1. Introduction
Research on graphene quantum dots (GQDs) has grown exponentially in recent years, Graphene quantum dots (GQDs), are graphene fragments small enough to cause exciton confinement in 3–20 nm particles and quantum-size effect. Although graphene is a zero band gap nanomaterial and hence non-luminescent it has an infinite excitation Bohr radius and affords quantum confinement in finite sized specimens [1]. The band gap in GQDs is non-zero and can be tuned by altering the size and the surface chemistry of the dots [2].
The preparation methods of GQDs can be classified into two categories: top–down and bottom–up methods. The strategy of top-down methods is to cut down large graphene sheets, carbon nanotubes, carbon fibers or graphite into small pieces of graphene sheet, while in the bottom–up methods, small molecules are used as starting materials to build the GQDs. Until now, for top-down approaches, nanolithography technique, acidic oxidation, hydrothermal or solvothermal microwave assisted, sonication-assisted, electrochemical, photo-Fenton reaction, selective plasma oxidation and chemical exfoliation methods have been used to synthesize GQDs; while for the bottom–up techniques, using benzene derivatives as starting materials through stepwise solution chemistry methods, carbonization as starting materials through microwave-assisted hydrothermal method, fullerenes as starting materials through ruthenium-catalyzed cage-opening and unsubstituted hexaperihexabenzocoronene as starting materials through the process of carbonization, oxidization, surface functionalization, and reduction successively have been utilized to prepare GQDs successfully [3]. GQDs provide an effective alternative to colloidal inorganic semi-conductive quantum dots (QDs), which have attracted much attention in the past two decades due to their electronic and optical properties [4]. However, QDs are highly toxic due to the release of heavy metals, such as cadmium, selenium, tellurium and zinc, from their core and their coating. In the early research on GQDs, tremendous effort was devoted to developing methods for the preparation of GQDs and exploring their properties [5], [6], [7], [8], [9], [10]. Their application in the analytical field has not been explored until very recently. Sensors based on GQDs can achieve a high level of performance due to their novel properties.
In the recent years, graphene quantum dots (GQDs) has become an emerging class of the nano-carbon family, due to their low toxicity, easy preparation, high chemical stability, environmental friendliness, their luminescence and ability to transfer photoinduced electron [11], [12]. It has shown great promise applications in the fields of bioimaging [13], [14], photo-luminescence [15], catalysts [16], and fluorescent sensor [17]. In addition, GQDs have been recognized as both, excellent electron donors and acceptor, making them interesting candidates for producing electrode materials [18], [19]. GQDs can be functionalized [20] especially with oxygen containing groups such as hydroxyl, carboxyl and epoxy groups which can greatly enhance their hydrophilia and biocompatibility. Therefore, GQDs have significant potential for bio- and immunosensing applications.
On the other hand, there is a continuous demand for fast and simple analytical methods for the determination of many clinical, biochemical and environmental analytes useful as markers of illness or contamination. In this respect, immunosensors that rely on antibody-antigen (Ab-Ag) interactions provide a promising means of diagnostics due to their specificity and sensitivity. Immunological sensors are based on recognition of the coupling of an Ag with an Ab to form a complex. Immobilization of one of the aforementioned elements onto a sensing surface will cause a signal change upon formation of a complex with the corresponding Ab or Ag. Highly-sensitive immunosensors can thus be constructed using enzymatic reactions involving fixed enzyme-labeled Ag. Immunosensors can be competitive or non-competitive, homogeneous or heterogeneous. Non-competitive immunoassays are usually more sensitive and more specific than competitive ones. Immunosensors can be classified as electrochemical, optical, piezoelectric, thermometric or magnetic, according to the type of transduction [21].
Optical immunosensors involve coupling immunoassay techniques with surface plasmon resonance (SPR) technology [22], [23]. A change in refractive index of the medium upon interaction of Ag (tumor marker) with Ab immobilized on the sensor surface can be measured [24]. Alternatively, Ab could be immobilized on the surface of an optical fiber and change in refractive index, fluorescence or luminescence correlated with the amount of Ag interacting with the Ab. Piezoelectric immunosensors are generally based on evaluating the change in frequency of oscillation as result of change in mass on interaction of Ag with Ab immobilized on quartz crystal [25].
Electrochemical immunosensors allow quantitative estimation of tumor markers by detecting the change in potential (potentiometric) [26], current intensity (amperometric) [27] or capacitance (capacitive) [28] as a result of Ab-Ag interaction. Potentiometric immunosensors generally use techniques that are non-dependent on labels and can directly detect the change in potential due to Ab-Ag interaction at the surface of the detection device. The first potentiometric immunosensor for an ovarian cancer-related tumor marker was developed for hCG by Yamamoto and co-workers in 1978 [29] using a non-labeled antibody. Capacitive immunosensors detect alterations in capacitance following an immunoreaction (Ab-Ag interaction) on the application of a potential pulse. Suwansa-ard and co-workers in 2009, developed a capacitive immunosensor for a flow injection-based label-free detection of CA125 in human serum [30].
Amperometric immunosensors have been by far more extensively studied as compared to others. This could be attributed to their ease fabrication, miniaturization, robustness, and cost-effectiveness and detection sensitivity. On the other hand, piezoelectric immunosensors, provide low detection limits (~ pico-molar or femtomolar concentrations), but have not replaced amperometric immunosensors because of their high cost, difficulty in mass production or miniaturization and problems with mechanical and electromagnetic interference. Hence, the next section will focus on the historical development of electrochemical immunosensors and more specifically amperometric immunosensors
Amperometric immunosensors are based on measurement of the current intensity from electrochemically active redox species produced during the reaction. In immunoreactions, analytes (Ag/Ab) do not act as redox partners themselves; hence an enzyme-labeled Ag or Ab may be used to provide electroactive species as reactants or products. The current generated gives an indirect measure of the analyte concentration. Enzymes such as horseradish peroxidase [31], [32] and alkaline phosphatase [33] are most commonly used to catalyze such electrochemical reactions.
The determination of the levels of cancer biomarkers provides crucial information for the screening diagnostics of cancer, and the development of sensing devices or other analytical methodologies for their sensitive and reliable point-of-care measurement. It has been a significant challenge for the early cancer detection and treatment monitoring, since high sensitivity, accuracy, minimal technical expertise, rapid and easy system maintenance should be obtained [34]. In this review examples from the literature describing cancer biomarker immunosensors based on GQDs were collected. These systems use a biorecognition element according to the definition given above. GQDs-based immunosensors for detection of different biomarkers were selected from latest research articles from 2000 to July 2016to be included in this review. Type of modifiers for GQDs, injection and detection techniques, labels, target analytes and the corresponding sample matrix were discussed in detail, paying special attention to the sensitivity of assays. In the case of GQD-based immunosensors for detection of cancer biomarkers, in our knowledge, only six studies have been published til now. Therefore, this review would like to explore the reason and possible problems involved in this research area. In general, we summarize below examples of GQD applications in electrochemical immunosensing reported so far in the literature, and compared with the other prepared immunosensors based on nanomaterials. Finally, we stress their potential for future development in this research field.
1.1. Do graphene quantum dots improve immunosensing technology?
The present paper has the objective of reviewing the state of the art of recent GQDs based immunosensors employed in cancer biomarkers analysis covering the period of 2000-July 2016 for comparison of their analytical performance, and also considering their advantages and limitations according to the transduction principle. Some approaches are also reported in order to improve the analytical performance of these immunosensors. The study of the analytical performance of GQD-based immunosensors is reported in the following discussions through the estimation of associated analytical figures of merit and taking into account the configuration of the various GQD-based immunosensors applied to the detection of cancer biomarkers considered.
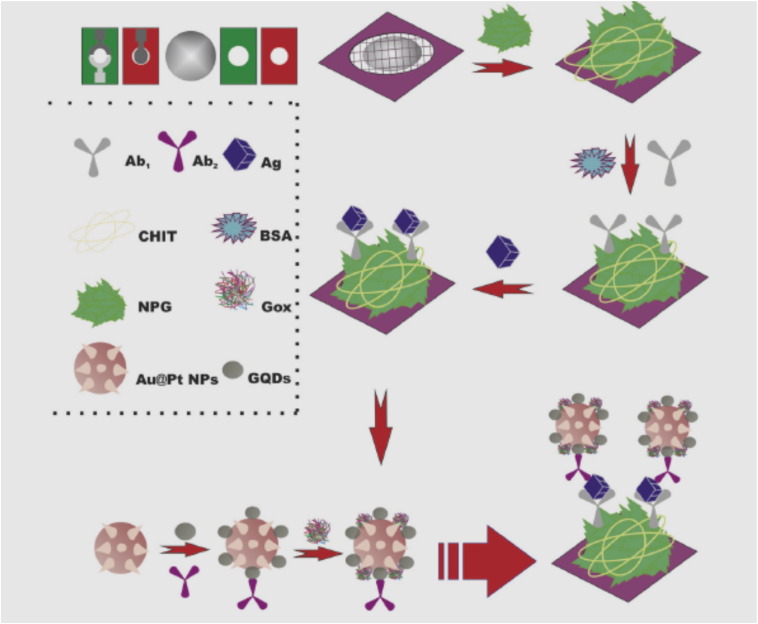
In the first report, a highly sensitive 3D origami device combined with electrochemiluminescence (ECL) immunosensor was introduced by Li and coworkers [35] for sensitive point-of-care testing of carcinoembryonic antigen (CEA). This immunodevice was developed based on nanoporous gold/chitosan modified paper working electrode (NGC-PWE) as sensor platform and green-luminescent graphene quantum dots (GQDs) functionalized Au@Pt core-shell nanoparticles (GQDs/Au@Pt) as signal label. According to this report, firstly, the origami device was fabricated by directly screen-printing electrodes on wax-patterned pure cellulose paper. Secondly, the NGC hybrids were prepared and introduced for binding of primary antibody with high loading amount. The ECL nanoprobe (Ab2/GQDs/Au@Pt) was designed by covalent assembly of signal antibody (Ab2) on GQDs tagged Au@Pt core-shell nanoparticles. After a sandwich-type immunoreaction, the GQDs/Au@Pt labels were captured onto the NGC-PWE surface (Scheme 1). The amplified ECL signals were achieved by efficient catalysis of the glucose oxidase (GOx) towards the oxidation of glucose to in situ generate H2O2 as co-reactant and the enhancement of Au@Pt to the ECL reaction of GQDs-H2O2. Under the optimized experimental conditions, the proposed strategy exhibited excellent high sensitivity for the determination of CEA ranging from 1.0 pg/mL to 10 ng/mL with the detection limit of 0.6 pg/mL. This immunosensor was tested for detection of CEA in clinical serum samples.
 Scheme 1. The schematic representation of the fabrication procedure for 3D origami ECL immunodevice [35]. Re-drawing by Corel Draw 6.0 software.
Scheme 1. The schematic representation of the fabrication procedure for 3D origami ECL immunodevice [35]. Re-drawing by Corel Draw 6.0 software.The application of the above-discussed immunosensor by using GQDs was compared with the other reported immunosensors which prepared based on nanomaterials. Table 1 compare the analytical performance of above discussed sensor with the other reported immunosensors for detection of CEA. Also, Table 1 [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76] includes the figures of merit of current immunosensors (literature published between 2009 and July 2016) for comparison of their analytical performance when used to the detection of CEA. As can be seen, carbon based nanomaterials has been used for detection of CEA. Compartment the results of the above discussed article with the previously reported method indicated that, the GQDs based prepared ECL immunodevice don't exhibited excellent performance for highly sensitive detection of CEA than others. On comparison with previously constructed immunosensor the dynamic range of GQD-based immunosensor was much smaller and a much more improved LOD was achieved employing GQDs. However, the proposed GQD-based immunosensor prepared by Li and coworkers can be expanded readily for detecting other cancer biomarkers and has the potential for reliable diagnosis of cancer and other diseases. Therefore, integration of some modification agents into GQDs can be increases Faradic currents. In conclusion of this case (GQD-based immunosensors for CEA), the hybridization of GQDs with other organic or inorganic materials [e.g., polymers, carbon and bimetallic nanoparticles, iron based magnetic nanoparticles, mesoporous silica and polymer dots] through covalent binding could considerably extend the possibilities for GQD-immunosensor development.
Table 1. Analytical parameters of recent immunosensors for CEA.
| Modification type | Linear range (ng/mL) | Limit of detection (ng/mL) | Ref. |
|---|---|---|---|
| Anti CEA/GQDs/Au@Pt | 0.001–10 | 0.0006 | [35] |
| Anti-CEA/AuNPs-GP-CS/Ag/AuE | 1.0 × 10− 6–1.0 | 2.0 × 10− 7 | [36] |
| Anti-CEA/AuNPs/SiO2-Thi/AuNPs/Cys/AuE | 1.0–100 | 0.34 | [37] |
| Anti-CEA/AuNPs/PBNPs/CS-MWCNTs-AuNPs/GCE | 0.30–120 | 0.1 | [38] |
| Anti-CEA/AuNPs/NiHCFNPs/AuNPs/GCE | 0.50–10 | 0.1 | [39] |
| Anti-CEA/AuNPs/CS/NG/GCE | 0.20–120 | 0.06 | [40] |
| Anti-CEA/AuNPs-GP/Fc-CS-TiO2/GCE | 1.0 × 10− 2–80 | 3.4 × 10− 3 | [41] |
| Anti-CEA/AuNPs-Ph-GCE | 1.0 × 10− 5–100 | 3 × 10− 6 | [42] |
| Anti-CEA/GO-Thi-AuNPs/GCE | 1.0 × 10− 7–1000 | 5.0 × 10− 8 | [43] |
| Anti-CEA/AuNPs-Thi-GR/GCE | 1.0 × 10− 2–0.10 | 4 × 10− 3 | [44] |
| Anti-CEA/AuNPs/PATP/AuE | 1.0 × 10− 6–10 | 1.5 × 10− 8 | [45] |
| Anti-CEA/IL-GS-AuNPs/GCE | 1.0 × 10− 6–100 | 1 × 10− 7 | [46] |
| Anti-CEA/SPA/AuNPs/HDTs/GE | 1.0 × 10− 3–100 | 1 × 10− 4 | [47] |
| AuNPs/MWCNTseChit/GCE | 0.3–2.5 | 0.01 | [48] |
| AuNPs/Fe3O4eChit/PB/GCE | 0.1–20 | 0.01 | |
| Poly-OAP/anti-CEA/AuNPs/Au | 0.5–20 | 0.1 | [49] |
| AuNPs/GCE | 0.01–50 | 0.01 | [50] |
| ChiteCNTseAuNPs/GCE | 0.1–2.0 | 0.04 | [51] |
| AgNPs/DNA/Thi/SCPE | 0.03–3 | 0.01 | [52] |
| AuNPs/NBeERGO/GCE | 0.001–40 | 0.00045 | [53] |
| Anti-CEA-HRP/Con A/pDA/3D-G/GCE | 0.1–750.0 | 0.09 | [54] |
| Anti-CEA/nano-Au/MWCN-CHT/GCE | 0.3–20 | 0.01 | [55] |
| Anti-CEA/CS-CNTs-GNPs/GCE | 0.1–200.0 | 0.04 | [56] |
| Anti-CEA/Au-CHT/(MWNT-PEIeAu/PB)5/GCE | 0.5–160 | 0.08 | [57] |
| Anti-CEA/colloid Au/chitosan/SPCE | 0.50–25 | 0.22 | [58] |
| Anti-CEA/AuNPs/SiO2-Thi/AuNPs/Cys/AuE | 1.0–100 | 0.34 | [59] |
| Anti-CEA/AuNPs/PBNPs/CS-CNTs-Au/GCE | 0.30–120 | 0.10 | [60] |
| Anti-CEA/AuNPs/CS/NG/GCE | 0.20–120 | 0.06 | [61] |
| Anti-CEA/AuNPs/rCu-GO/GCE | 0.01–120 | 0.004 | [62] |
| CS–CNTs–GNPs nanocomposite film | 0.1–2.0 | 0.04 | [63] |
| [Ag–Ag2O]/SiO2 nanocomposite material | 0.5–160 | 0.14 | [64] |
| Thi@NPG/AuNPs | 0.01–100 | 0.003 | [65] |
| AuNP@nafion/FC@CHIT | 0.01–150 | 0.0031 | [66] |
| HRP-anti-CEA-NGGN | 0.05–350 | 0.01 | [67] |
| Carboxylated g-C3N4/TiO2 nanosheets | 0.01–10 | 0.0021 | [68] |
| Anti-CEA-ZnO NP/GOD/pDA/rGO/GCE | 0.01–80 | 0.0033 | [69] |
| Anti-CEA/Au NP-HRP-MB/G/GCE | 5–60 | 5 | [70] |
| Anti-CEA/Au NP-Ag-NP/rGO/GCE | 0.0001–200 | 0.008 | [71] |
| Anti-CEA/AuNP-rGO/SWNT-PEI-Pd/Pt-GOD/GCE | 0.0001–160 | 3 × 10−5 | [72] |
| Anti-CEA/AuNP-rGO/MnO2-PDDA-GS/AuE | 0.0003–8 | 0.08 | [73] |
| Anti-CEA/AuNPs/PTC-Arg/Au@rGO/Au electrode | 0.0001–10 | 0.0003 | [74] |
| Anti-CEA/AuNP-hemine-AgNP-rGO-GOD-GCE | 0.0001–160 | 3 × 10−5 | [75] |
| Anti-CEA/Pt-Ag NPs/rCu-Au NP/μPADs | 0.001–100 | 0.0003 | [76] |
The above-described immunosensor demonstrate the broad potential of bio-conjugated GQDs for amplified transduction of bio-molecular recognition events. Given the optical and electrochemical applications, GQDs labels provide the basis for ultrasensitive assays of CEA. The remarkable sensitivity of the new GQDs-based sensing protocols opens up the possibility for detecting CEA. Such highly sensitive bio-detection schemes could provide early detection of diseases or a warning of a terrorist attack. The use of GQDs for detecting CEA is still in its infancy, but the lessons learned in ultrasensitive CEA detection should provide useful starting points. The successful realization of the new signal-amplification strategies requires proper attention to nonspecific adsorption issues that commonly control the detectability of bioaffinity assays. Proper washing and surface blocking steps should thus be employed to avoid amplification of background signals (associated with non-specific adsorption of the GQDs). Due to the minority of research being on development new GQDs-based immunosensors, more techniques should be involved in this area. With GQDs, it will be possible for immunosensors to be applied to pre-warning and real-time detection of CEA.
In this case, we have discussed the promising future of incorporating GQDs into the field of immunosensing, specifically their current roles in detection of CEA. The advantages of GQDs on immunosensing and enhancement of signal were compared with other reports, together with their analytical application on biological systems. But, there are many challenges ahead that must be addressed before GQDs can be successfully integrated into immunodevice. The main advances required, in our opinions, include the following.
-
(i)
Advanced techniques and facile methods are needed to increase the sensitivity of GQDs-based immunosensors towards signal amplification and highly sensitive detection of CEA.
-
(ii)
Further research is required into various functionalized GQDs to promote cell adhesion, sensitivity, selectivity, and so on which is necessary for immunosensing.
-
(iii)
More specialized coatings to minimize nonspecific bonding are needed for CEA immunosensing.
-
(iv)
Protocols and further experiments should be conducted to determine the exact nature of the nano-toxicity of GQDs.
-
(v)
Innovative solutions are required to reduce fabrication and running costs of GQDs micro- and nano-fluidic devices to make them economically viable for detection of CEA in real samples and cell lines.
-
(vi)
Results raise doubts about the repeatability, reproducibility, and comparability of GQD- based immunosensors, as, with every emerging technology, standards need to be established to avoid the doubts about the lack of reproducibility, repeatability, and compatibility across platforms and laboratories.
-
(vii)
Finally, we conclude that there is a bright opportunity for further advances and developments of immunosensors based on functionalized GQDs, especially through further miniaturization and integration into lab-on-chip systems. The design of implantable devices with the ability to monitor CEA in vivo and in real time is promising for the application of immunosensors, even though it is yet to be explored. Scientists therefore have a great deal to do to address the performance of immunosensors for CEA detection in the future.
In the second report, Yang and coworkers [77] reported a GQD-coated porous PtPd nanochain (pPtPd)-labeled ECL immunosensor using gold-silver nanocomposite-functionalized graphene for the detection of tumor markers in serum. To this end, AgNPs were attached to a PVP-coated graphene sheet and post-surface decorated with AuNPs to obtain a GN-Ag-Au hybrid nanomaterial that was deposited onto a GCE surface. Then, the primary antibodies were dropped onto the modified GCE. Separately, GQDs and pPtPd were conjugated (pPtPd@GQDs) through NH3+ groups present at the porous nanochains and COO– groups in the GQDs. This was followed by incubation with the secondary antibodies (Ab2) to obtain pPtPd@GQDs/Ab2. Next, the ECL immunosensor was incubated with the sample (CA199) at different concentrations and, finally, with pPtPd@GQDs/Ab2 to obtain a sandwich-type structure. The anodic ECL of the assay was measured in the presence of tripropylamine (TAP) as co-reactant. In this work, carbohydrate antigen 199 was used as a model analyte. Under optimal conditions, the ECL immunosensors exhibited a detection range (0.002–70 U/mL) and a low detection limit (0.96 mU/mL).
In the third research work, Wang and coworkers [78] explored the detection of avian leucosis virus subgroup J (ALV-J) by using a doubly-assisted signal-amplification electrochemical immunosensor based on GQD-apoferritin-encapsulated Cu NPs. A bare GQD-modified GCE was incubated with Ab1 and then with variable concentrations of ALV-J. Next, previously prepared Fe3O4@GQDs/Ab2-Cu-apoferritin/BSA bioconjugates were dropped onto the electrode surface and incubated. For preparation of immunosensors, 6 μL homogeneous suspension of GQDs (2 mg/mL) was added onto the bare GCE surface. After the GQDs coated electrode was dried, it was immersed into EDC (400 mM) and NHS (100 mM) solutions to activate the carboxyl groups of GQDs for 4 h. Then after washing with PBS, it was incubated in Ab1 solution at 4 °C for 6 h. To block the non-specific binding sites, 6 μL of 1.0 wt% BSA was placed onto the electrode for 1 h at 37 °C. Then, various concentrations of ALVs-J were incubated with the immobilized Ab1 for 1 h at 37 °C. Thereafter, the prepared Fe3O4@GQDs/Ab2–Cu-apoferritin/BSA buffer solution was dropped onto the electrode surface and incubated for 1 h. Before electrochemical detection, the fabricated electrode was immersed into 5 mL HCl solution (pH = 2) for 20 min to release Cu from the cavity of apoferritin. After the sandwich-type assembly was formed, Cu was released from the apoferritin cavity for detection by DPV. The dots were used for both conjugation of ALV-J Ab1 and immobilization of ALV-J Ab2.
In the case of ALV, several analytical methods have been reported for the detection of ALVs-J, including immunohistochemistry [79], enzyme-linked immunosorbent assay [80], and surface plasmon resonance [81]. However, these methods often require a few days to complete the detection. Therefore, according to obtained results (short response time and high sensitivity) with the above-discussed GQDs-based immunosensor is a good candidate to replacement with the other methods. Compartment of the results shows that, GQDs-based immunosensors open up new possibilities for future application in early stage diagnosis of tumor markers and cancer. Due to the super-physical and chemical properties of the GQDs, high-sensitivity detection and excellent diagnostics have been achieved. However, there are aspects such as the specificity and sensitivity in tumor markers sensing can be improved by developing novel GQDs such as bilayer functionalization and doping. By combining new surface modification techniques, GQDs composites, and biotechnologies, more theranostic method will be developed. We are convincing that the progress of GQDs-based device can open avenues for application in immunosensing and cancer.
In the fourth report, Al-Ogaidi et al. [82] developed an optical immunoassay for the detection of ovarian biomarker CA-125 based on CL resonance-energy transfer (CRET) to GQDs immobilized by electrostatic attraction onto an amino-modified glass chip. Scheme 2 depicts the immunoassay and the detection principle behind it. Capture antibodies (cAbs) were covalently linked to the dots via amide conjugation. In the absence of the antigen (CA-125), HRP catalyzed the production of reactive oxygen species (ROS) from H2O2 that oxidized luminol to its singlet dianion and produced excited electrons generating blue CL upon returning from the excited state to the ground state. In the presence of the antigen, exposure of the antibody-antigen complex formed (GQD-cAb + CA-125) to Ab-HRP led to the sandwich-type structure (GQD-cAb + CA-125 + Ab-HRP), where HRP was in close proximity to the GQDs. The fact that the dianion involved in the HRP-catalyzed reaction was close to the dots facilitated resonance-energy transfer between the two; this quenched the CL of the dots to an extent inversely proportional to the CA-125 concentration. In this sensor, graphene quantum dots (GQDs) are immobilized on a functionalized glass chip. Signal transduction is based on the CRET from the chemiluminescent reagent soluble in the aqueous solution to the GQDs on the solid chip. The use of GQDs as the energy acceptor avoids the photo-bleaching problem, which is usually associated with organic dyes. This provides more flexibility for selection of the energy donors. Moreover, GQDs are typically hydrophilic, and are rich in the carboxylic acid moiety, which is convenient for bioconjugation. The capture antibody is linked to the GQDs on a transparent solid substrate, which has a potential for further development of high-throughput and automated sensor chips. The immunoassay exhibited a LOD of as low as 0.05 U mL1 and a wide linear range from 0.1 U mL1 to 600 U mL1 for CA-125 in the buffer solution.
 Scheme 2. Scheme of the assembly of the immunoassay and the detection principle [82]. Re-drawing by Corel Draw 6.0 software.
Scheme 2. Scheme of the assembly of the immunoassay and the detection principle [82]. Re-drawing by Corel Draw 6.0 software.Table 2 [83], [84], [85], [86], [87], [88], [89] summarized all of reports in immunosensing of CA 125 and compare analytical results with above discussed GQD-based immunosensor. It can be observed from Table 2, that the dynamic range and LOD of the above-discussed GQD-based immunosensor is better than others which a much more improved dynamic range and LOD was achieved employing a GQDs. On the other hand, it is an excellent substitute for a FRET-based assay because it eliminates the need for an external excitation source. In addition, constructed GQD-based immunodevice exhibited a similar performance in the presence of 50% blood plasma with a LOD of 0.08 U mL1. The as-developed sensing platform can be further fabricated in an array chip-based device for high throughput multiplex detection.
Table 2. Immunosensors for ovarian tumor marker CA 125 showing their performance characteristics, immobilization strategy, the mediator used and response characteristics.
| Modification type | Linear range (U/mL) | Limit of detection (U/mL) | Ref. |
|---|---|---|---|
| Ag immobilized on AuNP stabilized with cellulose acetate membrane | 0–30 | 1.73 | [83] |
| Anti-CA125 was immobilized on carbon nanofibre (CNF) | 2–75 | 1.8 | [84] |
| Ab immobilized on gold microspheres and polythionine | 4.5–36.5 | 1.3 | [85] |
| Ab immobilized on gold electrode with ferrocene carboxylic acid liposomes as secondary Ab label | 0.001–300 | 0.0005 | [86] |
| Ag immobilized with AuNPs in titania sol-gel | 2–14 | 1.29 | [87] |
| Copper oxide nanoflake-modified screen printed gold electrode | 0–500 | 0.77 | [88] |
| Gold electrode decorated with MPA | AuNP@SiO2 | QD | CA125 mAb | 0–0.1 | 0.0016 | [89] |
Presently, a number of high quality in vitro diagnostic (IVD) technologies are commercially available that are being used in clinical laboratories for diagnosis CA 125. These analyzers are available in a variety of portability, sensitivity and throughput options. Various platforms including ADVIA Centaur (Siemens), Access 2 (Beckman Coulter), ARCHITECT i (Abbott Diagnostics), AxSYM (Abbott Diagnostics), VITROS (Ortho Clinical Diagnostics), AxSYM (Abbott Diagnostics), Elecsys (Roche Diagnostics) and VIDAS (BioMérieux) for the ovarian cancer biomarker CA 125 have been compared in Table 3, in terms of detection limit, linear range. Interestingly, compartment of the obtained results by GQDs-based immunosensor (above discussed) with some of commercial immunodevice for CA 125 show that, dynamic range and LOD of the proposed immunosensor by Al-Ogaidi and coworkers is suitable and therefore, is a good candidate to replacement. However, some of in vitro studies are necessary.
Table 3. Commercially available diagnostic technologies for ovarian cancer diagnosis.
| Company | Assay type | Limit of detection (U/mL) | Dynamic range (U/mL) |
|---|---|---|---|
| Ortho Clinical Diagnostics | Immunometric immunoassay; chemiluminometric technology | 1.2 | 0–1000 |
| Roche | Electrochemiluminescence immunoassay “ECLIA” | 0.6 | 0.6–5000 |
| Beckman Coulter | chemiluminometric technology | 0.5 | 0.5–5000 |
| Abbott | Chemiluminescent microparticle immunoassay(CMIA) | ≤ 1 | 0–1000 |
| Abbott | Microparticle enzyme immunoassay (MEIA) | 2–600 | – |
| Biomerieux | Enzyme-linked fluorescent assay (ELFA) | 4 | 4–600 |
| Siemens | Paramagnetic particle-based chemiluminometric technology | 2 | 2–600 |
Therefore, according to obtained analytical results by Al-Ogaidi, the unique properties of nanostructures have led to simple, highly-sensitive, electroanalytical procedures that could not be accomplished by standard electrochemical methods. However, the preparation of more novel and sensitive GQD-based immunosensor will largely depend on rapid progress in the synthesis of GQD with unique shapes and structures. So, much effort needs to be devoted to synthesizing novel GQD derivative with unique shapes and structures.
From the Table 2, Table 3, we conclude that there is a bright opportunity for further advances and developments of GQDs-based immunosensing, especially through further miniaturization and integration into lab-on-chip systems. The design of implantable immunosensors with the ability to in vivo detection of CA 125 and other biomarkers related to ovarian cancer and real time analysis is promising for the application of immunosensors, even though it is yet to be explored. Therefore, scientists have a great deal to for to address immunosensors based on GQDs in the future.
In the fifth published article, a new and versatile signaling transduction strategy in the fluoro-immunoassay through regulating the interaction between Gr and GQDs was proposed by Zhao and coworkers [90], which was applied to an immunosensor based on luminescence resonance energy transfer (LRET) for determination of human immunoglobulin G (IgG). The principle of this strategy is illustrated in Scheme 3. Gr as an acceptor and mouse anti-human immunoglobulin G (mIgG, antibody)-conjugated GQDs as donors were chosen to fabricate a LRET immunosensor for detecting human immunoglobulin G (IgG, antigen). When adding Gr to the GQDs covalently functionalized with mIgG (mIgG-GQDs) solution, both the p-p stacking interaction between Gr and GQDs and the nonspecific binding interaction between mIgG and the Gr surface would bring Gr and GQDs into LRET proximity to facilitate the luminescence quenching of GQDs based on LRET. In the sensing process, the addition of human IgG would bind the mIgG due to the specific antibody-antigen interaction, which effectively increased the distance between mIgG-GQDs and Gr surface, thus hindering the LRET process and then producing a restoration of luminescence. In this “on” state, the overall luminescence response depended on the amount of human IgG in the samples, thus providing an effective basis for quantitative determination of targets. Furthermore, the binding interaction between mIgG and human IgG can ensure the specificity of this design. The results showed that the presented sensor exhibited high sensitivity and good reproducibility in human IgG detection. What is more, it can be used for determination of other antigens by simple replacement of antibodies. It is expected that this novel strategy will open new opportunities for development of the LRET technique and promote the application of carbon-based nanomaterials in immunoassays.
 Scheme 3. Schematic illustration of a universal immunosensing strategy based on regulation of the interaction between Gr and GQDs [90]. Re-drawing by Corel Draw 6.0 software.
Scheme 3. Schematic illustration of a universal immunosensing strategy based on regulation of the interaction between Gr and GQDs [90]. Re-drawing by Corel Draw 6.0 software.Table 4 includes the figures of merit of current immunosensors (from literature published between 1973 to July 2016) for comparison of their analytical performance when used to the detection of IgG [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105].
Table 4. Analytical parameters of recent immunosensors for IgG.
| Type of sensor | Linear rage (nM) | LOD (pM) | Ref. |
|---|---|---|---|
| Anti-IgG/Ag@Au NPs/Cys/Nafion/Pt | 2.3–960 | 10 | [91] |
| Anti-IgG/COOHMWCNTs/Fe3O4 NPs/Au | 30–1000 | 25 | [92] |
| Anti-IgG (γ-chain)/MUA/Au | – | 30 | [93] |
| Anti-APAP/GPO/SPCE | 1.5–1512 | 25.7 | [94] |
| AuNPs-C/GCE | 2–250 | 1.8 | [95] |
| Anti-IgG/DNA nanopyramid/Au | 0.010–10 | 0.0028 | [96] |
| Anti-IgG/GPO/SPCE | 2.5–100 | 1.99 | [97] |
| Nanocrystalline diamond-Aptamer | 0.19–268 | 0.19 | [98] |
| Aptamer-based piezoelectric quartz crystal microbalance biosensor | 0.016–1.33 | 16 | [99] |
| Silver nanoparticle hybrid probes | 0.0026–5.3 | 0.211 | [100] |
| Aptamer-Au NP-screen-printed electrode (SPE) | – | – | [101] |
| Surface plasmon resonance | 0.0625–6.25 | 21.5, 12.9 | [102] |
| Aptamer-MWCNTs/ionic liquid/chitosan nanocomposite-GCE | 0.5–30 | 37 | [103] |
| Aptamer-MB | 0.0048–20 | 4.6 | [104] |
| Aptasensors-CdSe/ZnS functionalized MoS2-Enzyme | 0.0005–0.5 | 0.18 | [105] |
a NPs, nanoparticles; Cys, cysteine; Pt, platinum electrode; MWCNTs, multiwalled carbon nanotubes; Au, gold electrode or gold chip; MUA, mercaptoundecanoic acid; Anti-APAP, Anti-acetaminophen antibody; SPCE, screen printed carbon electrode; AuNPs-C, gold nanoparticle colloid carbon spheres; GCE, glassy carbon electrode.
In the final (sixth) published work, an ultrasensitive label-free electrochemiluminescent immunosensor was developed based on graphene quantum dots [106]. In this report, Au/AgrGO was synthesized and used as electrode material to load a great deal of graphene quantum dots due to the large surface area and excellent electron conductivity. In this report, Au/Ag-rGO was prepared using the following procedure. 80 mg aminated graphene powder was dispersed in 50 mL ultrapure water and under continuous ultrasound for 10 h. Then, 40 mg AgNO3, 5 mL 1% HAuCl4 and 5 mg PVP were added into the above solution. 4 mL 50 mM sodium citrate was added dropwise to reduce AgNO3 and HAuCl4 after 6 h of stirring. After 24 h of stirring, the Au/Ag-rGO suspension was filtered and then dried at 35 °C in vacuum. After aminated graphene quantum dots and acarboxyl graphene quantum dots were modified onto the electrode, the ECL intensity was much high using K2S2O8 as co-reactant. Then, antibody of PSA was immobilized on the surface of modified electrode surface through the adsorption of Au/Ag towards proteins, leading to the decrease of the ECL intensity (Scheme 4). According to this report, the ECL intensity decreased linearly with the logarithm of PSA concentration in the range of 1 pg/mL to 10 ng/mL. The detection limit achieved is 0.29 pg/mL. The immunosensor results were validated through the detection of PSA in serum samples with satisfactory results.