1. Introduction
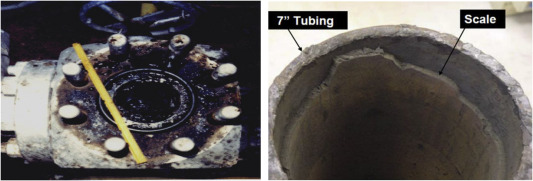
In the last few decades, several solutions have been proposed to remove and mitigate formation damage to improve the productivity and injectivity of oil and gas wells. Scales are one of the most severe forms of formation damage; it can deposit scales in accessible and relatively less accessible areas. The scale is an accumulation of different materials that can lead to clogging and prevent fluid flow in the wellbore, production tubing, valve, casing, perforations and downhole equipment (Crabtree et al., 1999). The scale problem can arise anywhere along water paths from surface equipment to the reservoir itself. The scale deposit can take place in surface water injection facility, injection wells, formation, production well, topside production facilities, pipelines, and at disposal wells (Bader, 2007). Water is the major source of all scales and when water is produced along with oil and gas, different types of scale are expected to form in the reservoir or in the production tubing. The scale can deposit in the form of a thick layer in the wellbore tubing that reduces the production diameter of the tubing, which results in clogging and blocking of the flow (Fig. 1). This can cause a severe increase in the pressure drop and this result in a decrease in the productivity of the well. The production capacity can reach zero within a few hours and could cause a huge treatment cost (Olajire, 2015). The precipitation of scale can also cause formation damage in the reservoir, blockage of different pipelines, enhancing corrosion rate and can pose a threat to safe production operations (El-Said et al., 2009). In the case of water injection wells, the scale could deposit in the formation of pores that can reduce the injectivity with time (BinMerdhah et al., 2010). Scale deposited in the formation can reduce the permeability and porosity of the formation. The variation in rock permeability depends on injection rate and temperature (Haghtalab et al., 2015; Moghadasi et al., 2004). Several types of scale deposition in the well and surface facilities cause a decrease in production capacity and injectivity.
 Fig. 1. Typical scale deposition in pipelines (Nasr-El-Din and Al-Humaidan, 2001).
Fig. 1. Typical scale deposition in pipelines (Nasr-El-Din and Al-Humaidan, 2001).Scale inhibitors (SIs) are a class of specialty chemicals that is used to slow or prevent scale formation in water systems. There are different types of scales and they are usually prevented by using scale inhibitors (Bin Merdhah, 2010; Lu et al., 2010; Tung et al., 2004). In most of the cases, scale dissolver are required to remove the scale, even after utilizing scale inhibitors as a primary control method. There are different scenarios where scale inhibitors are not totally effective in scale prevention such as when scale formation is not predicted accurately in advance and when placement of inhibitor is non-optimal owing to reservoir heterogeneity (Jordan et al., 2014). Once the scale deposition takes place, it must be removed using scale dissolver.
Scale deposition in downhole surfaces is initiated due to formation of local brine in the environment and the low solubility of some of the inorganic salts that are produced. There are 3 mechanisms that led to the formation of scale deposits both in offshore and onshore. It can be due to; 1) mixing of two incompatible brines, 2) change in conditions (temperature and pressure), and 3) brine evaporation (BinMerdhah et al., 2010). In oilfield applications, water is of great importance since scale will only occur if water is produced. All types of natural waters are rich in ions due to the dissolution of different mineral components. The formation contains significant amounts of Ca2+, Mg2+, Sr2+, and Ba2+ with total dissolved solids approaching 400,000 mg/L. The scale deposit usually starts when two or more incompatible water mix with each other (Olajire, 2015). The water systems are called incompatible when they interact with each other chemically and precipitate the minerals when mixed. Seawater (if it contains a high concentration of sulfate ions and low concentration of barium and calcium ions) and formation water (if it contains a low concentration of sulfate ions and high concentration of calcium and barium) are typical examples of incompatible brines (BinMerdhah et al., 2010). The mixing of two such water can lead to precipitation of barium sulfate and calcium sulfate. Other brine incompatibilities could result in depositing sulfide scales where hydrogen sulfide can react with iron, zinc, and lead. In water injection wells, the solubility of some salts in saturated injected water can reduce as it travels to high-temperature zone. This could also result in deposition of scale along the well stings. Similarly, a decrease in pressure can lower the solubility of various minerals in the water. For every 7000 psi decrease in pressure, the solubility of minerals can decrease by a factor of two (Crabtree et al., 1999). The solubility of some minerals such as carbonates changes with the presence of acid gases, such as CO2 and H2S.
The scale deposition depends on several factors such as temperature, pressure, chemical reaction equilibria, pH, contact time, evaporation, and ionic strength(Yap et al., 2010). The scale deposits can be as a single mineral phase, but usually, scales are composed of the combination of different elements. Several organic compounds such as naphthenic acids and their salts, aromatic compounds, sulfur-containing compounds, resins, paraffin and unsaturated hydrocarbons can also affect the formation of scale in downhole conditions(Lakshmi et al., 2013). The most common types of scales encountered in oil and gas production include sulfates (Ba, Sr, Ca), oxides/hydroxides (Fe, Mg), carbonates (Ca, Mg, Fe), and sulfides (Fe) (Li et al., 2009; Senthilmurugan et al., 2011). The typical scale compositions in sandstone and carbonate reservoirs are given in Table 1.
Table 1. Typical scale in carbonate and sandstone reservoirs.
| Carbonate | Sandstones | ||
|---|---|---|---|
| Scale Type | wt% | Scale Type | wt% |
| Iron sulfide | 29.2 | Calcium Carbonate | 33.5 |
| Iron Oxide | 28.1 | Iron Oxide | 30.3 |
| Silicon Oxide | 10.4 | Silicon Oxide | 28.5 |
| Iron Hydroxide | 9.0 | Iron Sulfide | 1.7 |
| Iron Carbonate | 5.5 | Iron Carbonate | 2.5 |
| Dolomite | 4.6 | Barium Sulfate | 1.1 |
| Calcium Carbonate | 3.8 | Magnesium Oxide | 0.6 |
| Calcium Sulfate | 3.6 | Aluminum Oxide | 0.6 |
| Chlorite | 2.2 | Strontium Oxide | 0.5 |
| Sodium Chloride | 1.4 | Aluminum Silicate | 0.4 |
| Barium Sulfate | 1.3 | Chromium Oxide | 0.2 |
| Aluminum Silicate | 0.9 | Others | 0.1 |
| Molybdenum oxide | 0.2 | ||
The selection of an appropriate chemical formulation to dissolve and remove scale is a challenging task due to the diversity of the scale minerals in a single well. The different types of scales have varying reactivity in acid media and chemical dissolvers. Additionally, the co-deposition of mixed scales in the well is also common, where an insoluble mineral covers a soluble mineral. Another issue in chemical scale removal is the precipitation of reaction byproducts following scale dissolution. This is more common at high pressure and high-temperature conditions where dissolution and precipitation kinetics are competitive.
Antony et al. reviewed the scale properties and mechanism of scale formation during reverse osmosis in water desalination and wastewater treatment(Antony et al., 2011). Li et al. (2017) reviewed the scale formation and control strategy using scale inhibitors (Li et al., 2017). Kelland (2014) also reviewed several scale inhibitors for oil and gas wells (Kelland, 2014). Scale inhibitors are applied to prevent scale formation, while scale remover (dissolver) is used to dissolve the scale. If the scale problems still arise even after utilizing scale inhibitors as a primary control method, scale dissolver (remover) is used to remove the scale. This review discusses the different types of scale dissolvers. Olajire (2015) reviewed the oilfield scale management technology (Olajire, 2015). The review mainly discusses the thermodynamic and kinetic prediction of mineral scale formation. Various chemicals that are applied as scale inhibitors in oil and gas industry are discussed in that review. Crabtree et al. (1999) reviewed the physical causes of scale formation during production operations (Crabtree et al., 1999). Some inhibitor systems that are used to prevent the formation of scale are also mentioned. Although the methods of scale removal by mechanical and chemical methods are compared, the chemicals used have not been discussed in detail. In addition, there are many recent advancements in oilfield scale removal. This review focuses on different chemicals that are used in oilfield applications for the removal of different types of scale. The current review has highlighted the conditions for the formation of the most common oilfield scales and identified several types of chemicals that are used for the scale removal. In addition, new green materials that are recently introduced are also reviewed. Future perspectives of oilfield scales are also discussed.
2. Scale types and methods of formation
Scale formation differs significantly from one well to another, but it can also vary along the depth of the same well. Oilfield scales can be classified in different ways; however, the most common scales include, but not limited to, sulfides (mainly iron sulfides), oxides (mainly iron oxides), sulfate, and carbonate scales. A relatively unusual scale zinc sulfide has been reported (Berry et al., 2012). Table 2 describes the important and common oilfield scales. The tendency for scaling of some scales, such as calcium sulfate and barite, is independent of brine pH. However, the scaling tendency of the majority of scales such as iron sulfides and carbonates depends on brine pH. It is worthy to mention that iron is the most effective ion, which is responsible for balancing the negative charge of the Mg-OH and Si-OH hydroxyl group surfaces. Therefore, increasing the pH will cause what is called the ion shielding on the edges of Mg-OH and Si-OH hydroxyl group surfaces and the negative value will increase as pH increases (Jones, 1981; Tan et al., 2013). Most of the scales are either water soluble or acid soluble. However, some scales are neither soluble in water nor in acids. Sodium chloride is a typical example of water-soluble scale. Calcium carbonate, iron sulfide, and iron oxide are acid soluble scales.
Table 2. Different types of scales.
| Sulfides | |
|---|---|
| Pyrrhotite | Fe7S8 |
| Troilite | FeS |
| Mackinawite | Fe9S8 |
| Pyrite | FeS2 |
| Marcasite | FeS2 |
| Greigite | Fe3S4 |
| Sphalerite | ZnS |
| galena | PbS |
| Carbonates | |
| Calcite | CaCO3 |
| Vaterite | CaCO3 |
| Aragonite | CaCO3 |
| Siderite | FeCO3 |
| Dolomite | CaMg(CO3)2 |
| Aragonite | CaCO3 |
| Sulfates | |
| gypsum | CaSO4.2H2O |
| Anhydrate | CaSO4 |
| Barite | BaSO4 |
| Hemihydrate | CaSO4.5H2O |
| Celestite | SrSO4 |
| Iron scales | |
| Ferrous Hydroxide | Fe(OH)2 |
| Ferrous Hydroxide | Fe(OH)3 |
| Hematite | Fe2O3 |
| Magnetite | Fe3O4 |
| Akaganeite | α-FeOOH |
| Goethite | β-FeOOH |
| Lepidocrocite | γ-FeOOH |
| Hibbingite | Fe2(OH)3Cl |
| Miscellaneous | |
| Brucite | Mg(OH)2 |
| Periclase | MgO |
| Nickel Ferrite | NiFe2O4 |
2.1. Sulfide scales
2.1.1. Iron sulfide
Iron sulfide scale is one of the major scales formed particularly in sour oil and gas wells. Physically, it can vary from viscous fluid to dry black powder. The scale characteristics are usually a function of pH, temperature, scale age, and pressure. Formation of iron sulfide scale takes place due to the reaction between hydrogen sulfide and iron. Hydrogen sulfide gas can react with ferric ions and cause iron sulfide precipitation. There are various sources of delivery of hydrogen sulfide and iron in the wells. Hydrogen sulfide can be produced as a free gas in sour wells. The other sources of hydrogen sulfide include, but not limited to, degradation of drilling mud additives, reduction of sulfate ion, thermal degradation of organic sulfur-containing compounds, acid treatment of sour wells, and sulfate-reducing bacteria (SRB) (Chen and Huang, 1986; Seto and Beliveau, 2000; Singh et al., 1989). Thermal degradation of sulfate containing minerals can also generate H2S.
The sources of iron are many such as tubular, minerals, clays, propping agents, formation brine and several other fluids lost during drilling and completion operations. The main source of iron can be leaching of iron from minerals present in the formation or corrosion products. During acidizing operation, acid can be contaminated with iron at any stage starting from the surface tank to well tubulars. This contamination can lead to deposition of iron in the formation or in the wellbore. Acids easily dissolve the rust of the storage tanks (Hall and Dill, 1988). Later, during the acid injection process, acids can also dissolve corrosion and mill scale products. Iron containing minerals in the formation are another common source of iron in the formation. Hematite is a sedimentary mineral and common in sandstone reservoirs. Some sandstone contains up to 2 wt% hematite (Ma et al., 2016). The proppant was introduced during hydraulic fracturing processes and it can also be an additional source of iron in the well. Iron sulfides having iron as a major component are soluble in acids, however, if the sulfur ratio is higher it becomes insoluble in inorganic acids (Nasr-El-Din et al., 2001a).
Depending upon formation mineralogy, pressure, temperature, brine, pH, and time of exposure, there are various forms of iron sulfide scales based on the iron to sulfur ratio. Iron sulfide scale can be either in mono-sulfide phases such as pyrrhotite, troilite, and mackinawite or disulfide phase such as pyrite and marcasite (Chen et al., 2016). The iron sulfide scales can be in a soft form such as pyrrhotite or in a hard form such as pyrite. In the same well, several types of iron sulfide scales could exist. Hard scales are usually present in the shallower depth. At higher temperature, the iron sulfide scale predominantly consists of troilite and/or pyrrhotite (Hafiz et al., 2017).
Iron sulfide scale causes many operational problems in oil and gas industry. They are present in all types of well such as producer, injector, disposal, and supply wells due to the presence of iron and H2S (Elkatatny, 2017). Iron sulfide scale can be accumulated near wellbore and can alter the performance of supply wells, injectors, and gas wells (Cusack et al., 1987; Patton, 1993; Walker et al., 1991). It can also affect the performance of downhole tools such as production logging tool. The increased in corrosion rate due to iron sulfide scale poses a threat to the safe operation of the pipeline valving system. It also causes the precipitation of asphaltene near wellbore area and reduces the effective permeability of oil flow. Iron sulfide accumulation in the formation can also change the wettability of the rock from water-wet with oil-wet (Elkatatny, 2017). In production tubing, the formation of iron sulfide scale in the form of thick lining can block the flow. It also causes damage to various wellbore equipment such as heat exchangers, turbines, production tubing, pipes, pumps, and valves. If not properly handled, the iron sulfide scale could result in shutting down the wellbore.
Iron sulfide scale can be coated with oil that results as a diffusion barrier in the reaction of the acid with scale. To mitigate this type of scenarios, formulations also contain some surfactants that enhance the contact between scale and treatment chemical. A long exposure of iron sulfide scale to H2S will result in scale rich in sulfur that will be difficult to remove. In addition, other corrosion products can reduce the solubility of iron sulfide scale in non-acidic chemicals. Iron sulfide scales change the surface charge to more positive making the rock more oil-wet and alter the relative permeability and reduce productivity. In sandstone rocks containing iron minerals, the removal of these scales from the reservoir rock has been reported to enhance oil recovery by more than 20% (Mahmoud, 2018; Mahmoud et al., 2017b; Mahmoud and Abdelgawad, 2015).
2.1.2. Other sulfide scales
Some other uncommon sulfide scales, such as zinc and lead sulfides, are also reported in the literature (Al-Harbi et al., 2017; Berry et al., 2012). Lead and zinc sulfide scales are deposited in the upper part of the well, produced water control valve, and in subsurface safety control valves. These scales were mainly found in the wells located in the Gulf of Mexico and North Sea (Baraka-Lokmane et al., 2015, 2016; Lopez et al., 2005). The solubilities of zinc and lead sulfides are much lower compared to the solubility of iron sulfide in water. Fig. 2 shows the solubility of three sulfide scales in 1M NaCl at different pH values. At all pH, the solubility of the iron sulfide is the highest followed by the solubility of zinc and lead sulfides. The solubility of lead and zinc sulfides increase with an increase in temperature and salinity (Barrett and Anderson, 1988). This indicates that during the production stage, cooling of brine could lead to deposition of zinc and lead sulfide scales.
 Fig. 2. Solubility of different sulfide scales in 1M NaCl at 25 °C (Collins and Jordan, 2003).
Fig. 2. Solubility of different sulfide scales in 1M NaCl at 25 °C (Collins and Jordan, 2003).Wurtzite, a form of zinc sulfide scale, was found in a well in the Gulf of Mexico (Berry et al., 2012). Deposition of zinc sulfide scale takes place in several ways. The main source of zinc sulfide was believed to be the formation of brine and different completion fluids. The zinc ions come from the partial dissolution of formation mineral, such as sphalerite (ZnS), in the formation/injected brine. The concentration of zinc ion in Gulf of Mexico fields is reported to be as high as 245 ppm. Zinc bromide used in completion fluid as weighting material can also be a source of zinc. These completion fluids are used for hydrostatic pressurecontrol. Any fluid loss to the formation during completion operations can lead to the introduction of zinc ions in the formation. The reaction of zinc with hydrogen sulfide results in deposition of zinc sulfide scale in the well. It was reported that in the presence of 2 ppm hydrogen sulfide a loss of 500 bbl (with a concentration of 17.2 lb/gal) zinc bromide within the reservoir can deposit a significant zinc sulfide scale (Biggs et al., 1992).
A similar scale deposition of zinc sulfide has been reported in a field of North Sea. Hydrogen sulfide gas is the most common source of sulfide ion in the downhole environment. The very small concentration of H2S can cause a significant scale deposition in the presence of zinc ions. Degradation of different drilling fluids and corrosion inhibitors can produce sulfide ions. However, degradation of drilling fluids is not enough to deposit a significant zinc sulfide scale during several years of production. Zinc sulfide is primarily affecting the performance of topside equipment such as hydrocyclones and low-pressure separators (Collins and Jordan, 2003). On the other hand, the major source of lead sulfide scale is the partial dissolution of formation mineral such as galena (PbS). The concentration of lead ions in Gulf of Mexico field is around 70 ppm (Collins and Jordan, 2003).
2.2. Sulfate scales
Sulfate scales are common in the applications related to seawater injection. Sulfate scales are considered one of the hardest forms of scale having the lowest solubility in acids and are difficult to remove. Sulfate scales are deposited due to combining of two different waters (one contains sulfate ion and another with barium, strontium or calcium) (Chilingar et al., 2013). Fig. 3 shows the solubility product constant (Ksp) for different sulfate scales. The solubility product constant is an equilibrium product constant of a solid dissolving in an aqueous solution. The higher the Ksp values, the higher the solubility of liquid in that solution. Among sulfate scale, gypsum has the highest solubility product constant at all temperatures where barium sulfate has the lowest Ksp.
 Fig. 3. Solubility of sulfate scale (Li et al., 1995).
Fig. 3. Solubility of sulfate scale (Li et al., 1995).2.2.1. Barium sulfate
Barium sulfate scale has a solubility of 2.3 mg/L of water and a solubility product (Ksp)of 10−9.99 at 25 °C (Kuwahara, 2011; Morris and Paul, 1990). At the same temperature, the solubility of gypsum is 2080 mg/L (Nasr-El-Din et al., 2004). The barium sulfate scale can form from two main sources. The first source is the injection of seawater into the zone where there is an abundance of barium ions causing barium sulfate scale deposition. This scale deposition takes place due to the incompatibility of seawater (rich in sulfate ions) and connate water (rich in barium ions). Drilling operations could also result in deposition of barium sulfate scale in the production tubing and in the formation. Barium sulfate is a common weighting material that is used in well drilling operations. During the drilling operation, some of the barium sulfates will invade the formation and deposits barite scale in the formation resulting in a reduction in the permeability of the rock (Bageri et al., 2017).
The BaSO4 scale formation during injection of seawater and formation water in sandstone core is shown in Fig. 4 using SEM micrograph. Most of the scaling deposition took place in the front sections of the cores. It is likely that most of the scaling ions deposited within the front section of the cores immediately after flooding leaving behind only a few ions to precipitate in the rear section of the core. During the production operations, these barite scale particles are produced with oil and form a scale around the production tubing, casing, and valves.
 Fig. 4. SEM images of a sandstone core (a) unscaled core (b) Containing BaSO4Scale (BinMerdhah et al., 2010).
Fig. 4. SEM images of a sandstone core (a) unscaled core (b) Containing BaSO4Scale (BinMerdhah et al., 2010).2.2.2. Strontium sulfate
Strontium sulfate is common with different oil production facilities. The main reason for strontium sulfate scale formation is the supersaturation of water containing strontium sulfate (Amiri et al., 2014).
2.2.3. Calcium sulfate
Calcium sulfate has been reported as one of the major scales that can cause significant problems in producer and injector wells (Al-Khaldi et al., 2011). Calcium sulfate scale can also be deposited in the reservoir and can alter the permeability and porosity of the reservoir (Mahmoud, 2014b). Calcium sulfate scale mainly deposits around the electrical submersible pump in the reservoir (Oddo et al., 1991). The precipitation of CaSO4 is complicated as it has three crystal forms known as gypsum, hemihydrate, and anhydrite. Gypsum is usually formed at low temperatures (<50 °C) while anhydrate typically deposits at high-temperature conditions. The solubility of calcium sulfate increases with temperature up to 40 °C (Moghadasi et al., 2007). Further increase in the temperature reduces the solubility of CaSO4. The prediction of the exact form of calcium sulfate scale at a given condition is difficult. However, above 40 °C, the relative solubility of anhydrite is low compared to gypsum. Therefore, at high reservoir temperature anhydrite is the predominant form of CaSO4 scale. The solution pH and pressure also affect the solubility of gypsum in water. Usually, calcium sulfate is less soluble in high pH solution and low pressures (Carlberg, 1973; Delorey et al., 1996). The ionic strength can also affect the solubility of CaSO4. The maximum solubility of CaSO4 is determined by the ionic strength of the solution. Calcium sulfate scale could be a result of acid stimulation, water injection, and/or combined hydrocarbon/water production. However, the main reason of CaSO4 scale is also the chemically incompatible water.
2.3. Carbonate scales
The solubility product constant of different carbonate scales is shown in Fig. 5. Magnesium carbonate has the highest solubility constant at low temperatures, while strontium carbonate has the lowest. Calcium carbonate scale is the most common among carbonate scales and is the most widespread in topside production facilities and upper part of the production tubing (Lakshmi et al., 2013). Calcite is the most stable form of CaCO3 scale at reservoir conditions. Therefore, calcite is more common compared to other calcium carbonate scales such as aragonite and vaterite. The carbonate scale formation is a direct function of pH, temperature, calcium ion concentration, bicarbonateconcentration, and ionic strength. The chemical equilibrium between ions and CO2 in the water determines the calcium carbonate scale deposition. The calcium carbonate scale deposition can take place through a combination of calcium and bicarbonate ions. The deposition of CaCO3 scale increases with increasing temperature, the pH, and lowering the pressure (Hamid et al., 2016; Ramstad et al., 2005). Other than calcium carbonate, iron carbonate scales have also been reported in the different downhole equipment (Amiri et al., 2013). The main source of siderite (iron carbonate) scale is also the change in pressure and temperature conditions that leads towards the loss of dissolved CO2. As a result of this loss, pH of the system increases and solubility of these minerals decreases in the flowing fluid (Jordan et al., 2014).
 Fig. 5. Solubility of carbonate scale (Li et al., 1995).
Fig. 5. Solubility of carbonate scale (Li et al., 1995).Other scale types also exist in the oilfield, but they are not common such as phosphate scale, silicates scales, and elemental sulphur. Calcium phosphatescale is very common in water treatment plants and in cooling water systems. In cooling water, due to the extensive use of water, the orthophosphateconcentration increases. In addition, contamination of water with fertilizers based on phosphates leads to the formation of calcium phosphate that is insoluble in water (Pierce and Grattan, 1989). Phosphate scales are not common in the oilfield.
One of the major scales that are commonly found during steam injectionoperations are the silicates. In some fields, produced water that contains lithium is used in the steam operation. During steam, injection lithium reacts with the silicates and forms lithium silicate scale. This shows the importance of treating produced water to avoid scale formation (Zalewski and Bulkowski, 1998). Iron silicate scale was observed in downstream operations of steam injected reservoirs in a siliceous reservoir. This type of scale causes corrosion and plugging of the surface facilities (Daniels et al., 1997). Wang and Wei (2016)reported the formation of silicate scale in steam generator tubing and calcite was found to be more dominant compared to silicate scales. Silicate scales can be formed as well during alkaline injection or in alkaline/surfactant/polymer flooding (Wang and Wei, 2016). Sodium hydroxide is usually used during water flooding or enhanced oil recovery operations to minimize surfactant adsorption. Sodium hydroxide reacts with quartz to produce silicate scales that are insoluble in water. Sodium hydroxide dissolves silica first before it precipitates (Basbar et al., 2013).
Elemental sulphur is present as dissolved species in virtually all deep sour gas reservoirs. Sulphur precipitation is induced by a reduction in the solubility of sulphur in the gas phase beyond its thermodynamic saturation point because of the decrease in pressure and temperature. The change in pressure and temperature occurs during production operations and can result in sulphur deposition in the reservoir wellbore and surface facilities (Hands et al., 2002; Shedid and Zekri, 2006). The solution to this problem is to control the gas production rate that will maintain the sulphur solubility in the gas and prevents its precipitation (Mahmoud, 2014a).
3. Scale removal chemicals
Both chemical and mechanical methods are used in removing the scale of oil and gas wells. Scale removal techniques should be economical, fast, non-damaging to the wellbore tubing and formation, and must be able to prevent the precipitation. The strength of the scale and texture play an important role in the selection of the appropriate removal technique. Mechanical methods have been used frequently to remove different types of scale deposition. By mechanical means, the easily accessible scale is milled and removed. This also depends on the location and nature of the scale. Mechanical methods are only applicable if the scale is present in the wellbore and can be easily milled out. The mechanical methods to remove the scale are expensive due to the complications that is associated with drilling processes. Mechanical methods are even not applicable if the scale is present in the formation. Due to the complexities associated with mechanical methods, the de-scaling job is preferably carried out using chemical methods.
The chemical methods are more cost-effective in removing different types of scales and can be used for descaling job in both wellbore and formation. In chemical methods, several inorganic and organic chemicals are used to remove the scale. The best scale removal chemical can be selected by knowing the exact composition of the scale, and its physical/chemical properties. The effective composition of the scale dissolver in one well might not be effective in other wells due to the different nature of the scales. Poor selection of chemicals can even speed up the recurrence of scale. The scale removal efficiency using chemical methods mainly depends on the accessibility of scale to the chemicals. Surface area to volume ratio is the critical parameter in efficiently and cost-effectively in removing scale. Scales having the large surface area, high porosity, and with hair-like projection have a very high removal rate since chemicals could have large access to the scale particles.
Formation permeability can also affect the placement of scale dissolvers in the zone affected by the scale since high permeability zone can divert the scale removal chemicals to another way. Some gelling materials like viscoelastic surfactant are needed to improve the placement of scale dissolver in the targeted zones. In most of the cases, scales are usually coated with a hydrocarbon that is hindering the contact of acid with scales. In such cases, acid-to-scale contact can be improved by using certain types of surfactants. The selection of surfactant is critical as it might generate acid-crude oil emulsion.
HCl is the most commonly used acid to remove the scale from oil wells. However, owing to its environmental effect and difficulties in handling, several other chemicals have been used in laboratory and field scale. This section highlights several chemicals that have been used for scale removal. An ideal scale dissolver should be thermally stable at downhole temperature, have high dissolving capacity, must be less corrosive, and should not generate free H2S gas after dissolution (Chen et al., 2016). The scale dissolution performance of different scale dissolvers is discussed below.
3.1. Hydrochloric acid
Hydrochloric acid is being used in descaling job in the field and considered as one of the most powerful scale removers since most of the scale minerals have high acid solubility (Al Tolaihy et al., 2010). The use of HCl can be very expensive at high-temperature conditions where additional additives are required to control the reaction rate (Olajire, 2015). Few iron sulfide scales such as troilite and pyrrhotite dissolve in HCl, while pyrite and marcasite is difficult to dissolve in HCl (Wang et al., 2013b). Zinc sulfide also dissolves in HCl, however, better performance was achieved using different additives (Berry et al., 2012). Some oxidizing agents such as H2O2 are required to increase the solubility of zinc sulfide in HCl. Carbonate scales have a very high solubility in HCl and can effectively be removed using HCl. However, the spent acid solution containing scale by-products also acts as an initiator for reformation of scales. Sulfate scales such as barium, calcium, and strontium sulfates have very low acid solubility. At 25 °C and ambient pressure, the solubility of calcium sulfate in HCl is only 1.8 wt% (Moghadasi et al., 2007). The use of some convertor such as Na2CO3 and NaOH can convert gypsum to acid-soluble compounds that can be removed using an acid. The solubility of calcium sulfate in HCl is below 2% at ambient conditions (Olajire, 2015). Some convertors such as NaOH, Na2CO3, and KOH are also used to convert several acid insoluble scales into acid soluble scales.
The generation of H2S and corrosion of production string are major barriers to the application of HCl. Hydrochloric acid is very corrosive to steel, particularly at high temperatures. Acid corrosion inhibitors tend to adsorb on the scale surface that can result in higher metal loss and reduces the access of acid to the scale (Wang et al., 2013a). The adsorbed inhibitor can also block the pore spaceand could result in reducing the relative permeability of oil and gas (Crowe and Minor, 1985). The generation of H2S gas by the reaction of HCl and iron sulfide scale could be a serious issue for well integrity and may cause additional operational risks (Chen et al., 2009). Hydrogen sulfide scavengers must be added to control the free H2S generated during the descaling job using acids. The H2S scavengers also hinder the HCl dissolution reaction in a similar way as corrosion inhibitors do. The HCl dissolution capabilities can reduce up to 50% using 5% aldehyde-based H2S scavengers (Wang et al., 2013a).
The acid loss to formation can cause severe damage to formation rocks as well. The acid can react with the carbonate formation and cause an increase in the pH. The iron sulfide scale precipitate once the pH increases above 1.9 (Wang et al., 2013a). These precipitates can also clog the near-wellbore area and lower the productivity of the well. There are some sludging tendencies when the spent acid contacts with the crude oil (Huang et al., 2002). Therefore, the focus has shifted to use non-acidic treatments in scale removal. HCl can only dissolve specific types of scale and is ineffective for other types of scales. For example, it can dissolve and remove FeS from the well but unable to completely dissolve and remove FeS2 (Elkatatny, 2017; Wang et al., 2015). Owing to the toxicity of H2S and corrosion of production string, the focus has been shifted to find alternative chemical solutions to remove the iron sulfide scale, particularly at high temperatures.
3.2. Organic acids
Different organic acids such as acetic acid, formic acid, maleic acid and citric acid have also been proposed as alternatives to HCl for high pressure high temperature (HPHT) reservoirs (Da Motta et al., 1998; Nasr-El-Din et al., 2001b; Smith et al., 2000; Van Domelen and Jennings Jr, 1995). The majority of the organic acids have very low dissociation constants compared to HCl (Metcalf et al., 2004). They are an attractive choice for scale removal owing to lower corrosion rates and long reaction time. However, organic acids are more expensive than HCl and their performance in dissolving scale particularly carbonate is not as effective as HCl (Da Motta et al., 1998). The chemical structure of some organic acids used for scale removal is shown in Table 3.
Table 3. Chemical structure of some organic acids reported in scale removal application.
| Maleic Acid |
 |
| Formic Acid |
 |
| Acetic Acid |
|
| Glutamic Acid |
 |
| Succinic Acid |
 |
| Citric Acid |
 |
Acetic and formic acids are weak acids and they are weakly ionized. Commercially acetic acids are available up to 100%, however, for field applications they are normally diluted to 15% (Van Domelen and Jennings Jr, 1995). At concentrations above 15%, one of the reaction products (calcium acetate) can precipitate owing to its limited solubility. The concentration of formic acid is also kept below 15% due to the limited solubility of calcium formate (Van Domelen and Jennings Jr, 1995). The dissolving capabilities of organic acids are much lower compared to HCl. The calcium carbonatedissolving capacities of formic acid and acetic acids are 76% and 58% of the dissolving capacity of HCl, respectively. The mixture of acetic and formic acids is a viable option for HPHT wells made of high-alloy steel. A mixture of acetic acid (5 wt%) and formic acid (7 wt %) is 4 times more efficient compared to 10 wt% acetic acid alone in dissolving calcite scale. Citric acid has been used in oil field applications specifically as an iron control agent (Hall and Dill, 1988). Chemically, it has three carboxylic and one hydroxyl group. The major issue with citric acid is the low solubility of calcium citrate (0.0018 mol/1000g water) which further decreases with the increase in temperature (Li et al., 2008). Other organic acids include maleic acid (dicarboxylic acid and a cis-isomer of butenedioic acid), glutamic acid (an α-amino acid that contains α-amino and α -carboxylic acid), succinic acid (dicarboxylic acid also termed as butanedioic acid) and gluconic acid.
Mixtures of organic acid and mineral acid can have a synergetic effect in addition to lowering the amount of mineral acid for specific applications. The mixtures of organic and mineral acids are economically much better than the HCl-based system. Several types of research report the use of a mixture of HCl and an organic acid to dissolve scales, particularly at high temperature to balance the limitations of each class. The organic-inorganic acid mixture is effective for high-temperature carbonate formation. However, organic acids are not effective in removing several types of iron sulfide scale at different temperatures and pH conditions.
3.3. Chelating agents
Chelating agents are the molecules that contain electron-donating group to form a coordination bond with metal ions. Deprotonated chelating agents are negatively charged molecules that can sequester metal ions through coordination bond and the process is called chelation. Because of sequestration, stable ring-like structures are formed that surround the metal ions by capturing all coordination sites of metal ions and minimizing its interaction with other ions in the solution. The properties of metal ions and chelating agent determine the stability of the metal-ligand complex. The affinity of chelating agent for metal is characterized by its stability constant (a higher stability constant indicates a more stable chelated product). The stability constant depends on several factors such as the size of the ring, a number of rings, pH of the chelating agent, and nature of the donor and the central metal atom (Tariq et al., 2017). Most of the chelating agents sequester ions are efficient in basic solution. In acidic form, they could not sequester effectively due to the occupation of hydrogen ions by coordinating functional group (Fredd and Fogler, 1998). As pH increases, the deprotonation results in maximum chelating ability. However, at high pH, the OH− can also occupy the coordination sites and reduce the chelation ability.
Biodegradability of chelating agents is an important consideration in selecting the chelating agent for a specific application. The chelating agent can remobilize heavy metal from sediments to drinking and groundwater (Nowack, 2002; Sillanpää and Kurniawan, 2011). The biodegradability of chelating agents is related to the number of nitrogen atom present in the molecule. The chelating agents containing single nitrogen atom are readily biodegradable. The chelating agents containing more than two nitrogen atoms have poor biodegradability (Sýkora et al., 2001). The substituent also affects the biodegradability of chelating agents and increases in the order ‒COCH3, ‒CH3, ‒C2H5, ‒CH2CH2OH, ‒CH2COOH (Sýkora et al., 2001).
Chelating agents are an attractive alternative to organic and inorganic acids for removal of scale from formations. Chelating agents are more environmentally friendly, readily biodegradable and less corrosive to well tubular and other downhole equipment. The main advantage of chelating agent is their very low corrosion rate compared to HCl. Owing to low corrosion, a lesser quantity of corrosion inhibitors is required. Due to their environmentally friendly nature, chelating agents are the preferred choice to remove scale from sensitive downhole equipment such as an electrical submersible pump (Bageri et al., 2017). However, the cost of the chelating agent itself is higher compared to inorganic acids. The first successful barium sulfate removal job using a chelating agent is tested in North Alwin region of North Sea (de Vries and Arnaud, 1993).
Most of the chelating agents used in the oil industry are aminopolycarboxylic acids. In this group of chelating agents, nitrogen is located at the center of the molecules and carboxylic acid groups behave as the arms of the chelating agent. Chelating agents showed superior properties over several organic and inorganic acids. They have the good dissolving power, low corrosion, better iron control, low sludging tendencies and are they more environmentally friendly (Almubarak et al., 2017a). The common chelating agents that have been utilized include but not limited to ethylenediamine tetraacetic acid (EDTA), hydroxyethyl ethylene diamine tetraacetic acid (HEDTA), hydroxyethyl iminodiacetic acid (HIDA), l-Glutamic acid N,N-diacetic acid (GLDA), Diethylene triamine pentaacetic acid (DTPA), Nitrilotriacetic acid (NTA), and Methylglycinediacetic acid (MGDA). The structures of these chelating agents are shown in Table 4.