Graphical Abstract
Keywords
1. Introduction
The development of pressure ulcers (PUs) in bedridden patients is an iatrogenic complication that significantly reduces quality of life by causing pain and prolonging hospital stays [1,2]. PUs heal slowly, are difficult to treat and have a high risk of recurrence [3]. The reported PU incidence in hospitals is approximately 12% but ranges from 8 to 40%, depending on country, type of hospital (academic, non-academic) and department [4]. PUs are wounds induced by sustained tissue deformation caused by a combination of pressure, shear, temperature and humidity [5]. This deformation can directly damage cell structures or impair blood perfusion, lymphatic function and transport between interstitial spaces which causes ischaemia, tissue damage and cell death [6,7]. The modified Reswick and Rogers curve indicates that the PU risk is dependent on cell deformation, time and individual characteristics. PUs usually occur in areas of the body where only a small layer of tissue is situated between the bone and the surface such as the sacrum, coccyx, heels, ankles and thighs or where medical instruments, with hard surfaces, contact the skin such as with oxygen masks or instrumental wires.
PUs can be prevented by taking timely measures to avoid prolonged tissue deformation [8,9]. Identifying patients at risk is important to provide these patients with frequent body turns, pressure-reducing support surfaces and to secure a healthy skin condition [10]. Tissue deformation is ideally monitored directly to assess the PU risk. Unfortunately, this deformation can only be measured at low resolution with bulky short-time measurement devices such as ultrasound, MRI and CT and only in high resolution with even more impractical ex vivo micro-CT imaging [11]. Therefore, in a clinical setting tissue deformation can be estimated for example by using interface pressure maps of the skin on the mattress. A higher interface pressure often causes higher internal pressures, generally causing a larger tissue deformation with corresponding higher PU risk. However, every patient has a different anatomy, fat distribution and tissue condition, which changes the tissue tolerance for pressure and the correlation between interface pressure and tissue deformation [12]. Thus, although interface pressure should be kept at a minimum, the critical interface pressure for PU development over time is different for every patient and is also dependent on the body part that is strained by the pressure.
Regarding the contribution of the microclimate to tissue deformation, research has shown evidence of a negative effect on PU formation for both low and high temperatures as well as low and high humidity [7,[13], [14], [15]]. Consequently, the goal should be to limit extreme temperature and humidity levels of the tissue. Current guidelines [10] advise all bedridden patients to be turned on an individualized schedule depending on their mobility, but patients should reposition themselves or with assistance at least every 4 hours, which, besides pressure relief, also positively affects humidity and temperature. Tracking of the turns and the positions and thereby the location of the load on the body allows to relieve specific body parts that are disproportionally strained [16,8]. However, frequent monitoring and executing patient turns is a significant time investment for the already over-busy nursing staff. Therefore, technical solutions that can assist with this monitoring task are highly welcomed.
Sensor technology equipped with body position detection (BPD) is crucial for monitoring the PU risk. BPD has three important advantages compared to plain pressure, temperature or humidity measurements. First, the body position itself provides a significant amount of information on the duration of the pressure load on the skin and an estimation of the location of the tissue at risk. For PU prevention, information about the angle of the trunk is important to estimate the side of the body that is loaded with pressure. For example, BPD can detect whether it is the tissue around the hip or coccyx that is strained. Second, it enables plain pressure, temperature or humidity values to be assigned to an anatomical position, which allows the tissue at risk to be tracked over time. Additionally, this would allow the plain values to be combined into an advanced PU risk model factoring for different (personal) anatomical thresholds. An additional advantage of BPD is that the turn frequency has been well-researched [17], [18], [19], [20] and thus the 4-hour time-interval threshold can be used to send out timely warning signals. Finally, the value and necessity of BPD have been shown by a study which monitored general patient movement and could not find a correlation between PU formation and movement [21]. The authors postulated that high-frequency small movements increase both shear forces that act on the skin and cause friction, elevating temperature and moisture levels, which increases tissue deformation. Thus, it is important to differentiate between effective and non-effective movements.
Previous reviews also focussed on sensor technology as means of PU prevention. In 2015, Marchione et al. [22] summarized the available sensor techniques. Therefore, our current review continues from this point in time. Mansfield et al. [23] published a survey on various approaches for preventing pressure ulcers and also included active prevention strategies. Both the review and the survey were broad and did not focus on BPD. Silva et al. [24] concentrated on reviewing the data processing abilities and specifications of the algorithms. Finally, Moore et al. specifically focussed on movement detection and reported a large heterogeneity between studies and found a lack of consensus on defining clinically relevant movements based on the included articles. Therefore, we focussed on a detection method that is more likely to be clinically relevant. Consequently, in this systematic review we assessed the clinical applicability of sensor types that can detect in-bed body positions.
2. Methods
2.1. Database search
The scope of this systematic review concerned BPD sensor systems for the prevention of PUs. Therefore, the search was conducted on databases focusing on the fields of medicine and computer science. The list of these databases and their electronic addresses is presented in figure 1. The search was conducted on October 25th 2022. Synonyms of pressure ulcers and words related to sensor, technology and measurements were included in the search string that is presented below (complete strings per database are included in the appendix):
 Fig. 1
Fig. 1(decubitus OR bedsore OR pressure ulcer OR pressure-sensitive-mat) AND (sensor OR devices OR early diagnosis OR sensor* OR sensing OR sense* OR bedsens* OR early-detect*) AND (technology OR monitor OR monitoring OR measurement OR measuring* OR measurer*)
2.2. Manual screening of title and abstract
After the databases were searched, the title and abstract were manually independently in- or excluded by two scientists (TvH and AMvD) using the following in-and exclusion criteria:
Inclusion:
-
•
Test subjects and patients of all age groups measured in-bed
-
•
Continuous automatic body position monitoring systems
-
•
Articles from 2015 onwards
Exclusion:
-
•
Lack of in-bed BPD
-
•
Animals
-
•
Less than 3 test subjects or patients
-
•
Case reports, narrative reviews, expert opinions, editorials, conference proceedings, patents
-
•
Study protocol only
-
•
Redundant -> the author has published a more recent paper describing the same with a larger dataset
-
•
Cameras -> privacy concerns, issues when a quilt is used and low light noise [25]
2.3. Data extraction
After the title and abstract screening was completed, the included articles were entirely read for the following properties:
Authors •Publication year •Hardware used for monitoring the patient •Manufacturer •Medical •CE •Study population •Type of population •Were the participants instructed? •Detectable body positions •Number of hours/samples •Reported BPD accuracy •Location of the sensor •Extensiveness of accuracy report
3. Results
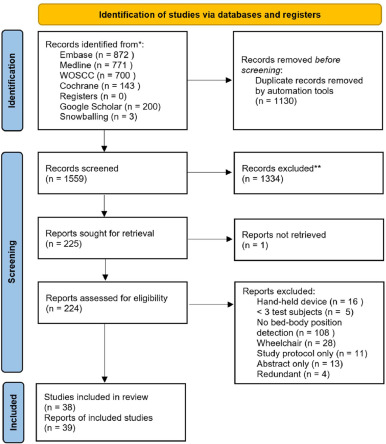
The database search resulted in 2689 articles of which 1130 duplicates were removed. During selection, 1334 articles were rejected based on reading the title and abstract. After reading the full text, 185 of the 224 articles were rejected based on exclusion criteria that were not previously identified during the title and abstract screening. Thus, 39 articles were included in this systematic review. The PRISMA flow diagram is shown in figure 1. Data from the included articles were extracted and are shown in table 1.
Table 1. Overview of the sensor types
| Sensor Type | Detection | Position | Applicability |
|---|---|---|---|
| Inertial sensors | + High repositioning detection accuracy including body turn angle |
- Wearable - Risk of rolling on the sensor - Detached sensors - Contact with the skin - Higher risk of infection |
+ Warning system present + Medical CE approval |
| Piezoresistive sensors |
+ BPD and pressure - Sensitive to drift |
- On top of the mattress - Reduced comfort |
+ Adjustable dependent on application |
| Piezoelectric sensors |
+ Also detects heart rate and respiratory rate - Limited BPD categories |
+ Under the mattress | - Signal is easily disturbed |
| Load sensors | - Limited BPD, no prone position | + Inside or under the bedframe |
+ Durability + Ease of use |
| Radio wave-based sensors | - Susceptible to interference | + No patient contact | - External antenna's |
| Capacitive sensors | + Pressure and BPD | - On top of the mattress |
+ Accurate pressure reading possible - Bulky |
+ represents advantages, whereas – are disadvantages
The 39 included articles were categorized into 6 subsections based on sensor types: the two most studied sensors were inertial (n=14) and piezoresistive sensors (n=12), followed by piezoelectric sensors (n=3), load sensors (n=4), radio-wave-based sensors (n=3) and capacitive sensors (n=3). First, the working mechanism per sensor type is discussed, followed by the sensor variance, validation, clinical use and availability. Table 1 presents an overview of the properties of each sensor type.
3.1. Body positions
The results presented in table 2 and 3 show the large variety in the type and number of bed positions that were discerned. The most common positions that were detected are supine, left lateral and right lateral followed by the prone and fowler supine position. Additionally, variations in the positions of the extremities in the lying positions were adopted such as left and right foetus, supine with a bent left or right leg and supine with arms parallel to the body or with the extremities wide. Finally, positions were categorised based on the location in bed, for example, lying on the left side of the bed, supine lying to the left or sitting on the side of the bed. The inertial sensors used the torso angle directly with a threshold turn angle of -20 or +20 degrees to differentiate between the supine and left or right lateral, whereas the transition angle from, for example, left lateral to prone was not specified for other sensor types. Additionally, some sensors used a movement score, in which they differentiated between large and small movements.
Table 2. Different body positions from the extracted articles as described in detail in table 3
| # | Position | Number of articles |
|---|---|---|
| Prime positions: | ||
| 1 | S=Supine | 27 |
| 2 | L=Left lateral | 25 |
| 3 | R=Right lateral | 25 |
| 4 | P=Prone | 15 |
| 5 | FS=Fowler Supine (sitting) | 8 |
| 6 | O=Out of bed | 1* |
| Extremity positions: | ||
| 7 | Fl=left foetus (legs pulled up) | 4 |
| 8 | Fr=right foetus (legs pulled up) | 4 |
| 9 | SL=supine with bent left leg | 2 |
| 10 | SR=supine with bent right leg | 2 |
| 11 | Sp=supine arms parallel to body | 1 |
| Location in-bed: | ||
| 12 | Ss=sitting on the side of the bed | 2 |
| 13 | LL2=Lying on the left side of the bed; | 1 |
| 14 | LR2=lying on the right side of the bed | 1 |
| 15 | LL=supine lying to the left | 1 |
| 16 | LR=supine lying to the right | 1 |
*One article included out of bed as a position, other articles did not report it separately
Table 3. Detailed overview of the included articles
| 1st Author | Pub. Year | Sensor type | Manufacturer / name | Medical CE | Study pop | Population type | Instructed | Body positions | Hours/samples | Reported Accuracy | Extensive report |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inertial sensors | |||||||||||
| D. Pickham [8] | 2018 | IS | Leaf Healthcare | yes | 1312 | 718MP, 594FP | no | Body angle | 103,000 hours | not spec. | - |
| T.L. Yap (2) [26] | 2022 | IS | Leaf Healthcare (not specified) | yes | 992 | 629MP, 363FP | no | Body angle | 16.06-17.44 days per patient |
Margin of error: ±2.5 degrees |
- |
| J. Maguire [9] | 2021 | IS | Leaf Healthcare | yes | 154 | 83MP, 71FP | no | Body angle | 74,523 hours | not spec. | - |
| S. C. Schutt [27] | 2018 | IS | Leaf Healthcare | yes | 138 | 68MP, 70FP | no | Body angle | Base: 4,322+ int: 3,532 hours | not spec. | - |
| T.L. Yap (1) [19] | 2019 | IS | Leaf Healthcare | yes | 44 | 13MP, 31FP | no | Body angle | 11,632 hours | not spec. | |
| B. S. Renganathan [28] | 2019 | IS | PRESENSE | no | 40 | Patients | no | Body angle | 774+676 hours | not spec. | - |
| A. R. Budarick [29] | 2020 | IS 2x + cap | Delsys + Xsensor | yes | 25 | 13M, 12F | yes | Body angle | unknown | not spec. | - |
| P. Alinia [30] | 2020 | IS 5-9x | MTx 3-DOF orientation trackers (Xsens Technologies) | no | 30 | 19M, 11F | yes | B | 278 episodes of 20 seconds |
Location dependent Best: 98.4% Worst: 64.8% |
Conf. + RSP per sensor position |
| L. Nuksawn [31] | 2015 | IS | tri-axial accelerometer | no | 20 | 10M, 12F | yes | S,L,R, FS, standing, walking | unknown | 85.7% | no |
| R. M. Kwasnicki [32] | 2018 | IS 3x | ADXL330, InvenSense ITG-3200, Honeywell HMC5843 | no | 16 | 9M, 7F | yes | B,Fl,Fr,Sp,Pp | 256 random postures |
With calibration: 99.5% (4 postures) 92.5% (8 postures) |
Conf. |
| E. B. Monroy [33] | 2020 | IS 2x | Tactigon ONE | no | 7 | 3M, 4F | yes | S,L,R,FS,SR,SL | 500,000 samples | 99% | Conf. |
| Z. Zhang [34] | 2015 | IS | biosignalsplux sensor | no | 7 | 5M, 2F | yes | B | unknown | 99% | Conf. |
| G. Cicceri [35] | 2020 | IS | Raspberry pi + Adafruit LSM303 | no | 6 | Healthy | yes | B, FS, movement | 8,707 samples | 99.1% | Conf. |
| R. K. Megalingam [36] | 2016 | IS | Arduino + ADXL 335 | no | 5 | Healthy | yes | Body angle | unknown | not spec. | - |
| Piezoresistive sensors | |||||||||||
| G. Matar [37] | 2020 | PR | Sensor Products 64 × 26 mattress | no | 12 | 10M, 2F | yes | B | 1,116 images | 97.9% | Conf. |
| R. Hudec [38] | 2021 | PR | Textile: yarn and velostat | no | 21 | 18M, 3F | yes | B | 630 samples | 82.2% | Conf. |
| R. Onose [39] | 2017 | PR | Garment-textile | no | 20 | 4M, 16F | yes | no BPD | unknown | not spec. | no |
| H.K. Diao [40] | 2021 | PR | FSR 32 × 32 | no | I: 16 + O: 5 | 9M, 7F | yes + no | B | 1,056 samples | Instructed: 95.1%; overnight: 86.4%; | yes |
| D. Hayn [41] | 2015 | PR | ADXL 345 + FSR-406 | no | 14 | Patients | no | move/non mov | 8,111 hours | not spec. | - |
| M. B. Pouyan [42] | 2017 | PR | 2 pressure mats | n/a | 13 | Healthy | yes | S,L,R | 20.024 data points | 80.4-85.5% | RSP per patient |
| M. Heydarzadeh [43] | 2016 | PR | Vista Medical Boditrak | yes | 10 | Healthy | yes | B,Fl,Fr | 60.000+ images? | 98.1% | Conf. |
| F. J. Costello [44] | 2021 | PR | 32 × 64 pressure mat | n/a | 13 | Healthy | yes | S,L,R,Sw,Ss,Sr,SR,SL,Fl,Fr | 20,024 data points | 98.6% | Conf. |
| T. H. Kim [45] | 2019 | PR | FSR 16 × 8 | no | 7 | 6M, 1F | no | B,LL,LR | 258 postures | 90% | APP |
| Y. W. Hung [46] | 2015 | PR | FSR 18 × 12 | no | 6 | Healthy | yes | S,L,R, movement | 900 images | 95.9% | inconsistent |
| Y. S. Hong [47] | 2018 | PR | 18 FSR 408, 27 FSR 406 | no | 5 | Elderly | yes | S,L,R | 374 tests | threshold 300: 87.3% | no |
| A. P. Rodríguez [48] | 2020 | PR | Vista Medical Boditrak | yes | 8 | Healthy | yes | S,L,R | 232 real, 6,032 augmented | 99.0% | RSP per patient |
| Piezoelectric sensors | |||||||||||
| W. Viriyavit [49] | 2020 | PE + PR | 2 FSR 402; Piezoelectric Diaphragms | no | 3 | Patients | yes | S,LL2,LR2,FS,O | 459 hours, 5,335 samples | 97.1% | Conf. |
| M. X. Liu [50] | 2018 | PE | piezoelectric film in the mattress | no | 12 | 6M, 6F | no | B | 8 hours (40 min * 12 subjects) |
Uncorrected: 90% corrected: 97% |
APP |
| M. Enayati [51] | 2018 | PE | 4 hydraulic tubes incl. pressure sensors | no | 58 | Healthy | yes | B | unknown | K-fold:100%, LOSO: 75% | no, ext. |
| Load sensors | |||||||||||
| N. Zahradka [52] | 2018 | Load | iLoad Pro | no | 54 | Healthy | Yes | S,L,R,FS,Ls,Rs, movement | 54 pos. + 54 movement |
Position: 74.9%, Movement.: 79.7% |
Conf. |
| N. Pupic [53] | 2022 | Load | Load cell + 3 IMU | no | 18 | 10M, 8F | Yes | S,L,R + bins | 2963 samples |
LOSO: 84.0% ± 12.2% 15° bin: 52%-56% |
No, ext |
| G. Wong [54] | 2020 | Load | Load cell: DLC902-30KGHB | no | 20 | 8M, 12F | yes | S,L,R | 4932 observations | 94.2% | Conf. |
| D. M. Minteer [55] | 2020 |
Load + IS |
custom | no | 10 | 3MP, 7FP | no |
movement/ repositioning |
105 hours; 137 movements | 85% (Gown: 80%, Load: 89%) | -/no |
| Radio wave-based systems | |||||||||||
| J. Liu [25] | 2019 | RW | RFID tag matrix | no | 12 | 8M,4F | yes | B,Fl,Fr | 120 samples | 96.7% | Conf. |
| S. A. Shah [56] | 2016 | RW | Leaky Coaxial cable | no | I: 3 + II: 6 | Healthy | yes | S,Lateral,FS | n/a | not spec. | no |
| V. Nguyen [57] | 2016 | RW | IR-UWB | no | 6 | 3M, 3F | yes | B | n/a | 88.9% | No, ext |
| Capacitive sensors | |||||||||||
| S. Rus [58] | 2017 | Cap | Multi-capacitance | no | 14 | Healthy | yes | B,FS | 3741 samples | LOSO: 90.4% dispersed subset: 85% | Conf. |
| S. Fryer [59] | 2022 | Cap | Xsensor Foresite | Yes | 12 | Patients | No | Movement | n/a | ∼80% | No |
| D.J.C. Matthies [60] | 2021 | Cap + PZ | 12 Pressure Tiles + 8 Capacitors | no | 11 | 8M, 3F | yes | B,Ss | n/a |
LOSO: 85.0% 50% split: 99.5% |
Conf. |
Sensor types: IS=Inertial Sensor; PR=Piezoresistive sensor; PE=Piezoelectric sensor; Load=Load sensor; RW=Radio wave based sensor; HS=hydraulic sensor Cap= Capacitive sensor; Population type: M=male; F=female; MP=male patient, FP=female patient; Instructed: Volunteers were instructed to attain certain positions B=Main/Basic positions: Supine (S), Left lateral (L), Right lateral (R), Prone (P); Body positions FS=Fowler's supine, O=Out of bed; SR= Supine with bent right leg; SL; Supine with bent left leg, Sp=Supine arms parallel to the body, Pp=Prone arms parallel to the body, LL = supine lying to the left, LR = supine lying to the right, LL2=Lying on the left side of the bed; LR2=lying on the right side of the bed, Fl=Left foetus, Fr=Right foetus, Ss=Sitting on the side of the bed, Ls=Sitting on the left side, Rs=sitting on the right side; Reported accuracy: K-fold cross-validation: body positions were randomly picked from all subjects; LOSO=Leaf One Subject Out cross-validation, all body positions of one person were excluded from the training set Extensive report: Conf.=confusion matrix; RSP=Recall, Specificity, Precision; APP=Accuracy per posture; no, ext=an extensive report was provided, but not on the classification distribution
3.2. Inertial sensors
Inertial sensors consist of a triaxial-accelerometer (3D acceleration), a gyroscope (3D angular velocity and orientation) and sometimes a magnetometer (compass direction), which combined can detect motion and estimate body trunk angles [55]. The sensor is usually positioned on the sternum but can be attached to the abdominal area, extremities or hip as well [29,30,32].
The Leaf sensor and the PRESENSE system are two sensors equipped with a warning system that use the body turn angle as a threshold for differentiating between left, right and supine positions. These sensors are directly attached to the skin of the upper chest when in use [8,19,26,28]. The Leaf system is completely single-use [26] and therefore with larger recurring cost, while the PRESENSE uses a disposable ECG sticker with a multiple-use inertial sensor [28]. Both systems discriminate positions according to degrees of turn angle across specified thresholds (20 degrees and 12- or 15-minute tissue decompression time) and automatically reset after self-repositioning [8,27].
The sensors without a warning system use a smart algorithm to determine body positions instead of using the turn angle of the trunk. In four studies, two or more sensors were combined with a smart algorithm to determine the body positions [32,33] with two studies also including the position of the extremities [30,35]. The first sensor was positioned on the sternum or abdominal area, whereas the eventual second and additional sensors were positioned on the wrists, hip or ankles. The sensors that were not used on patients were usually placed on the clothing, although the way of attachment was not always specified [32]. Finally, some systems used one central computing unit connected to multiple types of sensors including ECG and breathing rate to obtain more parameters for the health status of the patient [34,36].
Of all of these sensors, only the leaf sensor and PRESENSE are used clinically. Most studies with inertial sensors that used the body turn angle did not specify the accuracy of the system, however, one study reported a turn angle accuracy of ±2.5 degrees for the Leaf sensor [19]. The systems that were validated for BPD report a high classification accuracy between 99% and 99.5% [32], [33], [34], [35]. Nuksawn et al. [31] achieved a lower accuracy (85.7%), however, their system was also able to differentiate between standing and walking, increasing the number of categories and thus increasing the detection difficulty.
We found the most comprehensive clinical studies with inertial sensors. The largest study was by Pickham et al. [8] with 1312 patients which compared the PU prevalence with and without the sensor active. The PU incidence was significantly lower in the intervention group (0.7%) compared to the control group (2.3%). In 2022, Yap et al. [19] included 992 patients in a clinical trial, on a high-spec foam mattress, and found no new development of PUs when using 2, 3 or 4-hour turn-interval warnings (intervention) compared to a 5.2% baseline incidence. Three other studies focused on compliance with the 2-hour turning protocol. In 2019, Yap et al. observed an increase in mean compliance from 61.4% to 81.5% in a population of 44 residents [26]. Schutt et al. (2018) found a comparable increase in compliance from 64% to 98% in a population of 75 patients [27]. Renganathan et al. (2019) found the greatest increase in compliance from 24% to 98% in a small population of 40 patients [28]. Finally, Maguire et al. found that extending personalised turn intervals up to 4 hours could be safely implemented without increasing PU incidence [9].
Due to direct patient contact for the majority of inertial sensors, both frail skin and an adhesive allergy were exclusion criteria, because some can cause skin tears or an allergic reaction [8,27]. Another complication was the patient rolling on top of the sensor which can be uncomfortable or can in itself induce PUs [42,54]. Moreover, sensors were reported to detach due to resident picking behaviours, moist skin under the sensor or skin products applied before sensor application [26].
3.3. Piezoresistive sensors
Piezoresistive sensors are sensors that can detect changes in electrical resistance relative to the applied force, which can be converted to pressure values (in mmHg) [61]. These sensors are often located on top of the mattress underneath the bedsheet since this enables the most direct pressure distribution measurements. Piezoresistive sensors are versatile as their number and positioning can be adjusted to fit the application. For example, some are placed homogeneously covering the whole mattress, providing a detailed pressure map of the patient whereas others are positioned in certain patterns aiming to obtain the most critical data to estimate body positions with the least number of sensors to reduce cost [40,47].
The piezoresistive BPD accuracy, tested in a maximum of 21 healthy volunteers, ranges between 75.9% and 98.6% [37,38]. The results in table 1 show that piezoresistive sensors with BPD have not been used on patients yet. However, pressure mats have been used with patients for their pressure mapping capabilities, without a BPD- or warning system [62], [63], [64].
From the selection of piezoresistive systems that can detect body position, only one was a ready-built commercially available system [43]. The other systems were self-built systems that frequently use the same basic components such as the commercially available FSR-406 sensors while using a different number and placement of the sensors [37,40,[45], [46], [47]].
There are several disadvantages of piezoresistive sensors. First, the position of the sensors on top of the mattress reduces comfort [41]. Second, according to Pouyan et al. [42], for high precision, this sensor type has to be calibrated each time that it was used because it suffers from drift. Finally, they require storage space when not in use and, may spread infection, due to indirect contact with the patient, if not properly cleaned [54].
3.4. Piezoelectric sensors
Piezoelectric sensors can detect vibrations such as small movements, respiratory rate and heart rate [21]. The sensor is usually placed under the mattress in the thoracic region of the patient, but it cannot detect absolute pressure due to charge leakage [65].
Viriyavit et al. [49] combined two piezoelectric sensors and two piezoresistive sensors attached to a ready-made sensor panel to detect in-bed weight distribution in a fall prevention study. A low number of sensors was used to keep the system low-cost. The system was tested on three subjects resulting in 5335 samples and can discern five body positions including off-bed, sitting, lying centre, lying left and lying right with an accuracy of 97%.
A different approach to BPD is to use the ballistocardiograph (BCG) signal -movement generated by the heart - to identify the four basic body positions. Liu et al. [50] used a piezoelectric film integrated into the mattress whereas Enayati et al. [51] used 4 water tubes fitted under the mattress. Depending on the angle of the body, the BCG signal becomes weaker or stronger from which the body position can be deducted. Both Viriyavit and Liu et al. achieved a BPD accuracy of 97% in a lab-testing environment, however, the systems were neither tested on patients nor were they commercially available yet. Enayati et al. [51] achieved 100% accuracy with the K-fold test method and 75% accuracy with the leave-one-subject-out (LOSO) test method. The system performance was affected by electrical devices and the type of bed [65] and it was unclear how BCG data were affected by clothing, pillow and blanket [50].
3.5. Load sensors
Load sensors are commonly placed in the bedframe or as pads under the bed wheels and can detect the weight (distribution) of the person in the bed. Combining the signals of the load sensors, deviations in the centre of mass during movement can be calculated, which subsequently can be used to estimate the orientation of the patient. The advantages of this sensor type are the lack of patient contact, the low costs and the low maintenance requirements [54].
Minteer et al. developed both a load sensor system and an inertial sensor (gown) [55]. The systems were simultaneously assessed by monitoring 10 immobile patients with both the camera, inertial sensor and load sensors resulting in an accuracy of 85% for ‘repositioning’ events. These events were defined as “a rotation of the patient's core body while lying in bed, to include adjusting individual limbs for cleaning purposes and/or comfort measures.” The authors reported eleven missed movements and two false positives for the gown sensor (total=65) and seven missed events with one false positive for the load sensor (total=72). Noticeably, only one accuracy was reported for the two systems together without providing separate values.
Wong et al. [54] proposed a PU prevention tool and advocated the benefits of using load sensors compared to other sensor types. They noted that the posture detection of a clinical 30-degree angle with support pillows was more difficult to detect than a 90-degree left or right lying position, resulting in a detection accuracy ranging from 73% to 94%. Pupic et al. [53] used a similar setup and achieved an accuracy of 85% for differentiating between left, right and supine. They also investigated the influence of the turn angle of the patient on the interface pressure exerted on the tissue around the sacrum, left- and right trochanter. They found that 15-degree steps provided clinically relevant differences in pressure but their load sensor system was only able to differentiate this small bin size with an accuracy of 52-56%.
Zahradka et al. [52] also demonstrated with 54 healthy volunteers that certain body positions could be determined with load sensors with an overall accuracy of 74.9%. However, similar to the other two studies with load sensors, they could not differentiate between supine and prone position. Furthermore, they found that the difference between lying and sitting could be well detected, but differentiating between lying positions resulted in a high classification error when not binding categories together.
3.6. Radio wave-based sensors
Systems based on radio waves consist of a transmitter that sends out radiation in the non-ionizing electromagnetic spectrum and a receiver that detects it. Because the body is an obstacle between the transmitter and receiver, it causes disturbances via reflection, refraction and scattering, which leads to different signal profiles for specific body positions [25,56]. There is no need for direct contact with the patient. Three variants are discussed below, although they are in the prototype phase and have not yet been validated on patients.
3.6.1. Radio-frequency identification (RFID) tags
In 2019, Liu et al. [25] positioned 500 passive (=battery free) RFID tags under a thin mattress which were powered and beamed by an RFID reader (antenna) that was positioned above the bed. The operating frequency was not specified. The tags were taped under a bed sheet or on the surface of a mattress and scattered the radio waves back to the receiver. Depending on the body position, a grayscale profile was created corresponding to the differences in signal strength caused by the presence of a body between the tags and the reader. According to the authors, they used an algorithm that can be used for the general population. The tags can detect two additional postures beside the basic positions: left and right foetus. Moreover, the tags can also estimate the respiratory rate.
3.6.2. Leaky Coaxial cable
Shah et al. [56] used a commercial Leaky coaxial cable (LCX) that communicates to a 2.4 GHz Wi-Fi-router. They used an LCX cable of 1 meter for one volunteer and a 3-meter-long cable for the detection of two or more volunteers in multiple rooms. They stated that an LCX cable is more robust than an antenna setup because of the directionality of the signals and they mention the proposed systems obtained high identification accuracy. However, the authors did not report the accuracy of the system and the test subjects had homogeneous heights and weights.
3.6.3. IR-UWB radar
Nguyen et al. [57] used Impulse radio ultra-wideband (IR-UWB) radar to detect postures, heart rate and respiration rate. The IR-UWB radar, a 40 × 40 inch (102 × 102 cm) panel, was positioned under the mattress and tested on six subjects. In this study, a frequency of 4.1 MHz (between AM and FM radio wave frequency) was used which is different from continuous radio wave-based techniques as it transmits information via bursts of short impulses instead of using a sine wave. This enables operation with time-of-flight instead of received signal strength indication (RSSI), which increases ranging measurement precision that, besides the basic body postures, can be used to detect heart rate and respiration rate. A posture detection accuracy of 88.9% was achieved for the basic positions.
3.7. Capacitive sensors
Capacitive sensors are situated on the mattress and can detect changes in electrical charge which enables pressure measurement or contact detection.
Rus et al. [58] described their system as a big touch screen with crossed wires that can detect mutual capacitance at the intersections of the wires. A very low radio wave frequency of 7.3 kHz was used. The basic positions were detected together with the fowler supine position. They reported a LOSO accuracy of 90.5% for the whole dataset but specified 94.7% and 85.0% for a similar and dispersed subset, respectively.
Matthies et al. [60] used both pressure sensors and capacitive sensors attached to a mattress protector. The system achieved an accuracy of 99.5% with a 50% split and 85.0% with the LOSO test method for the basic positions and sitting on the side of the bed.
Fryer et al. [59] used a capacitive sensor mat that measures absolute pressure. They used this to track large-scale movements that resulted in a clear change in the spatial distribution of pressure through changes in posture. They found that certain movement patterns correlated to acquired skin damage, demonstrating that the system could potentially be used for PU prevention.
4. Discussion
4.1. Summary of main results
We assessed the clinical applicability of different sensor types that can detect body positions in bed. Most articles on BPD systems included inertial sensors and (piezoresistive) pressure sensors. Alternative types were piezoelectric sensors, load sensors, radiofrequency-based techniques and capacitive sensors. Recently, two inertial sensors were shown to reduce PU incidence [8,19,9] and increase turn compliance [26], [27], [28]. These findings strengthen the benefits of technical support for nurses in PU prevention. Although inertial sensors achieve high BPD accuracy and reported promising clinical evidence, they have some serious drawbacks concerning comfort and clinical usability [66]. That is why it is desirable to further develop other sensor types for body position monitoring that do not need (direct) patient contact and do not require extra effort of nurses to operate.
4.2. Warning system
For active PU prevention, sensors must be equipped with a warning system [24]. A warning system should alert nurses if a patient deteriorates from low-risk to high-risk or it should assist with turn-protocol compliance. High-risk patients could be detected with no-contact lower accuracy systems such as piezoelectric, load and radio wave-based sensors whereas strict turn-protocol monitoring of high-risk patients could be achieved by using higher accuracy, extremity measuring, techniques such as piezoresistive, capacitive and inertial sensors. Currently, most of the BPD systems are in the prototype stage and do not have an active warning system. However, for BPD systems this should be easy to implement, because turn guidelines have already been investigated. Although personalised alarm thresholds are still under review, a 4-hour turn frequency is recommended for general use and has been shown to reduce PU incidence [10,19].
4.3. Body position categories and accuracy
For most use cases, it is important to have a BPD system with a low misclassification rate. However, the accuracy is affected by multiple factors. A few studies have reported different classification accuracies depending on the number of body position categories [32,40] and many based on the validation setup [51,58,60]. For example, one study performed accuracy tests in both a lab setting and a setting closer to clinical practice with longer measurements and random movements and found a 95% accuracy in a lab setting compared to 86% in a clinical setting [40]. Healthy, well-instructed individuals perform clearly defined movements with larger and more consistent shifts in pressure and weight distribution compared to patients, especially if a homogeneous test population is used. Furthermore, the four basic positions largely differ in their pressure maps and weight shifts, whereas in practice patients most likely position themselves in all the gradual steps in between as well. Movement thresholds used for healthy volunteers will thus likely be different for slowly moving uninstructed patients, lowering detection accuracy in clinical practice. Generally, accuracy seems to drop 10% in a setting where volunteers could move randomly in comparison to instructed volunteers. Additionally, some authors use sensor calibration for every new patient in a lab setting to improve accuracy [32,42], but this would drastically increase nurse workload in a clinical setting [67].
Next, we notice that most authors, except one [54], tested their systems in a lab setting with a flat mattress, whereas most bedridden patients are required to have a minimum head of bed angle (HOB) of 30 degrees or more [68]. This is an issue because the pressure map and weight distribution of someone positioned in a 30-degree HOB compared to a 0-degree HOB differ significantly [69,70]. Furthermore, in none of the articles the legs were raised 30 degrees to achieve semi-fowler positions and in several articles no pillows were used in the training data [37,40,60], despite this being common practice. These differences in the way the algorithms were trained and tested most likely lead to a lower accuracy in clinical practice, reducing usability for PU prevention.
Unfortunately, the number and type of categories differed between published articles, decreasing comparability between systems. The number of categorised body positions relates to the accuracy of the system [32]. Fewer categories are more robust and thus result in a higher classification accuracy whereas distinguishing a high number of categories is more difficult [51]. It is easy to increase the reported accuracy by limiting or avoiding positions that are difficult to detect during testing. To avoid bias, a more representative value is to report a confusion matrix that reports the predicted and true labels per category. This allows for the assessment of the classification accuracy of specific categories, better predicting real-world accuracy.
Finally, the rationale of the chosen positions was missing or lacking clinical relevance in most of the articles. The sensor systems were often not able to differentiate between sitting and lying in bed and one category was missing completely from most articles: the 30-degree side-lying position, which is recommended by the EPUAP guidelines [10]. Currently, only articles with inertial sensors specify that they use a trunk turn threshold of 20 degrees and two articles with load sensors. One article used training data with a 30-degree trunk turn angle [54] whereas the other article reported accuracies for different bin sizes [53]. To increase clinical relevance and comparability between sensors, we suggest which positions are clinically relevant and should be included in future articles.