1. Introduction
Nerve injuries affect millions of people worldwide. These injuries often affect youth of employable age and leave them with a permanent disability of cognitive, motor or psychotic nature. They should deal with a reduced quality of life as well as several debilitating social and economic burdens [1], [2], [3]. Peripheral nerve injuries affect 13–23 patients per 100,000 as reported in 2015 [4]. They pose one of the major problems at trauma facilities. Etiologies associated with peripheral nerve injuries include penetrating injury, crush, traction, ischemia, and less common mechanisms such as thermal, electric shock, radiation, percussion, and vibration [1]. Injured nerves of the peripheral nervous system (PNS) have the ability to regenerate. However, spontaneous regeneration of the nerve, in the absence of any therapeutic intervention does not result in a good functional outcome [3]. Despite early diagnosis surgical intervention does not result in the recovery of functions as it was pre-injury. Traditional epineural neurorrhaphy promotes regeneration by direct contact, and can result in the formation of neuromas. Autologous grafts have high standards, but they still have limitations as the nerves are short aged, supply is limited. They also present the possibility of causing morbidity, neuroma formation at site of harvest, scarring and sensory loss [4]. Allogenic grafts could also be used but they would produce an immune response which will disable the cells that cure the injury [5]. The central nervous system (CNS) consists of the brain, spinal cord and retina. It is limited in terms of its spontaneous regenerative capacity, limiting the possible treatment strategies. The etiologies of CNS injuries are apoptotic and necrotic death of neurons (including photoreceptors), astrocytes and oligodendrocytes, axonal injury, demyelination, excitotoxicity, ischemia, oxidative damage, and inflammation [6]. Traumatic Brain Injury (TBI) can present symptoms ranging from headaches to paralysis. However, the current treatment approaches focus on preventing any further damage, rather than facilitating further regeneration [2]. Spinal cord injuries (SCI) occur majorly in traffic accidents and due to elderly patients falling. They result in paraplegia and quadriplegia, which cannot be effectively treated so far [7]. Moreover, diseases of the CNS are complex, and can cause decrease in cognitive, motor and sensory functions (as in the cases of Parkinson's disease, Alzheimer's and Multiple Sclerosis) and loss of vision due to retinal defects (Retinitis Pigmentosa and Age related Macular degeneration). Pharmacological intervention is restricted to simply delaying the progression of disease. There are additional limitations as well. The site of cell transplantation has an inhospitable environment. Furthermore, limited drugs and biologics can successfully cross the blood brain barrier thereby eliminating oral and intravenous drug administration methods (Fig. 1) [6]. Thus, we find the need for better approaches towards the treatment of nerve injuries. Tissue engineering centric approaches will enable regeneration, repair and replacement of tissue at the site of injury. Consequently, functionality will be restored, even for complex CNS injuries like TBI. Treatment strategies combining cell transplantation, molecule delivery and biomaterial scaffold constructions are considered the greatest hope for possible regeneration and functional recovery in SCIs [8]. Tissue engineering is achieved through the fabrication of a scaffold, which mimics all the properties of the tissue which should be repaired to favor cell penetration and tissue regeneration in three dimensions. Tissue engineering comprises three main components namely, the biomaterial used, the cells and the biomechanical and/or mechanical stimuli (Fig. 2).

Fig. 1. An overview of CNS and PNS injuries.
Types of nerve injuries and their possible effects: PNS injuries (at the left) are mainly categorized into three types—Neuropraxia (2.), Axonotmesis (3.) and Neurotmesis (4.) Injuries to the CNS (right) occur in the brain encompassing traumatic brain injuries (TBI) and neurodegenerative diseases and in the spine (SCIs).
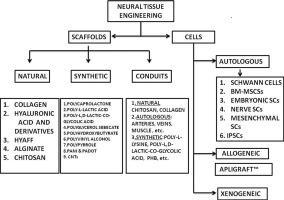
Figure adopted from references [11], [12], [13], [14]. Fig. 2. Overview of Neural Tissue Engineering approach. Two approaches: Conventional (Types of grafts) and Tissue engineering approach. Types of stimuli, cells and biomaterials are elaborated.
Fig. 2. Overview of Neural Tissue Engineering approach. Two approaches: Conventional (Types of grafts) and Tissue engineering approach. Types of stimuli, cells and biomaterials are elaborated.Many different types of scaffolds have been studied for neural tissue engineering, namely nanofibrous scaffolds, natural and synthetic scaffolds. The nanofibrous scaffolds have an increasing application in tissue engineering [9]. Many cell types like MSCs, iPSCs, ESCs cord blood cells and majorly, Schwann cells are used. Various fabrication techniques like phase separation and electrospinning are used (Fig. 3). Some of the growth factors used in case of neural tissue engineering include NGF (Nerve growth factor), NT-3 (Neurotrophin-3) and BDNF (Brain derived neurotrophic factor). Several products are commercially available for neural tissue engineering. Neuragen®, NeuraWrap™, NeuroMatrix™, NeuroFlex™ are some FDA approved collagen based nerve conduits available in the market. NeuroTube® is a nerve conduit made of Polyglycolic acid, while Neurolac™ is composed of Poly (D,L-lactide-co-ε-caprolactone) and Salutunnel™ is made of Polyvinyl alcohol. Neuragen® was the first commercially available, FDA-approved nerve conduit. These conduits have been reported effective for regeneration of the nerve. In the United States alone they result in the expenditure of 150 billion dollars in the annual healthcare dollar. Over 200,000 repair procedures are performed in the United States annually [10]. Thus, neural tissue engineering offers potentially great solutions on the healthcare front, as well as great economic prospects in the market.
 Fig. 3. Schematic diagram showing the apparatus for electrospinning: Electrospinning is a method for scaffold fabrication. The above mentioned parameters can be varied to make scaffolds with different properties.
Fig. 3. Schematic diagram showing the apparatus for electrospinning: Electrospinning is a method for scaffold fabrication. The above mentioned parameters can be varied to make scaffolds with different properties.2. Ideal properties of a scaffold
The scaffold used should be analogous to the natural ECM of the tissue and should support 3D cell cultures. To design an appropriate scaffold to repair the damage in a tissue we need to know the physical, chemical and mechanical properties of that tissue (Fig. 4). The following properties given below depend on one key parameter, which is the choice of biomaterial for scaffold fabrication. Depending on the requirements of the scaffold, the biomaterial is suitably selected [15].
 Fig. 4. The ideal properties of scaffolds used in Neural Tissue Engineering depend on the right choice of biomaterial and its corresponding chemical, physical and mechanical properties.
Fig. 4. The ideal properties of scaffolds used in Neural Tissue Engineering depend on the right choice of biomaterial and its corresponding chemical, physical and mechanical properties.2.1. Biocompatibility
Biocompatibility is a property of prime importance as it facilitates cell adhesion, proper functionality of cells and migration and proliferation of cells on the scaffold [15]. Surface modification of the scaffold can be done using bioactive molecules to make biomimetic materials. Bioactive molecules like long chains of ECM proteins including fibronectin, laminin, vitronectin and short peptide sequences are coated on the biomaterials. The surface modification favors cell adhesion and cell proliferation. Bulk modification of the biomaterial is more beneficial than surface modification. In bulk modification, the cell signaling peptides are integrated into the biomaterials and recognition sites are present both in the bulk and on the surface. Cell adhesion property depends upon surface properties like wettability and charge density [16]. Along with biocompatibility, toxicity profiles also play a crucial role in cell adhesion, growth and proliferation on the scaffold [17].
2.2. Biodegradable
One of the major advantages of synthesizing biodegradable scaffolds is that they eliminate the need for surgical removal of the scaffold and they are absorbed by the surrounding tissues in the body [17]. In case of neural tissue engineering, controlled biodegradable scaffolds are preferred as the scaffold is meant to support the growth of nerve cells and then be degraded by the body as subsequent repair takes place. A biodegradable scaffold will also facilitate the neighboring cells to produce their own extracellular matrix (ECM). It should be taken care of that the by-products of biodegradation should be non-toxic as well and that they are easily dispose-off [15]. The cytotoxic effects could also enable neuroma formation. Biodegradability should favor the elimination of chronic inflammation [16].
2.3. Porosity and pore size
An ideal scaffold should possess the appropriate shape and porosity required to mimic its natural tissue. High porosity and a pore size sufficient to aid in cell seeding, vascularization and diffusion of growth factors and nutrients into the scaffold and surrounding tissues is necessary. Ideally, 90% porosity and pore size of 100 μm–500 μm is standard for yielding good results [17]. These factors contribute to the architecture of the scaffold. It is crucial to scaffolds that they have interconnected pores to facilitate cell penetration and diffusion of nutrients to cells and ECM present in the scaffold. Diffusing out of waste products also depends on the porous interconnected structure. But while pores need to be large enough to accommodate diffusion of cells, nutrients and waste products, it should also be small enough to generate a highly specific surface area which results in efficient binding of cells to scaffold due to minimal ligand density [15].
2.4. Mechanical properties
The scaffold should have mechanical properties identical to that of the tissue at the implantation site. As this is not always feasible, materials with mechanical properties that can protect the cells from compressive or tensile forces without disturbing the biomechanical cues are used [17]. Polymeric nanofibers are highly advantageous with respect to this aspect. They have been shown to display unique mechanical properties, like the tensile modulus, tensile strengthand shear modulus. These parameters have been found to increase with subsequent decrease in the diameter of the fiber. Although the explanation behind this phenomenon has not been clearly elucidated yet, it has been postulated that there is an increase in macromolecular chain alignment as the fiber diameter is decreased, resulting in a higher crystallinity of the nanofiber. Such unique mechanical properties facilitate the modulation of cell behavior and provide strength to resist the forces exerted by cytoskeletal elements on the scaffold. Hence, optimal mechanical properties and porous scaffold architecture together play a crucial role in facilitating cell infiltration and vascularization which largely determine the making of a good scaffold [15].
2.5. Provide multiple cues
An ideal neural scaffold should be able to provide any one or a combination of cues including mechanical, biochemical, topographical and electrical cues. These cues help the scaffold to mimic the native extracellular matrix of the tissue in vivo, facilitates neurite outgrowth by providing better contact guidance and promotes and enhances cell adhesion, proliferation, migration and viability [16]. The mimicking of ECM will aid the surrounding cells to secrete their own ECM, which binds the cells to tissues and thus elicits signals that facilitate cell development and morphogenesis [17]. Electrical stimulation has been found to be especially successful in the case of neural tissue engineering. Neuronal repair is highly unique due to the complexity of the nervous system. Electrical cues have been found to enhance the nerve regeneration process and this has led to the increased usage of electrically conducive polymers like PPY and PANI in neural tissue engineering [18].
3. Scaffolds made from natural polymers
The extracellular matrix has numerous functions in the body. It provides structural and mechanical support to the tissues, facilitates migration of cells, holds the cells together, facilitates communication in cells so that daily cellular activities and wound healing can be performed uninterrupted [16], [19]. Natural polymers have advantage as scaffold design as they closely mimic the macromolecules that cells interact with in vivo. The frequently used natural polymers for scaffold design in neural tissue engineering are collagen, gelatin, hyaluronic acid, chitosan, chitin, elastin, and alginate (Table 1).
Table 1. Natural biomaterials and cells in neural tissue engineering.
| Natural Materials | ||||
|---|---|---|---|---|
| Material | Advantage | Disadvantage | Cells used | References |
| Collagen | Versatility, low antigenicity, inflammatory and cytotoxic response, biocompatibility, good water uptake capabilities, availability of several isolation methods, ability to tailor mechanical and cross linking properties. | Weak mechanical and structural stability upon uptake of water. | BMa-MSCsband Schwann cells. | [11], [15], [17], [19], [21], [22], [23], [24], [113] |
| Hyaluronic acid | Good biocompatibility, high water content, safe degraded products, limited immunogenicity, viscoelastic properties and ability to influence wound healing, metastasis etc. | Non-adherence of cells and water solubility. | Mouse MSCs and Schwann cells. | [22], [25], [26], [27], [28], [29], [30], [31], [32], [34], [114] |
| HYAFF | Biocompatibility, complete degradability, solubility in DMSO, stable on hydrolysis, strong interaction with polar molecules and ability to promote cell adhesion and proliferation. | Release of acidic degradation products, poor processability and loss of mechanical properties very early during degradation. | Schwann cells. | [36], [37], [38], [39], [115], [116] |
| Alginate | High biocompatibility, high biodegradability, non-antigenicity and chelating property. | Unstable mechanical properties and lack of the specific cell-recognition signals. | NSCsc ad Schwann cells. | [33], [40], [41], [42], [43], [118] |
| Chitosan | Biocompatibility, biodegradability, non-toxicity, inhibition of growth of fungi, yeast and bacteria and non-immunogenicity. | Some forms of chitosan may be toxic. | Schwann cells and BMSCd-derived Schwann cells. | [44], [45], [46], [47], [48], [49], [114], [119], [120] |
- a
-
BM – Bone marrow derived
- b
-
MSCs – Mesenchymal stem cells.
- c
-
NSCs – Neural stem cells.
- d
-
BMSC – Bone marrow stem cells.
3.1. Collagen
Collagen is one of the most widely studied constituents of the ECM due to its presence in all the connective tissues in the body. There are 28 types of collagen that have been identified, all characterized by a triple helical structure. Collagen molecules are composed of three alpha chains that assemble together. The amino acid sequence is characterized as Gly-X-Y-, wherein glycine is essential at every third position to enable the tight packaging structure of collagen. The presence of 4-hydroxyproline, formed during post-translational modification is a marker for collagen [20], [21]. Collagen is an eligible biomaterial for scaffolds pertaining to almost all types of tissues—it is used in bone tissue engineering, for skin, cornea potentially for the development of heart valves and other cardiovascular diseases, and so on [15], [19], [21]. The versatility of collagen is attributed to the widespread distribution of collagen in the body. Some other advantageous features of collage include low antigenicity, low inflammatory and cytotoxic response, biocompatibility, good water uptake capabilities, availability of several methods for isolation from various sources and the ability to tailor mechanical and cross linking properties as per need [17], [19]. Collagen 1 is suitable for implantation within the body because there are very few people who have humoral immunity against it. Moreover, a simple serological test can determine whether the use of this biomaterial will elicit an allergic response in the patient [22]. A self-organizing collagen guiding conduit was developed by Phillips et al., which is a Schwann cell containing conduit to be used at the site of injury in the Peripheral Nervous System. The implant was reported to show neurite extension from a dissociated dorsal root ganglia, and showed a greater regeneration over all [23]. Collagen-based scaffolds are fabricated through electrospinning, among other methods. Collagen does not have good mechanical and structural stability upon uptake of water. To prevent this, collagen can be used along with other natural and synthetic polymers to modify properties like mechanical strength, permeability rate, compressive modulus, cell number; cell metabolic activity etc. Different collagen concentrations bestow different properties to the scaffold [17], [21], [22]. For instance, to increase the strength of a collagen based, scaffold, its cross-linking with glutaraldehyde vapors, formaldehyde and epoxy compounds was done and the results obtained were that the scaffold mimicked the tensile strength of many commercially available products, like Beschitin™ and Resolute™. However, there are greater chances of cytotoxity and immune response using this [19]. Studies have reported that greater similarity of the nanofibrous collagen scaffold to the nerve tissue facilitates successful regeneration of neural tissue and thus makes an excellent scaffold for nerve regeneration [22]. Collagen scaffolds have been made as composites along with several synthetic scaffolds to enhance the mechanical strength of the scaffold. In one study, Poly (L-lactic) co-poly (3-caprolactone)-collagen nanofibrous scaffold was synthesized and bone marrow derived mesenchymal stem cells were incorporated in the scaffold. Results indicated that the differentiated MSCs on the PLCL/Coll scaffold had neuronal morphology with multipolar elongations and expressed neurofilament and nestin proteins, which confirm neuronal induction [24].
3.2. Hyaluronic acid (and its derivatives)
Hyaluronic acid (HA, also Hyaluranon) is a linear, non-branched non-sulfated glycosaminoglycan (GAG) and is composed of repeating disaccharides (β-1, 4-D-glucuronic acid (known as uronic acid) and β-1, 3-N-acetyl-glucosamide). It has sites for cell adhesion and is non-immunogenic [22], [25]. Hyaluronic acid has found many applications in tissue engineering, especially for its use as a hydrogel scaffold. HA is one of the main components of hydrogels, as it imparts the property of biodegradability to hydrogels made of non-biodegradable components like Poly-Ethylene Glycol (PEG) [25]. It plays a role pertaining to osteoarthritis, treatment, as an aid in eye surgery, and for wound regeneration. It is used in soft tissue replacement surgically and has several diagnostic applications. It serves as a diagnostic marker for cancer, rheumatoid arthritis, several liver pathologies as well as early organ rejection [26], [27]. Also, it has been explored a s a medium for drug delivery via several routes like nasal, oral, pulmonary, ophthalmic, topical, and parenteral. The possible targeting of cancer cells using HA has also been explored [28], [29], [30]. The several advantages of using HA for scaffold building are its excellent biocompatibility, high water content, capacity to degrade into safe products, limited immunogenicity, viscoelastic properties suitable for several tissue types and the ability to bind to specific cell surface receptors (CD44, RHAMM, and ICAM-1, etc.), thereby influencing several cellular processes. Thus, it is a plausible option for influencing processes like wound healing, metastasis, etc. [27], [29], [31] HA is especially advantageous for designing scaffolds for neural tissue regeneration due to its high abundance in the neural system (especially the Central Nervous System), making it an extremely biocompatible choice. Moreover, HA plays a role in wound healing. Several strategies for successful axonal regeneration using HA exist. HA also reduces glial scar formation. HA Hydrogel has a very porous structure, with interconnected pores that allow for the transportation of nutrition, penetration of cells, blood vessels and nerves. Use of HA scaffold in vivo has reported less glial scarring, smaller gliosis thickness and lesser glial fibrillary acidic protein (GFAP) positive cells around the scar area. A dominant disadvantage associated with the use of HA is that cells do not adhere to its surface. Thus, to overcome this, it is blended with other biomaterials which will enhance the binding of cells and consequentially increase neural tissue regeneration. An example is collagen-HA scaffold, which had desired mechanical properties for CNS regeneration and promoted differentiation of Neural Stem Cells (NSCs) for neural regeneration in vitro [32]. Another disadvantage of HA is its water solubility, which makes it necessary to add a cross-linking agent to convert it into injectable form [22], [33]. In one study, HA hydrogel that could be degraded by cell released enzymes was fabricated and seeded with mouse mesenchymal stem cells. The HA hydrogel was modified to contain acrylate groups and cross linked using matrix metalloproteinase (MMP) degradable cross linkers. Results indicated that faster MSC proliferation and migration occurred in the presence of RGD and MMP degradation sites in the hydrogel [34]. In another study, HA strands were cross linked using glutaraldehyde and coated with polylysine. This was then seeded with Schwann cells. Results indicated higher attachment of cells, water insolubility of HA and lesser biodegradability, hence making it a potent nerve graft [35].
3.3. HYAFF
HYAFF is an HA derivative obtained by esterification of HA with benzyl ester. Some favorable properties of HYAFF are its biocompatibility, complete degradability, solubility in DMSO, stable on hydrolysis, strong interaction with polar molecules and its ability to promote cell adhesion and proliferation of various cell types [36]. Moreover, HYAFF can be used to design films, non-woven fabrics, gauzes, sponges, tubes and microsphere, thus broadening its applications. It is used for tissue repair, controlled drug release, nerve regeneration, delivery of growth factors, wound healing, etc. [37]. Experiments have reported HYAFF based tube scaffolds are good substrates for nerve cell cultures and explants. It was reported that cells from rat sciatic nerves showed adhesion to the scaffold, followed by proliferation and colonization of the scaffold. Its properties are ideal for Peripheral Nervous System Tissue Regeneration [38]. The ability of HYAFF to support adhesion and proliferation of Schwann cells and endothelial cells obtained from peripheral nerve has been studied. Results indicated that HYAFF is a good choice of biomaterial for peripheral nerve cell culture and nerve explants [36], [39].
3.4. Alginate
It is a naturally derived, linear polysaccharide obtained from brown algae and bacteria. It is composed of (1–4)-linked β-D-mannuronic acid (M) and α-L-guluronic acid monomers (G). Alginate is pH dependent anionic and can thus interact with positively charged proteoglycans and other molecules, a property which can be utilized for delivery of cationic drugs. Also, alginate hydrogels are formed by making them interact with divalent cations in aqueous solution [33], [40]. The advantages of alginate as a scaffold are its high biocompatibility, high biodegradability, non-antigenicity and chelating property. The several types of scaffold that can be formed using alginate are summarized in a review by Sun & Tan et al. A study was conducted to evaluate the effects of alginate concentration on neurotropic factor release. The study inferred that NSC-seeded alginate beads with a high G, non-PLL-coated composition may be useful in the repair of injured nervous tissue, where the repair is facilitated by the secretion of neuroprotective factors, while the alginate beads coated with PLL and a high M concentration were fragile and susceptible to maximum breakage [41]. Alginate hydrogel (AH) has been used as a bioresourceable conduit supplemented with Schwann cells to replace nerve grafts. Enhanced neurite growth, viability and proliferation were observed [42]. Alginate conduits seeded with Schwann cells have also been fabricated along with the incorporation of fibronectin. Results indicated that regeneration rate, viability of the cells and axonal growth were enhanced using this conduit [43].
3.5. Chitosan
Chitin is naturally found in the cell walls of crustaceans such as insects, crabs, shrimps as well as the cell wall of bacilli and fungi [17], [44]. Chitosan is obtained by the alkaline deacetylation of chitin. The degree of deacetylation of chitin is process-dependent. The degree of deacetylation along with the molecular weight affects several properties like solubility, mechanical strength and degradation. The evaluation of chitosan in terms of mechanical properties, growth factor delivery, etc. concluded that it is an excellent choice for construction of scaffold [44]. It has found several applications like in bone tissue engineering, skin tissue engineering, etc. Several advantages of chitosan are its biocompatibility, biodegradability, non-toxic, inhibition of growth of several fungi, yeast and bacteria and non-immunogenicity. Due to the absence of any protein or lipid in chitosan structure, no antibody can be developed against it [45]. Chitosan has also been proved to be a good choice for neural tissue regeneration [46]. Chitin incorporated with other materials show an affinity for nerve cells. Chitin based nerve chambers can be used for effective nerve regeneration over long gaps as well as functional recovery. A dog sciatic nerve gap of 50 mm was repaired using chitosan-based scaffold. Chitosan based scaffolds promote the adhesion, growth and migration of Schwann Cells. This is very important because Schwann cells release neurotropic factors, express neuron-specific ligands and guide neurite outgrowth, thus playing an irreplaceable role in nerve tissue regeneration. Schwann cells also secrete and deposit ECM. Moreover, it has the mechanical strength necessary for effective neural regeneration. Chitosan conduits resulted in the sprouting of myelinated axons and successful recovery of nerve function [47]. Chitosan scaffold pre-seeded with Schwann cells was synthesized as a bio-artificial nerve graft for peripheral nerve regeneration. Good biocompatibility of the Schwann cells and chitosan fibers was observed [48]. In another study, chitosan conduit seeded with bone marrow stromal cell (BMSC) derived Schwann cells was fabricated. Results indicated the profound efficacy of the conduit in treating critical peripheral nerve defects by supporting nerve conduction, myelin regeneration and development of myelinated axons [49].
4. Scaffolds made from synthetic materials
Although the gold standard for the treatment of nerve injuries which are greater than 5 mm in size is said to be autologous nerve grafts, they have several shortcomings, including limited availability of graft tissue, donor site morbidity, neuroma formation, nerve site mismatch, and sometimes possible lack of functional recovery. Allogenic grafts pose the obvious disadvantage of immune rejection [17], [46]. To overcome the disadvantages posed by these grafts, synthetic scaffolds are being devised that exactly mimic the biological and mechanical properties of the cells and ECM in vivo. Due to the extreme versatility of the composition and formation of polymers, polymeric scaffolds are proving to very efficient scaffold materials [45], [46]. Polymeric biomaterialscaffolds can be used for peripheral and central nerve injuries, both in vivo and in vitro. Properties like biodegradability, non-inflammatory, non-toxicity, porosity and other mechanical properties can be easily engineered in these polymeric scaffolds, suitable to the characteristics of the in vivo tissue where the graft should be implanted [16]. The hydrophilicity of the synthetic polymer also plays a crucial role, as hydrophobicity elicits monocyte adhesion to the graft, leading to immune rejection of the graft. This property can also be easily tailored in synthetic polymers. Some widely used synthetic polymers for the fabrication of scaffolds include Poly -L-lactic acid (PLLA), Polycaprolactone (PCL), Polyhydroxybutyrate (PHB), Polyglycerol sebacate, Poly-D,L-lactide-co-glycolic acid (PLGA), Poly-L-lactate (PLA), Poly-3-hydroxybutyrate (PHB), Polyamide, Polydioxanone, Poly-e-caprolactone-co-ethyl ethylene phosphate (PCLEEP), Poly-D.L-lactide-co-caprolactone (PDLLA), Polyvinyl alcohol (PVA), Poly acrylonitrile-co-methylacrylate (PAN-MA) and copolymer of methyl methacrylate (MMA) and acrylic acid (AA) (PMMAAA) [50]. Conductive polymers like Polypyrrole (PPY) and polyanaline (PANI) are also gaining popularity in nerve tissue engineering by their ability to conduct electrical signals (Table 2).
Table 2. Synthetic biomaterials and cells in neural tissue engineering.
| Synthetic materials | ||||
|---|---|---|---|---|
| Material | Advantage | Disadvantage | Cells used | References |
| PCL | Biodegradable, biocompatible, possesses high elasticity, low toxicity, good mechanical properties and a slow degradation profile. | Cytotoxic effects on using organic solvents. | Adipose-derived multipotent SCs, hMSCsa, NCSCsb and Schwann cells. | [17], [51], [53], [54], [55], [57], [58], [114] |
| PLLA | Biodegradable, ultrafine continuous fibers, high surface-to-volume ratio, high porosity, varied distribution of pore size | Poor biocompatibility, release of acidic products on degradation, poor process ability and premature failure of mechanical features during degradation. | MSCs, NSCsc and Schwann cells. | [22], [59], [60], [61], [62], [114], [121], [122] |
| PLGA | Biodegradability, non-toxicity and film forming ability. | Plastic deformation and failure on exposure to long term strain, releases acidic products on degradation. | NSCs, NPCsd and Mouse embryonic fibroblasts. | [17], [22], [63], [114], [123] |
| PHB | Potential neural protective agent, high crystallinity, longer degradation time, and provides support for cell adhesion and proliferation (of osteoblasts, fibroblasts). | Poor biocompatibility. | OECse, MSCs, NSCs and hMSCs. | [66], [113], [124], [125], [126] |
| PVA | Non-toxic, hydrophilic and high mechanical strength. | Poor biocompatibility. | Dorsal root ganglia. | [67] |
| PPY | Exhibits rigidity, good biocompatibility and cell adhesion properties, non-toxic, non-allergic, non-mutagenic and non-haemolytic. | Insoluble, non-biodegradable and poor process stability. | Schwann cells. | [22], [68], [69], [114], [117] |
| PANI | Versatility, conductive, good biocompatibility and increased neurite outgrowth. | Inability to degrade and chronic inflammation. | hMSCs and NSCs. | [70], [71], [72] |
| PEDOT | Versatility, conductive, good biocompatibility and increased neurite outgrowth. | Inability to degrade and chronic inflammation. | hMSCs and NSCs. | [70], [71], [72] |
| CNTs | Superior conductivity, remarkable stiffness, high aspect ratio, maintains structural stability of scaffolds, biocompatibility, optimal nanotopography and induces conductivity. | Cytotoxicity and non-biodegradability. | hNSCs, hMSCs, Mouse embryonic NSCs and hESCsf. | [22], [72], [73], [74], [75], [76], [77], [78], [127] |
- a
-
hMSC – human Mesenchymal stem cells
- b
-
NCSC – Neural crest stem cells
- c
-
NSC – Neural stem cells
- d
-
NPC – Neural progenitor cells
- e
-
OEC – Olfactory progenitor cells
- f
-
hESC – human Embryonic stem cells.
4.1. Polycaprolactone (PCL)
PCL is a biodegradable and biocompatible polymer, which is cost efficient, possesses high elasticity, low toxicity, good mechanical properties and a slow degradation profile [17], [51]. It has been widely applied in tissue engineering including bone and neural tissue engineering. PCL fibers have most commonly been generated by the method of electrospinning [52]. But it has been experimentally observed that fabrication or bioactive molecule encapsulation using organic solvents may engender cytotoxic effects when the graft is being implanted in the body. A solvent free method, template synthesis was used where PCL fibers were synthesized using Alumina nanoporous membrane. NaOH was then used to dissolve the template and yield PCL nanowires [51]. PCL has been used in conjugation with natural polymers or coated with cells to improve its biocompatibility and cell adhesion properties. This will confer mechanical strength and biocompatibility to the scaffold. A tubular prosthesis of PCL in combination with aligned collagen was synthesized to direct aligned axonal growth of the nerve fibers. Aligned implants allow the regeneration of nerve fibers in a contact-oriented fashion which increases the growth of the fibers [53]. In one study, PCL fibers have been extruded and embedded in poly-2-hydroxyethyl methacrylate (HEMA) gel. The sonication of the PCL/HEMA composite was dissolved in acetone solvent leaching out PCL and leaving behind HEMA gel with oriented longitudinal channels embedded in it. The channel diameter can be controlled by altering the concentration of PCL used in the composite. The fabrication of such gels using synthetic polymers with oriented channels will provide support and contact guidance to regenerating neuritis and axons [54]. PCL nanotubes have also been used in conjugation with Adipose-derived (multipotent) stem cells to improve the effects on nerve regeneration [55]. A PCL scaffold seeded with human mesenchymal stem cells was shown to induce adipogenic, chondrogenic and osteogenic lineages [17]. In another study, nonwoven aligned nanofibrous nerve conduits were synthesized, composed of poly (L-lactide-co-caprolactone) and polypropylene glycol in a ratio of 70:30 by electrospinning seeded with neural crest stem cells (NCSC) obtained from ESCs and iPSCs. NCSCs are multipotent in nature and can give rise to cells of mesodermal and ectodermal lineages. Results indicated that NSCS-engrafted nerve conduits had faster regeneration potential of sciatic nerves. These cells also promoted axonal myelination. It was observed that NCSCs differentiated into Schwann cells and then integrated into the myelin sheath around axons [56]. PCL has also been used directly in conjugation with Schwann cells. Aligned PCL fibers fabricated by electrospinning seeded with human Schwann cells indicated enhanced peripheral nerve regeneration by promoting formation of bands of Bungner [57]. Aligned and random nanofibrous PCL/gelatin scaffolds fabricated by electrospinning were seeded with Schwann cells to assist the direction of growth of regenerating axons in peripheral nerves [58].
4.2. Poly-L-lactic acid (PLLA)
PLLA is a biodegradable and biocompatible synthetic polymer. Nano-structured PLLA scaffolds are widely used in neural tissue engineering owing to its analogous structure to the natural ECM of nerve cells, including ultrafine continuous fibers, high surface-to-volume ratio, high porosity, varied distribution of pore size from 50 to 350 nm. It contains ester linkages in the polymer backbone which lead to bio-functionalization of the polymer with various biomolecules by means of covalent conjugation. This polymer also poses several disadvantages including poor biocompatibility, release of acidic products on degradation, poor process ability and premature failure of mechanical features during degradation. In one study, PLLA conjugated with mesenchymal stem cells was made into a nanofibrous scaffold. It was shown to induce differentiation into different neurogenic lineages by culturing in differential media [22]. It has been experimentally proven that electrospun aligned PLLA fibers are shown to support neurite extension and axon regeneration better than electrospun random fibers, although random and aligned fibers yielded the same results with respect to NSC differentiation [59]. 3-D PLLA nano-structured porous scaffolds have been fabricated using liquid-liquid phase separation methods. The fabricated scaffold seeded with NSCs showed significant improvement in NSC differentiation and neurite outgrowth [60]. Several studies have proven that PLLA scaffolds efficiently support NSC differentiation and neurite outgrowth, thereby making them suitable to applications in neural tissue engineering. PLLA conduits incorporated with allogenic Schwann cells were studied to venture into the synthesis of bioactive nerve conduits which can potentially replace autologous nerve grafts and peripheral nerve regeneration [61]. A heparin/collagen encapsulating nerve growth factor multilayer was coated upon a PLLA scaffold using layer-by-layer self-assembly mechanism. The scaffold aimed to provide biomolecular as well as physical cues to the regenerating nerve. Sustained release of nerve growth factor for two weeks, as well as superior benefits to Schwann cell proliferation and its cytoskeleton, PC12 differentiation and neurite growth was observed as compared to the aligned PLLA nanofibrous scaffolds [62].
4.3. Poly-D,L-lactic-co-glycolic acid (PLGA)
PLGA is a copolymer of polylactic acid (PLA) and polyglycolic acid (PGA). It is amorphous biodegradable polyester and is widely used for fabrication of scaffolds in neural tissue engineering due its biodegradability, non-toxicity and film forming ability. PLGA is said to have similar properties to that of skeletal tissue. It has also been effectively employed as a scaffold for regeneration of heart valves, blood vessels and cartilage. It poses a disadvantage, when exposed to long term cyclic strain exposure; it undergoes plastic deformation and fails [17]. It also releases acidic products on degradation, leading to a decrease in pH of the tissue which may result in aseptic inflammation. To overcome this, the PLA to PGA ratio can be controlled during the synthesis of the polymer. Since PLA is more hydrophobic and crystalline in nature than PGA, the degradation profile of PLGA can be altered and made slower by using higher concentrations of PLA in the synthetic polymer. Another major disadvantage is the lack of natural adhesion sites, as it is a synthetic polymer. This can be rectified by using techniques like hydrolysis, aminolysis, blending and covalent attachment of adhesive peptides. It has been approved by the FDA for biomedical applications. Its other areas of application include pharmaceutical, medical engineering and various other industries. In one study, PLGA nanofibers were generated using electrospinning technique and NSCs were seeded onto the scaffold. Results indicated high efficiency of cell adhesion, nerve cell differentiation and neurite outgrowth [22]. In another study, the PLGA scaffold was conjugated with PEG (Polyethylene glycol) for improved mechanical strength and seeded with mouse embryonic fibroblasts reprogrammed into induced neural stem cells (iNSCs) for the treatment of spinal cord injury (SCI). The scaffold induced continuity of the spinal cord and led to a significant reduction in cavity formation. PEG, which is composed of repetitive oxygen vinyl has higher hydrophilicity, and hence forms a great composite with PLGA by overcoming its shortcomings. PEG has also been shown to protect some important axonal cytoskeleton proteins to promote repair in SCI. Thus, the composite scaffold exhibited greater iNSC adhesion, cell growth and proliferation [63]. A PLGA scaffold was fabricated with the objective of inhibition of neurite growth. This was achieved by silencing Nogo-66 receptor gene using small interfering RNA in BM-MSCs and SCs. The strategy was seen as effective for spinal cord injury treatment [64].
4.4. Polyglycerol sebacate (PGS)
PGS is a chemically cross-linked elastomeric polymer and confers a high amount of toughness to the scaffold. It can support several cells including fibroblasts, hepatocytes, endothelial, smooth muscle, cardiac muscle and Schwann cells [17]. In one study, non-linear elastic biomaterial scaffold was fabricated using a PGS/PLLA composite. The fabrication was done using core and shell electrospinning technique. The results indicated that elastomeric mesh aids the growth and proliferation of enteric neural crest progenitor cells (ENCs). The scaffold also exhibited soft, tissue like mechanical properties on studying the stress-strain curve [65].
4.5. Polyhydroxy butyrate (PHB)
PHB is biodegradable aliphatic polyester synthesized by several bacteria using renewable carbon sources. Since the monomeric component of PHB, 3-hydroxy butyric acid (3-HBA) is a stable analyte in human serum and microbial PHB is recognized by mammals, PHB is widely applied as a biomaterial and is FDA approved. A study reported that PHB is a potential neural protective agent and acts by reducing the number of apoptotic glial cells in mice. It has high crystallinity, resulting in a brittle nature of scaffold and longer degradation time. A PHB blend with poly-3-hydroxy butyrate-co-3-hydroxyvalate (PHBV) scaffold has been found to provide better support for adhesion and proliferation of osteoblasts and fibroblasts. In one study, PHB films were blended with poly-L-lactide-co-caprolactone (PLCL), which is a copolymer of PLA and PCL. PLCL has been used in several applications including controlled-release drug delivery systems, monofilament surgical sutures and absorbable nerve guides. It is an FDA approved polymer for biomedical applications. This scaffold was reported to support cardiovascular, cartilage and nerve regeneration. The composite scaffold possesses better physical and mechanical properties and promotes enhanced cell adhesion, differentiation and proliferation. The study used olfactory ensheathing cells (OECs), which are an important class of glial cells promoting nerve regeneration of olfactory system, which in turn plays a crucial role in neural growth of CNS and PNS. The scaffold was shown to display a much favorable fiber diameter and increased hydrophilicity, which led to better proliferation of OECs [66].
4.6. Polyvinyl alcohol (PVA)
PVA is a non-toxic, hydrophilic and biocompatible polymer. Although it has high mechanical strength, its biocompatibility is not satisfactory. Hence, composites of PVA are made with natural polymers to enhance the overall physiochemical and biological properties of the fabricated scaffold. In one study, PVA fibers were blended with chitosan and the scaffold was fabricated using electrospinning. The results generated showed that the scaffold had balanced properties apt for neural cell regeneration [67].
4.7. Conductive polymers
Neural tissue engineering requires simultaneous biochemical, topographic and electrical cues for complete and efficient adhesion, differentiation and proliferation of cells. To enhance topographic cues, aligned fiber scaffolds are being generated by electrospinning that favors neurite extension unidirectional [68]. Biochemical cues are enhanced using composite scaffolds of synthetic and natural polymers, or by embedding cells like Schwann cells, stem cells or growth factors on the surface of the scaffold. Since neurons are involved in electrical signal transmission, electrical cues play a crucial role in directing the development of axons and neurite growth. Conductive polymers are those with electrons present in their backbone, which enables them to conduct electricity. Skeletal and nerve cells respond to electrical stimulation. Some common conductive polymers used are Polypyrrole (PPY), Poly-3, 4-ethylenedioxythiphene (PEDOT), Polyaniline (PANI) and carbon nanotubes.
4.7.1. Polypyrrole (PPY)
PPY is a conductive polyacetylene derivative that is used in drug delivery systems, nerve regeneration and biosensor coatings for neural probes. Its characteristic features include exhibits rigidity, insolubility and poor process stability which occur due to the conjugation in the molecular backbone of PPY. Its non-biodegradability poses a major challenge to its use in neural tissue engineering, but this could be overcoming by making composites of PPY [68]. Hence, it is usually conjugated with other synthetic or natural polymers to obtain a stable, biocompatible and biodegradable scaffold. PPY is more suited for peripheral nerve injury regeneration, has good biocompatibility and cell adhesion properties. It is also non-toxic, non-allergic, non-mutagenic and non-haemolytic, making it ideal for biomedical applications. In one study, PPY conductive meshes were formed on random and aligned electrospun PLGA fibers. The results indicated that the scaffold induced better neuronal growth and differentiation than PLGA scaffolds without PPY coating [22]. In another study, PPY were polymerized on electrospun PLA and PCL templates generating conductive core-sheath nanofibers. The results indicated that PPY deposited on PCL was much smoother than that of the PLA fibers. This occurred due to the presence of a methyl group on PLA side chain, making it less hydrophilic in nature than PCL. Increased neurite extension was observed due to electrical stimulation. Also, it was greater on PCL/PPY scaffolds owing to the smooth texture, as neurons have smooth surfaces due to the myelin sheath present on them. The efficiency of electrical stimulation is said to be caused due to an increased adsorption of ECM glycoproteins onto the surface of PPY, which results in better cell adhesion and growth [68]. In one study, the effect of electrical stimulation on Schwann cells was studied using Polypyrrole in conjugation with chitosan. Results indicated that the PPY/Chitosan scaffold supported cell adhesion, spreading and proliferation. In the presence of electrical stimulation, enhanced expression of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) was observed. Hence, nerve regeneration can be significantly enhanced using electrical stimulation on conductive scaffolds by means of increased neurotrophin secretion [69].
4.7.2. Polyaniline (PANI) and poly-3,4-ethylenedioxythiophene (PEDOT)
PANI and PEDOT are versatile conducting polymers. In one study, it was shown that differentiation of human mesenchymal stem cells (MSCs) was increased primarily due to the use of hybrid macroporous hydrogels made of PANI and PEG diacrylate. Dispersing the conductive polymer with a biological matrix facilitates easier fabrication of conductive scaffolds. In another study, the characteristics of scaffold deposited with PEDOT were also studied, and it was observed that they increase axon growth in nerve conduits. PANI and PEDOT were chemically synthesized and then added to a solution of collagen, after which the cell suspensions were made. The 3-D conductive collagen gels exhibited good cyto-compatibilty and increased neurite outgrowth as compared to non-conductive collagen gels [70]. An electrospun conductive nanofiber scaffold was fabricated using PANI with PCL/Gelatin. This scaffold was then seeded with nerve stem cells and electrical stimulation was applied. Results indicated enhanced attachment, proliferation and neurite outgrowth of NSCs [71]. PEDOT has been cross linked with the polymer Polystyrene sulfonate (PSS) to obtain a conjugated polymer scaffold. Human neural stem cells were then seeded onto this scaffold. On electrical stimulation, elongation and differentiation of NSCs into neurons and formation of longer neurites was observed [72].
4.7.3. Carbon nanotubes
They are a type of conducting polymers that need to be functionalized to aid neural signal transport, dendrite adhesion and elongation. They can be functionalized by substitution reactions like replacing carbon atoms from tube wall using boron or nitrogen [22]. They exhibit characteristic qualities like superior conductivity, remarkable stiffness and high aspect ratio. They also have an innate ability to absorb strain and induce conductivity. It maintains the structural stability of scaffolds when incorporated [72]. One study demonstrated that polyethyleneimine (PEI) deposited with multi-walled CNTs (MWCNTs) exhibited increased neurite growth and elongation in all directions [22]. Another study used carbon nanotube fibers (CNF) made with the natural polymer agarose. The agarose/CNF composite is conductive, non-toxic and functionalization can facilitate improved cell adhesion and proliferation. Results indicated that it generated directed nerve growth and overall increased differentiation and proliferation [73]. CNT network patterns have been used for selective growth and structural polarization-controlled neuronal differentiation of human NSCs into neurons. The CNT patterns were found to provide synergistic cues, optimal nanotopography and biocompatibility for differentiation of hNSCs in physiological solution. Thus, CNTs have been proven to be a highly suitable scaffold for controlling hNSC growth [74]. CNT networks on solid substrates have also been used in the directed growth and differentiation of hMSCs. It has been reported that the hMSCs could recognize the arrangement of individual CNTs in the CNT network, which allowed directional growth of hMSCs following the direction of alignment of the CNTs. These hMSCs were found to exhibit enhanced proliferation and osteogenic differentiation [75]. Another form of CNT has been studied, where a CNT rope substrate was developed and seeded with neural stem cells. On electrical stimulation, it was observed that it had a positive impact on promoting neurite elongation and enhanced differentiation of NSCs into mature neuronal cells [76]. Successful single walled carbon nanotube (SWNT) scaffolds have also been demonstrated. Layer-by-layer (LBL) assembled SWNT- polyelectrolytemultilayer thin films seeded with mouse embryonic neural stem cells from cortex have been found to successfully differentiate into neurons, astrocytes and oligodendrocytes with clear formation of neurites. The biocompatibility, neurite outgrowth and expression of neural markers were found to be similar as cells differentiated on poly-L-ornithine (PLO), which is a common growth substratum for neural stem cells [77]. hESCs have also been seeded on CNTs and proved to be successful candidates for neuronal differentiation. A poly (acrylic acid) grafted CNT thin film was fabricated and compared with PLO surfaces. This scaffold exhibited enhanced neuron differentiation and cell adhesion [78].