstructing the use of lignocellulose as the primary carbon source in bioproduction is its structural complexity, which is being constituted of three polymers cellulose, hemicellulose, and lignin. Lignin is a non-sugar fraction and confers to the overall recalcitrance of lignocellulose to microbial degradation (Rowell et al., 2012). On the other hand, cellulose consists of long glucan polymers containing only glucose sugars, and hemicellulose in many cases is composed of long polymers of xylan branched with several other sugars such as glucuronic acid, and arabinose (Fig. 2). The two main lignocellulose fermentation technologies include the Simultaneous Saccharification and Fermentation (SSF) and the Consolidated Bioprocessing (CBP) (Devi et al., 2022). In the SSF approach, lignocellulolytic enzymes are added in a bioreactor containing the lignocellulose and the microbial cells, which will subsequently ferment the sugars and oligosaccharides released from the enzymatic hydrolysis of lignocellulose. Both degradation and fermentation occur in parallel, hence it is named Simultaneous Saccharification and Fermentation. Conversely, CBP bypasses the addition of such expensive enzyme cocktails, and relies on microbial cells producing the hydrolytic enzymes to degrade lignocellulose (Olguin-Maciel et al., 2020). In this case, both lignocellulose hydrolysis and fermentation are driven by the microbial cells.
The optimum temperature range for the function of the hydrolytic enzymes is ∼ 45 °C–75 °C (Iram et al., 2020), however SSF and CBP often depend on the use of mesophilic microbial cell factories such as S. cerevisiae, which grow optimally at lower temperature (Lian et al., 2018). This mismatch between the optimal enzyme and preferred microbial growth temperature is often overcome by performing the fermentation at temperatures lower than the optimal for the hydrolytic enzymes, requiring a higher enzyme dosage. Nevertheless, the implementation of moderate thermophilic microbial cell factories in SSF and CBP bioprocesses offers a significant advantage over the use of mesophiles, as both degradation and fermentation can occur close to their optimal temperature (Svetlitchnyi et al., 2013). Additionally, thermophiles can contribute to reduced contamination risks and bioreactor cooling costs as well as increased reaction rates favored by the increased temperature that the process is performed at (Turner et al., 2007).
More specifically, in a few cases P. thermoglucosidasius has been employed as a microbial workhorse to catabolize lignocellulosic substrates. Raita et al. (2016)used Palm Kernel Cake (PKC), a lignocellulosic by-product from the palm oil industry, which was efficiently converted to ethanol by P. thermoglucosidasiusTM242 (Raita et al., 2016). The hemicellulose fraction in PKC is rich in mannan and usually requires enzymatic treatment with additional endo-mannanase and β-mannosidase before obtaining fermentable sugars. Nevertheless, the authors managed to reduce the number of required enzymes in this process by using the capability of P. thermoglucosidasius to grow on hemicellulose oligosaccharides. First, they hydrolyzed the PKC without the β-mannosidase and fed the mannooligosaccharide-rich hydrolysate to P. thermoglucosidasius TM242 producing almost five times more ethanol from PKC than what a conventional Saccharomyces sp. strain produced. The Saccharomyces sp. strain was incapable of mannooligosaccharide consumption, therefore resulting in this ethanol production difference. In a different approach, Holland et al. envisioned further improving the hemicellulolytic capabilities of P. thermoglucosidasiusTM242 (Holland et al., 2019). They equipped P. thermoglucosidasius TM242 with the endo-1,4-β-xylanase (XynA1) from the P. thermoglucosidasius C56-YS93. This enzyme targets the xylan backbone by acting on the β-glycosidic bonds linking two xyloses. The heterologous expression of endo-1,4-β-xylanase led to increased xylan cleavage but the endoxylanolytic activity was probably limited by proteases targeting the endo-1,4-β-xylanase in the extracellular environment. The authors suggested that protease-deficient strains and higher enzyme titers via promoter screening could probably further improve the xylanolytic activity of the engineered P. thermoglucosidasius TM242. Maduvanthi et al. took a different approach to augment the hemicellulolytic capabilities of P. thermoglucosidasius MTCC®7446 as they performed a Design of Experiment (DoE) workflow to optimize the process conditions in which P. thermoglucosidasius MTCC®7446 fermented corn stover to produce ethanol (Madhuvanthi et al., 2022). The authors capitalized on the fact that P. thermoglucosidasius naturally shows hemicellulolytic activity, thus they chose to fine-tune the fermentation condition parameters via DoE. In their optimal set of conditions, they used a 15% v/v of inoculum concentration, temperature of 50 °C, and fermentation time of 72 h, to finally achieve a final titer of 9.04 g/L of ethanol by using a starting concentration of 20.16 g/L sugar. Despite the auspicious results, arguably emphasis should also be given to further augmenting the lignocellulolytic capabilities of P. thermoglucosidasius, which in turn would result in increased sugar availability for fermentation and could result in higher yields.
Although, P. thermoglucosidasius is very well capable of degrading hemicellulose, it lacks the cellulases required for cellulose degradation. Bashir et al. focused on the cellulose degradation of wheat straw. Their strategy involved a mix of genomic and plasmid-based expression of heterologous cellulase genes in the ethanologenic P. thermoglucosidasius LS242, which resulted in a low final ethanol titer of 0.02 g/L in their best-performing strain (Bashir et al., 2019). In a different study, Basak et al. studied the cellulase naturally produced by P. thermoglucosidasius NBCB1. Under optimized conditions, the cellulase showed impressive activity (305 U/mg) on Avicel, which is a representative analog of the crystalline part of the cellulose (Basak et al., 2023). Such an effective biocatalyst could not only be used in a lignocellulosic biorefinery, but also in a variety of relevant applications given that thermophilic cellulases are one of the most demanded types of enzymes with applications in for example the textile and food processing industry (Rodrigues and Odaneth, 2021).
3.1.3. P. thermoglucosidasius has a relaxed carbon catabolite repression system
Indisputably, lignocellulose valorization remains a challenging task influenced by a multitude of factors some of which are related to the complete and simultaneous utilization of the sugars stored in the polymers. Lignocellulosic hydrolysate contains both C5 and C6 sugars, however, in most cases their simultaneous consumption by microbes is not naturally feasible. Carbon Catabolite Repression (CCR) is an effect via which the cells suppress the catabolic pathways of the least preferred sugar to prioritize one type of sugar over the others (Titgemeyer and Hillen, 2002). Seemingly, this hierarchy in sugar consumption may compromise the productivity in the fermentation and is sought to be eliminated (Kim et al., 2010). In their study, Liang et al. studied the CCR in P. thermoglucosidasius DSM 2542 by cultivating the cells in a mixture of glucose and xylose (Liang et al., 2022a). According to their results, a small amount of the available xylose was consumed in parallel with glucose. This contrasts more typical examples of CCR regulation as the one observed in B. subtilis (Kleijn et al., 2010), which tends to be more stringent. This loosely controlled CCR mechanism in P. thermoglucosidasius was further targeted by the same group. In their follow-up study, the authors employed an Adaptive Laboratory Evolution (ALE) methodology (Liang et al., 2022b), to finally demonstrate close to simultaneous consumption of glucose, xylose, and arabinose. This was the result of a series of evolutionary mutations that occurred in the ribose operon repressor, the adenine phosphoribosyltransferase, and the phosphoenolpyruvate:carbohydrate phosphotransferase system (PTS). In a separate study on the sugar utilization of P. thermoglucosidasius, Bidart et al. (2023) revealed that a variety of carbon sources including sugars and oligosaccharides are imported into the cell via a PTS-dependent mechanism. On the other hand, maltose, xylose, and N-acetylmuramic acid are seemingly imported via a PTS-independent mechanism. Arguably, further investigation of the findings of the latter two studies may allow to construct P. thermoglucosidasius strains that can efficiently grow in a mix of sugars; an essential feature in lignocellulose fermentation.
3.1.4. The genetic potential of P. thermoglucosidasius for lignocellulose degradation
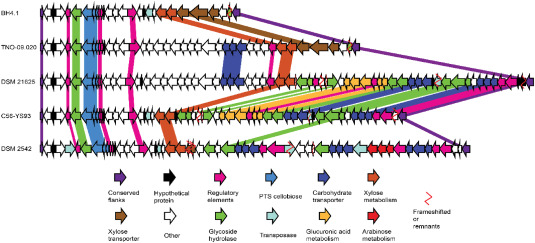
For reasons discussed previously, P. thermoglucosidasius is gradually attracting attention as a microbial platform for the conversion of lignocellulosic biomass to bio-based chemicals. It benefits from the fact that it can naturally degrade parts of the hemicellulose, shows a relaxed CCR, and can also consume the oligosaccharides in the hydrolysate thanks to a plethora of specific enzymes and transporters encoded from its genome (de Maayer et al., 2014). The genes responsible for the expression of hemicellulolytic enzymes belonging to glycoside hydrolase families as well as relevant hemicellulose-specific transporters are typically clustered in a genomic locus called hemicellulose utilization locus (HUS locus) (Daas et al., 2017; de Maayer et al., 2014). De Maayer et al. (2014) in their work showed that Geobacillus strains locate their HUS loci between a gene encoding an enoyl-CoA hydratase (echD) and a gene coding for 2-nitropropane dioxygenase (npd), currently referred to as nitronate monooxygenase (nmo). Here, we first analyzed the echD-npd islands of five P. thermoglucosidasius strains, however these loci do not contain genes with hemicellulose utilization relevance in this species (see Supplementary material). This was also previously concluded by De Maayer et al. (2014) when analyzing the echD-npd island of P. thermoglucosidasius TNO-09.020. However, unlike the previous analysis, which concluded that this strain has no HUS locus, we discovered that the genomic locus directly upstream from the npd/nmo gene does contain the genes with hemicellulose utilization relevance (Fig. 1). This alternative location of the HUS locus compared to Geobacillus was conserved across the P. thermoglucosidasius BH4.1, DSM 2542, DSM 21625, C56-YS93, and TNO-09.020 strains that we analyzed. Because all strains showed this conserved pattern of alternative location for their HUS locus, which were flanked by the same genes, we conclude that the HUS loci of P. thermoglucosidasiusspecies can be a found in between a dehydratase gene from the MaoC family and the npd/nmo gene.
 Fig. 1
Fig. 1 Fig. 2
Fig. 2The identified HUS loci of the five P. thermoglucosidasius strains display both similarities and differences in the genes they contain (Fig. 1). For example, both C56-YS93 and DSM 21625 strains possess genes for glucuronic acid metabolism, but they lack important genes such as the arabinose operon. On the other hand, DSM 2542 encodes for a full arabinose operon and more carbohydrate transporters than C56-YS93, but does not encode an endo-1,4-β-xylanase, while C56-YS93 does. Ultimately, there is no perfect P. thermoglucosidasius strain for hemicellulose bioconversion. Arguably, in order to attain a complete hemicellulose conversion, it is necessary to complement a strain with additional HUS components, which are not encoded by its own HUS locus. Indisputably, a characterization of the carbohydrate transporters and glycoside hydrolases encompassed within HUS loci would facilitate a rational strategy of integrating various parts from one HUS locus into another strain.
Xylan is the most commonly found polymer present in hemicellulose. It is degraded by specific enzymes coded from the HUS loci such as the endo-1,4-β-xylanase, and several other accessory enzymes (Fig. 2). These enzymes synergistically act on the xylan backbone and the branching sugars (de Maayer et al., 2014) to fully decompose the polymer into the resulting xylo-oligosaccharides. Once xylan is degraded, P. thermoglucosidasius can process the xylo-oligosaccharides intracellularly with β-xylosidases and xylanases to finally release xylose monomers, which then enter the main catabolic pathways. Although the xylanolytic pathways are known, most of the hydrolytic enzymes and carbohydrate transporters naturally expressed by P. thermoglucosidasiuslack biochemical characterization. In one of the few studies in the field, Wang et al. (2022) characterized an α-galactosidase naturally secreted by P. thermoglucosidasius T26, which showed maximum enzyme activity of 0.4976 U/mL under optimal conditions (60 °C, 2 day cultivation, 180 rpm). This debranching enzyme specifically hydrolyses the bond linking the α-galactosyl unit to the mannan backbone, a necessary hydrolysis step for lignocellulosic biomass that mostly contains galactomannan.
Further in vivo studies on the secretion of enzymes involved in lignocellulose hydrolysis could benefit from the use of a toolbox of signal peptides, used for engineered enzyme secretion (Bartosiak-Jentys et al., 2013). Indisputably, as more research groups engage in the research field of lignocellulose valorization using P. thermoglucosidasius, it is a matter of time until a number of well-characterized lignocellulolytic enzymes become available allowing us to implement them in whole-cell biocatalysis studies or other industrial practices.
3.2. CO-mediated hydrogenogenesis in P. thermoglucosidasius
Recent studies have revealed that certain strains of P. thermoglucosidasiuspossess the remarkable ability to oxidize carbon monoxide (CO) via the water gas shift reaction (WGSR; CO + H2O CO2 + H2), and utilize it as an energy source but not as a carbon source. (Aliyu et al., 2021b; Díaz et al., 2022; Inoue et al., 2019; Mohr et al., 2018b). The WGSR in P. thermoglucosidasius is catalyzed by a carbon monoxide dehydrogenase/energy-converting hydrogenase enzymatic complex (CODH/ECH), which oxidizes CO to CO2 and channels the electrons to the production of hydrogen. The CODH/ECH complex utilizes nickel and iron ions as cofactors (Mohr et al., 2019). A gene cluster of 15 genes is responsible for the assembly of a functional CODH/ECH complex in P. thermoglucosidasius (Adachi et al., 2020). Furthermore, a genetic analysis comparing hydrogenogenic and non-hydrogenogenic strains of P. thermoglucosidasius has revealed two unique loci being only present in hydrogenogenic strains. These unique loci were identified to code for proteins related to nickel and iron transport into the cell, highlighting the importance of these metal ions as cofactors for CO-mediated hydrogenogenesis (Aliyu et al., 2021a). Finding this metabolic feature in a facultative anaerobe is rather rare, given that it is typically observed in obligate anaerobes. The carbon monoxide dehydrogenase (CODH) and the specific enzymatic complex combination found in P. thermoglucosidasius of a CODH together with an ECH is only present in some strictly anaerobic Thermoanaerobacteraceae strains, such as Moorella glycerini. Horizontal gene transfer could be a plausible hypothesis to explain how this system evolved in P. thermoglucosidasius, however the evolutionary mechanisms behind the evolution of this system are unknown (Mohr et al., 2018a). Despite the general toxicity of CO to many microorganisms, certain strains of P. thermoglucosidasius can tolerate up to 100% CO concentrations (Aliyu et al., 2021b). It has been hypothesized that during the WGSR energy is generated by transmembrane ion translocation, which drives ATP synthesis (Fig. 3). However, how this process exactly occurs and what the yield of energy on CO is remains largely unknown. There is evidence from other organisms possessing a similar system, that during CO oxidation the ECH conserves energy through proton translocation mediated ATP synthesis, thus a similar phenomenon could be happening in P. thermoglucosidasius (Mohr et al., 2018a). Experimental evidence based on transcriptomics data revealed that during the WGSR in P. thermoglucosidasius, cellular functions such as motility, oxidative phosphorylation, ribosome synthesis, and central carbon metabolism were upregulated, indicating that the WGSR provided enough energy not only for maintenance but also for active metabolism (Aliyu et al., 2020).
CO2 + H2), and utilize it as an energy source but not as a carbon source. (Aliyu et al., 2021b; Díaz et al., 2022; Inoue et al., 2019; Mohr et al., 2018b). The WGSR in P. thermoglucosidasius is catalyzed by a carbon monoxide dehydrogenase/energy-converting hydrogenase enzymatic complex (CODH/ECH), which oxidizes CO to CO2 and channels the electrons to the production of hydrogen. The CODH/ECH complex utilizes nickel and iron ions as cofactors (Mohr et al., 2019). A gene cluster of 15 genes is responsible for the assembly of a functional CODH/ECH complex in P. thermoglucosidasius (Adachi et al., 2020). Furthermore, a genetic analysis comparing hydrogenogenic and non-hydrogenogenic strains of P. thermoglucosidasius has revealed two unique loci being only present in hydrogenogenic strains. These unique loci were identified to code for proteins related to nickel and iron transport into the cell, highlighting the importance of these metal ions as cofactors for CO-mediated hydrogenogenesis (Aliyu et al., 2021a). Finding this metabolic feature in a facultative anaerobe is rather rare, given that it is typically observed in obligate anaerobes. The carbon monoxide dehydrogenase (CODH) and the specific enzymatic complex combination found in P. thermoglucosidasius of a CODH together with an ECH is only present in some strictly anaerobic Thermoanaerobacteraceae strains, such as Moorella glycerini. Horizontal gene transfer could be a plausible hypothesis to explain how this system evolved in P. thermoglucosidasius, however the evolutionary mechanisms behind the evolution of this system are unknown (Mohr et al., 2018a). Despite the general toxicity of CO to many microorganisms, certain strains of P. thermoglucosidasius can tolerate up to 100% CO concentrations (Aliyu et al., 2021b). It has been hypothesized that during the WGSR energy is generated by transmembrane ion translocation, which drives ATP synthesis (Fig. 3). However, how this process exactly occurs and what the yield of energy on CO is remains largely unknown. There is evidence from other organisms possessing a similar system, that during CO oxidation the ECH conserves energy through proton translocation mediated ATP synthesis, thus a similar phenomenon could be happening in P. thermoglucosidasius (Mohr et al., 2018a). Experimental evidence based on transcriptomics data revealed that during the WGSR in P. thermoglucosidasius, cellular functions such as motility, oxidative phosphorylation, ribosome synthesis, and central carbon metabolism were upregulated, indicating that the WGSR provided enough energy not only for maintenance but also for active metabolism (Aliyu et al., 2020).
 Fig. 3
Fig. 3Hydrogenogenic strains of P. thermoglucosidasius could have several applications. For example, the energy generated in the WGSR could increase the yield of products in anaerobic processes when co-feeding CO. P. thermoglucosidasius could also be used for hydrogen production from CO, e.g. for the generation of high-purity hydrogen from syngas (a mix of CO and H2). Using P. thermoglucosidasius for such a process could potentially be advantageous over hydrogen-producing strict anaerobes, as the feedstock gas would not need to be cleaned of oxygen, reducing the costs (Mohr et al., 2019). Improved kinetics of the WGSR at high temperatures could also make thermophilic production of hydrogen from CO more efficient than in mesophilic systems (Díaz et al., 2022).
4. Bioproduction of biofuels, platform, and fine chemicals in engineered strains of P. thermoglucosidasius
In this section, a summary of the production of biofuel compounds, platform chemicals, and fine chemicals in genetically engineered strains of P. thermoglucosidasius is presented. While relatively high production of ethanol or lactate has been demonstrated, the titers achieved by P. thermoglucosidasius for other compounds may not yet be competitive with those produced by other mesophilic species.
4.1. Biofuels production in engineered strains of P. thermoglucosidasius
Nowadays, most of the world's ethanol production relies on mesophilic microbial cell factories that utilize sugar or, in rare cases, lignocellulosic-based feedstocks (Joshi and Mishra, 2022). Several authors highlighted the importance of finding a suitable bacterial species that can produce ethanol from lignocellulosic feedstocks in CBP, and the relevance of P. thermoglucosidasius as a potential candidate (Raita et al., 2016; Sheng et al., 2016; Thompson et al., 2008; Van Zyl et al., 2014; Zhou et al., 2016). In CBP, a lignocellulolytic microorganism is used as the workhorse to produce hydrolytic enzymes to break down the polymers composing lignocellulose into fermentable molecules, and then convert these into products in the same bioreactor. CBP can lead to cheaper and faster production in comparison with other methods. The pathway of ethanol production in P. thermoglucosidasius has been characterized, and this bacterium possesses a bifunctional AdhE enzyme that has both acetylate aldehyde dehydrogenase and alcohol dehydrogenase activities (Extance et al., 2013). Examples of engineered, ethanol-overproducing P. thermoglucosidasius strains can be found in the literature (Cripps et al., 2009; Raita et al., 2016; Zhou et al., 2016). The most successful strain has been P. thermoglucosidasius TM242, a type-strain derivative, in which the carbon flux was diverted to ethanol production by deleting L-lactate dehydrogenase (ldh) and pyruvate formate-lyase (pfl), in addition to the overexpression of the native pyruvate dehydrogenase (pdh). This strain could produce 14.46 and 14.83 g/L of bioethanol from glucose and cellobiose, respectively, achieving more than 90% of the theoretical yield of ethanol production on these substrates (Cripps et al., 2009; Raita et al., 2016; Zhou et al., 2016).
The optimum temperature growth of P. thermoglucosidasius and the bioethanol boiling point are close, thus opening the possibility to use gas stripping for continuous removal of bioethanol from the production culture. This approach would potentially reduce the downstream processing costs associated with the purification of products from dilute solutions. Furthermore, bioethanol concentrations above 15.78 g/L have been identified as detrimental to bioethanol productivity in the strain TM242, and therefore continuous removal from the culture would be beneficial to keep the maximum bioethanol productivity throughout the fermentation process (Calverley et al., 2021; Niu et al., 2015; Ortenzi, 2021). The feasibility of this approach has been analyzed in a 0.5 L continuous fermenter system coupled to a hot air microbubble stripping unit, in which it was demonstrated that ethanol concentration was kept below the toxicity limit, and therefore could potentially support a productivity of 14.9 g/L/h of bioethanol for the strain TM242 (Calverley et al., 2021). The authors concluded that the production of bioethanol under these conditions would be commercially viable. Moreover, P. thermoglucosidasius TM242 has been recently engineered to increase its ethanol tolerance limit to 25.98 g/L (Ortenzi, 2021). Furthermore, engineering of TM242 by deletion of the spo0A gene, yielded the strain TM444, a sporulation-deficient derivative with comparable ethanol production capabilities to the parental strain (Atkinson et al., 2011). The strain TM242 has previously been employed by TMO RENEWABLES LTD for ethanol production from a mixture of pre-treated hemicellulose and cellulose (Martin et al., 2008), however technical and commercial challenges led TMO RENEWABLES LTD to bankruptcy. Ultimately, the abovementioned advancements have contributed to positioning P. thermoglucosidasius as a prominent candidate for large-scale production of bioethanol, yet a comprehensive investigation of the scalability of this bioprocess is required.
Another promising biofuel molecule is isobutanol, which apart from fuel has other applications. It is currently mainly produced through chemical synthesis. Microbial production of isobutanol is a potential alternative, but native production levels are typically low. Therefore, microbial isobutanol production has primarily focused on engineered strains (Hussain et al., 2022; Lakshmi et al., 2021). A single study demonstrates the production of 3.3 g/L of isobutanol from glucose and 0.6 g/L from cellobiose in a P. thermoglucosidasius DSM 2542 engineered strain. The native valine biosynthesis pathway was modified to redirect the carbon flux toward isobutanol production. This study identified thermostable enzymes catalyzing the key steps in isobutanol biosynthesis, of which several were actually found in mesophilic organisms. The best performing identified candidates were acetolactate synthase from the mesophile B. subtilis(alsS), alpha-ketoisovalerate decarboxylase from the mesophile Lactobacillus lactis (kivD), and the native keto-acid reductoisomerase enzyme (ilvC) from P. thermoglucosidasius (Lin et al., 2014). Continuous removal of isobutanol by gas stripping has been demonstrated in E. coli and has proved to increase productivity due to alleviated product inhibition (Lakshmi et al., 2021). Combining thermophilic isobutanol production with continuous gas stripping could improve the efficiency of the bioprocess in P. thermoglucosidasius by tackling product inhibition and favoring thermodynamics.
4.2. Platform chemicals production in engineered strains of P. thermoglucosidasius
Platform chemicals, such as succinate, are versatile molecules with diverse applications and derived chemicals and materials. Bio-based succinate is being or has been produced at a commercial scale by several companies, although the global succinate market is dominated by the petrochemical-based industry (diLorenzo et al., 2022; Sun and Li, 2015). Metabolic engineering projects to increase succinate production have focused on mesophilic microorganisms. Basfia succiniproducens, E. coli and S. cerevisiae are the most successful mesophilic platforms in terms of succinate production (diLorenzo et al., 2022; Li et al., 2016; Sahoo et al., 2022). The first steps towards succinate production in P. thermoglucosidasius have been demonstrated. The deletion of the succinate dehydrogenase (sdh) gene in the type-strain P. thermoglucosidasius, resulted in increased succinate accumulation up to approximately 1 g/L (Lau, 2019).
2,3-butanediol (2,3-BDO) is another platform chemical primarily produced from fossil feedstocks. Because 2,3-BDO is a chiral compound with three stereoisomers, (2R,3R)-BDO, meso-BDO, and (2S,3S)-BDO, the chemical synthesis generates a racemic mixture complicating downstream processing. Bacterial production of 2,3-BDO with increased purity may offer advantages over traditional methods (Lee et al., 2021; Maina et al., 2022). Bacillus species can naturally produce (2R,3R)- and meso-BDO from pyruvate. However, in P. thermoglucosidasius the native enzymatic machinery for this conversion is not highly active. P. thermoglucosidasius DSM 2542 was engineered to produce 7.2 g/L of enantiomeric pure (2R,3R)-BDO from glucose by heterologous expression of the acetolactate synthase (alsS) from B. subtilis and the acetolactate decarboxylase (alsD) from S. thermophilus, achieving 72% of the theoretical yield and representing the first example of 2,3-BDO at a temperature above 50 °C (Zhou et al., 2020). A similar type-strain derivative was engineered by heterologous expression of the alsS from B. subtilis and the alsD from Bacillus cereus, in which the pyruvate metabolic flux was diverted to (2R,3R)-BDO production by deletion of the main competing pathways. This strain could produce 15.6 g/L of 2,3-BDO from glucose, with 97% enantiomeric purity for (2R,3R)-BDO (Table 3) (Sheng et al., 2023).
| Product | Genetic modificationsa | Titer | Feedstock | Source |
|---|---|---|---|---|
| Bioethanol | ΔldhL, Δpfl. O: pdh | 14.83 g/L | Cellobiose | Cripps et al. 2009 |
| Isobutanol | O: ilvC HO: bsalsS, llkivD | 3.3 g/L | Glucose | Lin et al. (2014) |
| Succinate | Δsdh | 1 g/L | Glucose | Lau (2019) |
| (2R,3R)-butanediol | ΔadhE, Δg3pdh, Δgdh, Δldh2, Δldh, Δpfl, ΔacoB1 | ∼15.6 g/L | Glucose | Sheng et al. (2023) |
| HO: bsalsS, bcalsD. | ||||
| 1,3-propanediol | ΔadhE, Δpfl, ΔldhL. O: pduCDEGH | 5.9 g/L | Glycerol | Chen et al. (2023) |
| l-lactate | ΔpflB::ldhL, Δaadh::ldhL, Δachd1, Δachd2. HO: bcpfk, bcpyk, | 151.1 g/L | Glucose | Liu et al. (2024) |
| d-lactate | ΔldhL::blldhD, ΔpflB::ldhD, Δaadh::ldhD, Δachd12. HO: bcpfk, bcpyk, | 153.1 g/L | Glucose | Liu et al. (2024) |
| Riboflavin | ΔpurR, ΔpurA, ΔccpN, Δlldh, ΔPpur::PYeD. HO: gtrib operon. ribC (G119D), purF (D292V). DR: tatAE, rex, prol, rsmF, kapD. | 5 g/L | Glucose | Yang et al. (2023) |
| τ-muurolol | HO: roseRS_3509, sshmgr, ssaca, sshmgs, ssmvk, ssdmd, sspmk, ssidi, gsfpps | 14 mg/L of caryophyllene equivalents | Waste bread | Styles et al. (2021) |
- a
-
O: overexpression; HO: heterologous overexpression.
More than half of the global 1,3-propanediol (1,3-PDO) production comes from bio-based sources (Grand View Research, 2022). Metabolic engineering strategies have been developed to increase the efficiency of its production, and nowadays the company DuPont use modified strains of E. coli to produce 1,3-PDO at a commercial scale (>135 g/L) (Nakamura and Whited, 2003). Recently, thermophilic production of 1,3-PDO (5.9 g/L) from glycerol, has been demonstrated in a DSM 2542 derivative. The carbon flux was redirected towards 1,3-PDO production in this strain by deleting the adhE, pfl, and ldhL genes, and glycerol assimilation was enhanced by overexpressing the native glycerol dehydratase (pduCDE) and its reactivator (pduGH) (Chen et al., 2023)
Lactic acid is an organic acid of industrial interest, which is naturally produced by various microorganisms. Lactic acid has two optical isomers and therefore bioproduction of lactic acid in microbial cell factories offers advantages over chemical production which generates a racemic mixture of l- and d-lactate. Under microaerobic or anaerobic conditions P. thermoglucosidasius produces lactate, ethanol, formate, and acetate as main fermentation products (Cripps et al., 2009; Díaz et al., 2022). Although mainly l-lactate is formed, D-lactate can also be produced in significant amounts. Production of l-lactate from pyruvate is catalyzed via the native l-lactate dehydrogenase (ldhL). In addition, both l- and d-lactate can be produced from dihydroxyacetone-phosphate via the methylglyoxal pathway (Fig. 4). Construction of l- and D-lactic acid producing derivatives of P. thermoglucosidasius DSM 2542 was achieved by combining targeted modifications with a semi-rational evolutionary approach to produce 151.1 g/L and 153.1 g/L of enantiomeric pure l- and D-lactate from glucose, at a yield of 98.7 and 93 %, respectively (Liu et al., 2024). To obtain the strain that produced D-lactate, DSM 2542 was rationally engineered by replacing the native ldhL by ldhD from Bacillus licheniformis BJQX, insertion of the 6-phosphofructokinase (pfk) and pyruvate kinase (pyk) genes from Heyndrickxia coagulans H2, deletion of the acetaldehyde dehydrogenase 1 and 2 (achd1 and achd2) and aldehyde-alcohol dehydrogenase (aadh) genes, deletion of the formate acetyltransferase (pflB) gene, and the insertion of two copies of codon optimized ldhD at the former positions of aadh and pflB. To obtain an l-lactacte producing strain, the three copies of ldhD previously introduced were replaced by two copies of the native P. thermoglucosidasius ldhL and one from H. coagulans H-2. Finally these l- and D-lactate producing strains were subjected to ALE to achieve the final phenotype. The authors concluded that this strain is up to industrial requirements for commercial lactate production, positioning P. thermoglucosidasius as a promising thermophilic lactate production platform (Liu et al., 2024).
 Fig. 4
Fig. 44.3. Fine chemicals and protein production in engineered strains of P. thermoglucosidasius
Fine chemicals, such as flavins or terpenes, are complex molecules with diverse applications in the pharmaceutical, food, and cosmetic industries, among others. Riboflavin (vitamin B2) is natively produced by most bacterial species, but its biosynthesis is tightly regulated, resulting in undetectable levels. Scientific progress during the last decades led to riboflavin overproducing strains and enabled a complete shift to microbial production, with B. subtilis being the predominant bacterial species employed for this purpose. Future riboflavin production could benefit from the use of thermophilic hosts, due to the significant amount of heat generated during its biosynthesis (Wang et al., 2018; You et al., 2021). Production of riboflavin at a titer of 0.5 g/L from glucose and 1 g/L from a mixture of glucose and xylose was achieved in an engineered strain of P. thermoglucosidasius DSM 2542. This was achieved by heterologous overexpression of the rib operon from Geobacillus thermodenitrificans in combination with a point mutation in the ribC gene to downregulate riboflavin consumption, engineering the purine pathway, redirecting the central carbon flux to the pentose phosphate pathway, and reducing the production of lactate (Yang et al., 2021). A follow up study constructed a similar P. thermoglucosidasiusriboflavin overproducing strain, with additional genetic modifications that released feedback inhibition of the pur operon and downregulated five extra genes (Table 3). This strain produced 5 g/L of riboflavin from glucose in a 5 L bioreactor over 28 h (Yang et al., 2023).
Terpenes are a highly heterogeneous class of biomolecules, with considerable structural and functional diversity. Nowadays, terpenes are primarily extracted from plants at low yields. Chemical synthesis of terpenes is complex and prohibited by regulations in the cases where the production of certain stereoisomers with undesired properties is uncontrollable (Helfrich et al., 2019a, Helfrich et al., 2019b; Liang et al., 2021). Consequently, the production of terpenes could benefit significantly from the use of (thermophilic) bacterial cell factories. As a proof of concept, 14 mg/L of caryophyllene equivalents of τ-muurolol were produced from waste bread, in an engineered P. thermoglucosidasius DSM 2542 strain. This strain was built by integrating in its chromosome a heterologous mevalonate pathway from Saccharolobus solfataricus and Geobacillus stearothermophilus, and a thermostable terpene synthase from Roseiflexus sp. Rs-1 (Styles et al., 2021). This study is the first to demonstrate bacterial production of terpenes at high temperatures and highlights the genetic flexibility of P. thermoglucosidasius to produce complex products.
The P. thermoglucosidasius strains Pharao and SKF4 were reported as sources of thermostable enzymes, such as amylases and proteases, which are relevant for biotechnological or industrial purposes (Saeed et al., 2021; Suleiman et al., 2020). Furthermore, the type strain DSM 2542 was found to express a novel cationic bacteriocin (Geo6) with antibacterial activity upon several species of thermophilic Gram-positive bacteria. Geo6 showcases extreme stability against a wide range of temperature and pH, which highlights its potential use in the food and pharmaceutical industries (Koniuchovaitė et al., 2023).
In conclusion, P. thermoglucosidasius is a versatile and promising candidate for the production of biofuels, platform and fine chemicals. For some products, such as ethanol or lactate, the titers achieved in modified strains of P. thermoglucosidasius could be competitive with established mesophilic production chassis. However, titers of other products are still far from being competitive with those of mesophilic organisms or even moderately thermophilic platforms, such us B. licheniformis in the case of 2,3-BDO (Baez et al., 2011; Lee et al., 2021; Pedrolli et al., 2015; Vemuri et al., 2002; Wang et al., 2018; Wang et al., 2011; You et al., 2021).
5. Conclusions
P. thermoglucosidasius has potential to become an industrial-relevant thermophilic cell factory within the bio-based industries in the foreseeable future. Genetic and metabolic engineering techniques developed in recent years have contributed to widen the spectrum of biofuels, platform and fine chemicals, which can be produced by engineered strains of P. thermoglucosidasius, highlighting the metabolic plasticity of the species. Additionally, P. thermoglucosidasius is a propitious platform for the production of low boiling point biofuels, such as bioethanol, where production can be combined with in situ recovery via gas-stripping. Moreover, lactate production by this bacterium has been improved to titers of industrial relevance. For the other products, the achieved yields are still lower than the ones reached by mesophilic model microorganisms, which can be justified as P. thermoglucosidasius is an emerging microbial host for which still a limited amount of knowledge on its metabolism and engineering has been acquired compared to mesophilic platform organisms. To speed up these developments, the systematic use of omics analyses could be fruitful, as shown recently by Chen et al. (2023). The availability of the metabolic model will be instrumental in these analyses. These data could support more effective, rational metabolic engineering strategies, which could also benefit from the emerging availability of faster and multiplex genetic engineering tools (Chen et al., 2023; Yilmaz et al., 2022). P. thermoglucosidasius also shows the potential for integration into lignocellulosic bioprocesses. The native hemicellulolytic machinery encoded by the HUS locus, the ability to uptake complex oligosaccharides, and the capacity to metabolize both C5 and C6 sugars are some distinct metabolic features exhibited by this species. They are all very relevant to the field of second-generation bioprocessing, and as shown in the examples discussed above, several metabolic engineering studies aimed to capitalize on them in order to augment the lignocellulolytic capabilities of P. thermoglucosidasius. Fundamental research in CO oxidation and hydrogenogenesis in P. thermoglucosidasius during the last years has contributed to the understanding of these mechanisms, however further investigation of these metabolic processes is essential in order to develop industrial applications such as the valorization of CO-rich waste off-gas streams at thermophilic temperatures. Ultimately, we believe that P. thermoglucosidasiuspossesses considerable potential that deserves further interest of the scientific and industrial biotech communities, which will further help to fully realize the capabilities of this promising thermophilic host.
CRediT authorship contribution statement
Miguel Paredes-Barrada: Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Panagiotis Kopsiaftis: Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Nico J. Claassens: Writing – review & editing, Project administration, Funding acquisition, Conceptualization. Richard van Kranenburg: Writing – review & editing, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare no competing financial interest. PK and RvK are employed by the commercial company Corbion (Gorinchem, The Netherlands).
Acknowledgements
PK acknowledges funding from Talent4BBI; a Marie Skłodowska-Curie COFUNDproject managed by BiOrbic, the SFI Research Centre in Ireland. Talent4BBIproject received funding from the EU Horizon 2020 research and innovation program under the grant agreement No. 101034323. NJC acknowledges support from a Veni grant (VI.Veni.192.156) from NWO (Netherlands Science Organisation).
Data availability
Data provided as supplementary materials
- †
-
These authors contributed equally.
- 1
-
These authors contributed equally.
© 2024 The Authors. Published by Elsevier Inc. on behalf of International Metabolic Engineering Society.