Introduction: objectives of intravascular imaging
Guidance of percutaneous coronary intervention
Intravascular imaging has earned its place at the table in interventional cardiology practice. Its present role is primarily in a posteriori checking of the result of percutaneous coronary interventions (PCI), as an add-on to conventional angiography.1 In PCI, one or more stents are implanted to resolve myocardial ischemia. PCI shows adequate acute success rates, but sees a persistent recurrence of symptoms in approximately 10% of patients in the first year (including periprocedural complications), rising to around 20% at three years 1, 2, 3. Around half of those repeat catheterizations address the target site.
In recent years, features on intravascular imaging have emerged that correlate with adverse events such as stent thrombosis and restenosis: inadequate stent expansion, dissections and thrombus 4, 5 can all be visualized with intravascular imaging. Catheter-based imaging is also used prior to intervention, to obtain measurements of vessel and lumen size, and length without the projection artifacts inherent to angiography. For these purposes, it is important to deliver high-contrast, easily interpretable images of the lumen and stent, as well as visualization of the vessel wall in high resolution.
Current commercially available technologies do this very well: Intravascular ultrasound (IVUS) [6] and intravascular optical coherence tomography (IVOCT; also called optical frequency domain imaging, or OFDI) [7] in particular are in practical use. The latter delivers higher resolution and higher image contrast, but at the cost of the necessity to flush blood from the artery. This step, combined with the inherently limited ranging depth, make IVOCT less reliable in large vessels, near major side branches, and in patients with kidney dysfunction (who do not tolerate the flush medium well). IVUS is easier to use and more versatile, but the images contain fewer details, less contrast, and require a longer learning curve to interpret. For the purpose of this review, we will assume familiarity with these techniques.
Recent meta-analyses, based on a range of registries and a number of randomized studies that compare intravascular imaging guidance to angiography alone, demonstrate a clear benefit from intravascular imaging on hard endpoints like death and myocardial infarction (MI), and also on repeat revascularization 8, ••9. Data also show improved procedural outcome metrics, like larger post-intervention minimal lumen area (MLA) [10], and effect on therapeutic decisions, such as number of implanted stents and stent length 11, 12. OCT demonstrated a favorable effect on functional assessment of the treated vessel [13]. These observations have led to proposals for intravascular imaging guided stenting 14, 15, criteria for which are currently being investigated in clinical trials.
Current techniques for plaque imaging
Despite promising recent indications for the clinical utility of current technology, the intravascular imaging field has always aspired to a higher goal: detecting atherosclerotic plaque for predicting future events and pre-emptive intervention. This ability is extremely valuable in the scenario of a patient presenting for coronary revascularization, who has one or more non-culprit lesions in addition to the one that generates his/her symptoms. These patients make up the other half of repeat revascularizations mentioned before: symptoms arose from a different site than was treated originally. Clinically, this target of “vulnerable plaque” [16] imaging has so far remained elusive. The key to finding vulnerable plaques is detecting the composition of plaques, in addition to their morphology. Unstable plaques are characterized by the presence of a heterogeneous lipid-rich necrotic core, covered by a thin fibrous cap (in the order of 50–100 μm) that is weakened by inflammation (thin-cap fibroatheroma; TCFA). These lesions frequently feature microcalcifications, increased microvessel density, and outward remodeling of the vessel. Vulnerable plaques can trigger events spontaneously, but also lead to higher complication rates in interventions. Detection is important for pre-emptive treatment as well as stent placement. Every defining characteristic of plaque vulnerability (thin cap, microvascularization, inflammation, lipids, plaque volume) has been investigated as a marker for detecting vulnerable plaque, driving technology development in catheter-based tissue composition imaging. Plaque burden (the ratio of plaque area and area enclosed in the outer vessel wall) is another important indicator of plaque stability, with plaques occupying >70% of the vessel being predictive of events 1, 17.
Angioscopy was the first optical technique to be applied to plaque imaging [18], but is rarely used today. It provides a video endoscopy image of the (flushed) vessel, showing thin-cap lipid-rich plaque as yellow, and has very good sensitivity for thrombus. It was also the first technique to show a prognostic association of imaging with cardiovascular events in small studies [19].
IVOCT has adequate resolution and useful tissue type contrast [20] for visualizing plaque microstructures, resulting in a rich literature on IVOCT imaging of coronary atherosclerosis. Cap thickness can be measured, and the presence of IVOCT-derived TCFA is a predictor of peri-procedural MI (PMI) 21, 22, a condition that is associated with worse long-term outcomes. IVOCT-measured cap thickness is associated with the prevalence of plaque rupture[23]. IVOCT-derived lipid-rich plaque was recently shown to be predictive of adverse cardiac events arising from non-culprit sites [24]. The technique also shows features of inflammation [25] and frequently plaque microchannels [26], but the reliability of these findings is unknown and their evidence level is low.
IVUS initially demonstrated the shortcomings of coronary angiography, by showing atherosclerosis in the vessel wall in addition to luminal narrowing. Because of its large penetration depth, it is the only intravascular technique that can directly quantify plaque burden. IVUS-derived plaque volume has been applied as the primary outcome measure in pharmaceutical interventions 27, 28. Even though plaque volume changes are typically small, there appears to be a robust association with clinical events [29]. IVUS remains a highly useful technique for PCI guidance, but lacks image contrast and resolution for vulnerable plaque characterization. IVUS signal analysis techniques 30, 31 have attempted to add tissue type information to the grayscale image, most prominently in the form of an analysis called IVUS-VH (virtual histology). IVUS-VH has been used extensively in plaque and drug studies 1, 17, 32 but has not achieved widespread clinical use, which can be at least partially attributed to its limited validation [33].
The clinical breakthrough of IVUS for imaging of lipid-rich plaque came with the introduction of a combined IVUS/near-infrared reflection spectroscopy (NIRS) device [34]. This combination catheter was the first commercial multimodal intracoronary imaging device. NIRS was developed and validated for intracoronary sensing of plaque lipid 35, 36, aiming for vulnerable plaque detection [37]. In small scale studies, coronary lipid as detected by NIRS was indeed found to be predictive of event risk 38, 39, more frequently involved in acute coronary syndrome (ACS) [40] and also associated with PMI [41]. Large clinical trials to establish criteria for intervention guidance are ongoing [37].
New developments in catheter technology: multimodal imaging
Associations between imaging markers and subsequent events exist on a population level, but may not be strong enough to direct interventions in individual patients. In more than a decade of vulnerable plaque imaging, no single technology has demonstrated the ability to predict whether a particular plaque will trigger an MI in the months or years following the index procedure. Different technologies are sensitive to different plaque components, as illustrated in Figure 1. The natural response of the engineering field has been to combine complementary technologies in a single catheter [42]. Most new developments in experimental intravascular imaging concern bi- or even trimodal catheters. Most of these novel devices are currently at the stage of preclinical tests; except for the NIRS/IVUS catheter, marketed by Infraredx, none are commercially available.
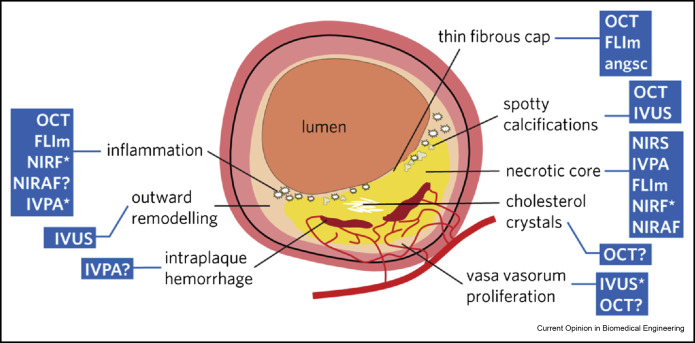
 Figure 1. (central illustration): The current model of a vulnerable plaque exhibits a range of features, all of which have been exploited to detect plaque vulnerability. The blue boxes list the different imaging modalities that can detect each feature: an asterisk means that exogenous agents have been used to achieve image contrast; a question mark indicates that sensitivity for this feature has been suggested but not robustly demonstrated. Note the long list of commercially unavailable technologies targeting the vulnerability hallmarks of inflammation and necrotic core. OCT: optical coherence tomography; IVUS: intravascular ultrasound; NIRS: near-infrared spectroscopy; FLIm: fluorescence lifetime imaging; NIR(A)F: near-infrared (auto-)fluorescence; IVPA: intravascular photoacoustics; angsc: angioscopy. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Figure 1. (central illustration): The current model of a vulnerable plaque exhibits a range of features, all of which have been exploited to detect plaque vulnerability. The blue boxes list the different imaging modalities that can detect each feature: an asterisk means that exogenous agents have been used to achieve image contrast; a question mark indicates that sensitivity for this feature has been suggested but not robustly demonstrated. Note the long list of commercially unavailable technologies targeting the vulnerability hallmarks of inflammation and necrotic core. OCT: optical coherence tomography; IVUS: intravascular ultrasound; NIRS: near-infrared spectroscopy; FLIm: fluorescence lifetime imaging; NIR(A)F: near-infrared (auto-)fluorescence; IVPA: intravascular photoacoustics; angsc: angioscopy. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)Optical and ultrasonic structural imaging
A combination catheter of the dominant structural imaging technologies offers a clear advantage over each technology on its own: IVOCT excels at high-resolution visualization of superficial structures, while IVUS provides an image of the entire vessel wall, including large plaques. This potential was recognized soon after the introduction of IVOCT in routine clinical practice, with the first prototypes appearing as early as 2010 [43]. Subsequent refinements of image quality and device engineering 44, 45, 46, 47 have resulted in experimental catheters that are suitable for in vivo imaging. Fusion (Figure 2) of the images naturally represents the vessel wall architecture in high resolution where available, and in lower resolution elsewhere.
 Figure 2. Integrated IVUS/OFDI catheter with co-aligned imaging beams, shown separately (OFDI: left; IVUS: middle) and fused (right). These images from autopsy human coronary artery specimens show how the resolution and contrast offered by OCT can support interpretation of images in terms of plaque characterization. OCT shows the plaque in the top row to be a fibrous plaque, while the bottom row displays an lipid-rich plaque, with a thick cap and inflammation at nine o'clock. IVUS affords assessment of the plaque burden, because the external elastic lamina is visible throughout. Unpublished image by Bouma et al., MGH.
Figure 2. Integrated IVUS/OFDI catheter with co-aligned imaging beams, shown separately (OFDI: left; IVUS: middle) and fused (right). These images from autopsy human coronary artery specimens show how the resolution and contrast offered by OCT can support interpretation of images in terms of plaque characterization. OCT shows the plaque in the top row to be a fibrous plaque, while the bottom row displays an lipid-rich plaque, with a thick cap and inflammation at nine o'clock. IVUS affords assessment of the plaque burden, because the external elastic lamina is visible throughout. Unpublished image by Bouma et al., MGH.IVUS and IVOCT are complementary in their resolution and imaging depth, and also in their image contrast mechanism: OCT relies on optical scattering, while IVUS derives its signal from ultrasound scattering. These wave–tissue interactions rely on different aspects of tissue microstructure, and hence assist in resolving ambiguities in image interpretation. One important confounder in IVOCT image interpretation is the distinction between calcified and fatty plaques, which both show as signal loss 48, 49. On IVUS, their appearance is highly distinct ••6, 45. Conversely, IVUS has poor contrast for different soft tissues (fibrous and fatty plaque), a difference that is much more easily seen on IVOCT ••7, 20.
A limitation of both IVUS and IVOCT is that they are structural imaging modalities: they rely on the scattered wave amplitude to create an image of the structure of the vessel wall. They do not carry direct information on tissue composition. This has to be inferred by image interpretation, which requires expertise and frequently gives rise to differing opinions. Direct identification of relevant tissue components facilitates this process. Polarization sensitive OCT 50, 51 is emerging as a way to visualize tissue organization with standard OCT catheters and a minimally modified imaging system. However, NIRS clearly shows the power of chemical sensing for detection of unstable atherosclerosis. We will now discuss a number of techniques based on fluorescence and absorption spectroscopy that attempt to add tissue type information that is not available with existing technologies.
Structural imaging with near-infrared fluorescence
Intravascular fluorescence imaging has been applied to add chemical tissue information to structural imaging. Near-infrared fluorescence (NIRF) was first explored with the use of indocyanine green (ICG), a clinically approved infrared dye with lipophilic properties which bind it to components of the necrotic core in atherosclerotic plaques, as well as local inflammation centers [52]. It can be systemically administered and is taken up into plaques in approximately an hour, following which it indicates the presence of lipid-rich plaque, compromised endothelium and neovascularization in excised human plaques [53]. In combination with OCT, ICG fluorescence allowed localization of these features in the structural image in a diabetic hypercholesterolemic pig model of coronary atherosclerosis [53]. These findings were compounded with ICG labeling of stent-induced inflammation in a healthy pig model [54], indicating that ICG adds reliable pathobiological information to structural images with a safe, non-targeted contrast dye. Targeted dyes would enable higher specificity in molecular tissue imaging, but have not been explored as intensively 55, 56.
Atherosclerotic plaques also exhibit near-infrared autofluorescence (NIRAF), and while the contrast mechanism remains unclear for now, studies demonstrate colocation of NIRAF, excited with 633 nm light, with pathological features on histology [57] and clinical OCT images [58]. The significance and usefulness of this signal remains to be investigated.
Combined OCT/fluorescence catheters have the advantage of relative simplicity: they are similar to conventional OCT catheters, with the exception of a double-clad fiber replacing the usual single-mode fiber. Tailoring the distal optics for optimal collection of fluorescence light may improve sensitivity, but this has not been extensively investigated. An IVUS/NIRF catheter has been pioneered and validated in rabbits, providing structural and ICG-based fluorescence imaging without clearing the blood from the artery. A triple modality IVUS/OCT/fluorescence catheter offers comprehensive IVUS/OCT structural imaging and chemical sensing, at the cost of increased catheter complexity [59].
Structural and chemical imaging with fluorescence lifetime
Fluorescence lifetime spectroscopy and imaging techniques can generate label-free optical molecular contrast that may be useful for detection of critical atherosclerotic plaque [60]. Plaques with thin fibrous cap [61], plaques rich in lipids 62, 63, and plaques with macrophage infiltration in the fibrotic cap [62]have been identified. When excited with UV light, several intrinsic constituents in normal and diseased arterial walls exhibit autofluorescence. The lifetime of the fluorescent state can discriminate biochemical components of atherosclerotic plaques 60, 64, 65, 66. This includes the structural proteins (elastin, collagen and crosslinks), lipid components in macrophages and lipid pools, and lipoproteins.
Intravascular application of these techniques has proven challenging, as they require practical fluorescence lifetime instrumentation and dedicated catheters. Recent advances in scanning point-spectroscopy time-resolved pulse-sampling techniques 67, 68, 69 have enabled development of a rotational fluorescence lifetime imaging (FLIm) system that can rapidly reconstruct a spectroscopic image of the vessel wall in a single pullback sequence 70, •71, 72.
Recent studies have explored the integration of FLIm with IVUS in a single rotational catheter to perform simultaneous assessment of coronary arteriesbiochemical features and morphology (Figure 3). Since UV light (i.e. 355 nm) has a limited penetration depth (estimated between 200 and 300 μm depending on tissue optical properties), FLIm does not allow interrogation of the entire wall thickness. However, this depth is sufficient in sampling the composition of the entire arterial intima and/or of the fibrotic cap. FLIm is sensitive to changes in collagen-lipid ratio within the intima, as well as to the presence of lipid pool underneath a thin fibrotic cap (<65–100 μm) which are hallmarks of plaques at risk of rupture. Consequently, FLIm can overcome IVUS limitations such as the inability to characterize the composition and inflammation state of fibrotic caps and to adequately discriminate between fibrous and lipid-rich plaques. A combination of FLIm and IVUS enables detection of biochemical features associated with vulnerable plaques, in addition to quantification of plaque burden, with the aim to improve characterization of complex pathologies.
 Figure 3. FLIm optical channel and IVUS transducer integrated into a single rotational catheter enable co-registered data acquisition (a). Ex vivo diseased human coronary sample mounted on custom holder for precise registration of imaging data with histology (b). Fibrous tissue and fibrous caps can be distinguished by slightly increased lifetimes in two fluorescence emissionchannels 390/40 nm and 450/45 nm fluorescence emission channels. Diffuse intimal thickening has shorter lifetimes than all other components in all wavelength ranges. Foam cell infiltration causes increased lifetimes at both 450/45 nm and 540/50 nm, as does necrosis (c). The dashed line indicates the location of the FLIm-IVUS cross-section (d) and CD68 image (e). This region includes a fibrous cap infiltrated with foam cells (purple arrowheads), fibrous cap (green arrowhead), and a thin fibrous cap overlying necrotic core (red arrowhead). The penetration depth of FLIm is approximately 250 μm, thus FLIm does not ‘see’ the deeper necrotic calcified core, but it is visible with IVUS (blue asterisk). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Figure 3. FLIm optical channel and IVUS transducer integrated into a single rotational catheter enable co-registered data acquisition (a). Ex vivo diseased human coronary sample mounted on custom holder for precise registration of imaging data with histology (b). Fibrous tissue and fibrous caps can be distinguished by slightly increased lifetimes in two fluorescence emissionchannels 390/40 nm and 450/45 nm fluorescence emission channels. Diffuse intimal thickening has shorter lifetimes than all other components in all wavelength ranges. Foam cell infiltration causes increased lifetimes at both 450/45 nm and 540/50 nm, as does necrosis (c). The dashed line indicates the location of the FLIm-IVUS cross-section (d) and CD68 image (e). This region includes a fibrous cap infiltrated with foam cells (purple arrowheads), fibrous cap (green arrowhead), and a thin fibrous cap overlying necrotic core (red arrowhead). The penetration depth of FLIm is approximately 250 μm, thus FLIm does not ‘see’ the deeper necrotic calcified core, but it is visible with IVUS (blue asterisk). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)Intravascular photoacoustic imaging
Photoacoustic imaging creates images of optical absorption by acquiring the ultrasonic signal emitted by optical absorption of short (nanosecond) laser pulses. The excitation wavelengths can be tuned to visualize specific tissue components only. The resolution of intravascular photoacoustic (IVPA) imaging is of the same order of magnitude as IVUS. Most studies to date have focused on visualizing atherosclerotic lipids 73, 74, 75, 76, a distinguishing characteristic of unstable plaque that offers endogenous photoacoustic image contrast deriving from vibrational overtone absorption in C H bonds in lipids [77]. Thus, IVPA targets the same plaque components as NIRS, but adds depth resolution, as illustrated in Figure 4. This means that IVPA allows a measurement of the thickness of the fibrous cap, where NIRS only flags the presence of lipid-rich plaque. The most commonly used wavelengths for lipid imaging are in the ranges around 1.2 μm 73, 74, 75, 76, 78, •79, 80 and 1.7 μm 81, 82, 83, 84, 85. The latter wavelength provided the first in vivo data in an atherosclerotic rabbit aorta [81], as well as real-time imaging in a live pig coronary artery [85]. It showed promise by a reduced interference of luminal blood [82], although flushing with (expensive) saline heavy water is preferred 84, 85, 86. Analysis of the response at a few wavelengths allows automatic discrimination between plaque and adipose lipids 78, 83; full spectroscopy provides the potential for plaque lipid subtyping [79]. Shorter wavelengths have also been proposed [87], but their validation remains tenuous.
H bonds in lipids [77]. Thus, IVPA targets the same plaque components as NIRS, but adds depth resolution, as illustrated in Figure 4. This means that IVPA allows a measurement of the thickness of the fibrous cap, where NIRS only flags the presence of lipid-rich plaque. The most commonly used wavelengths for lipid imaging are in the ranges around 1.2 μm 73, 74, 75, 76, 78, •79, 80 and 1.7 μm 81, 82, 83, 84, 85. The latter wavelength provided the first in vivo data in an atherosclerotic rabbit aorta [81], as well as real-time imaging in a live pig coronary artery [85]. It showed promise by a reduced interference of luminal blood [82], although flushing with (expensive) saline heavy water is preferred 84, 85, 86. Analysis of the response at a few wavelengths allows automatic discrimination between plaque and adipose lipids 78, 83; full spectroscopy provides the potential for plaque lipid subtyping [79]. Shorter wavelengths have also been proposed [87], but their validation remains tenuous.
 Figure 4. Intravascular photoacoustic imaging. (a) Principle of the technique; pulsed light (white arrow) gets absorbed by lipids (star) and emits an acoustic signal (blue wavefronts) that is registered by an ultrasound transducer on the catheter. (b) Merged IVPA/US image of the plaque at locations with large plaque volume (the front surface of the volume in (c)). (c) 3D reconstruction of pullback IVPA/US images acquired at 20 frames per second. (d) Histology at the imaging plane corresponding to (b). The dynamic range is 35 dB in IVUS image and 20 dB in the IVPA image [85]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Figure 4. Intravascular photoacoustic imaging. (a) Principle of the technique; pulsed light (white arrow) gets absorbed by lipids (star) and emits an acoustic signal (blue wavefronts) that is registered by an ultrasound transducer on the catheter. (b) Merged IVPA/US image of the plaque at locations with large plaque volume (the front surface of the volume in (c)). (c) 3D reconstruction of pullback IVPA/US images acquired at 20 frames per second. (d) Histology at the imaging plane corresponding to (b). The dynamic range is 35 dB in IVUS image and 20 dB in the IVPA image [85]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)IVPA imaging catheters, similar to the combined IVUS/fluorescence catheters discussed above, combine an IVUS transducer and an optical fiber for light delivery. There is the additional requirement of alignment of the optical and acoustic beams, as the ultrasonic transducer must receive the optically excited signal. It must also be able to withstand the optical power needed for real-time IVPA, which can be significant (100s of mW). Flexible catheters of sizes compatible with coronary imaging are now available 85, 88, including one that also receives light though the excitation fiber, adding NIRF to IVUS and IVPA [89].
Compared to IVUS signals, IVPA signals are weaker, and tend to contain lower frequencies [90]. Limits on the applied optical power mean that pulse energies cannot be indefinitely high in a real-time imaging scenario that requires pulse rates in the order of 10 kHz. C H bonds that provide lipid image contrast are also very common in the soft polymers that have acceptable ultrasonic and mechanical properties for catheter sheaths. The low tissue PA strength, sheath attenuation and PA generation by the catheter itself (which is detected as an echo) combine into a complex set of requirements on the design and materials used for IVPA devices, which has led to slow translation of the technology to in vivo imaging.
H bonds that provide lipid image contrast are also very common in the soft polymers that have acceptable ultrasonic and mechanical properties for catheter sheaths. The low tissue PA strength, sheath attenuation and PA generation by the catheter itself (which is detected as an echo) combine into a complex set of requirements on the design and materials used for IVPA devices, which has led to slow translation of the technology to in vivo imaging.
Challenges and outlook
Since its inception with the invention of IVUS [91], intravascular imaging has evolved to a field with many technological approaches that reveal many aspects of coronary atherosclerosis and catheter-based interventions. Table 1summarizes the capabilities of the different technologies discussed in this review. Most defining features of TCFA, the histopathological phenotype that is assumed to be the most likely candidate for vulnerable plaque can now be visualized. Multimodal imaging enables the assessment of a range of plaque structural and composition properties. IVUS/OCT provides comprehensive structural imaging, while absorption or fluorescence-based techniques add composition information. The baseline validation for these techniques is the qualitative similarity to histology: does the technology detect the target features?
Table 1. Applicability of different intravascular imaging technologies or combinations thereof. +++ good, ++ reasonable, + poor performance, – not possible (or not reported and unlikely).
| Imaging modalities | Stent features | Plaque features | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strut apposition | Edge dissection | Tissue protrusion | Strut coverage | Thrombus | Lipid content | Cap thickness | Plaque burden | Microvessel formation | Inflammation | Microcalcification | |
| OCT | +++ | +++ | +++ | ++ | ++ | ++ | ++ | + | + | ++ | ++ |
| IVUS | + | + | + | – | + | + | – | +++ | +a | – | ++ |
| IVUS-OCT | +++ | +++ | +++ | + | ++ | ++ | ++ | +++ | + | ++ | +++ |
| IVUS-NIRS | + | + | + | – | + | +++ | +b | +++ | – | – | ++ |
| IVUS-NIRF | + | + | + | +a | +a | +a | – | +++ | – | +++a | ++ |
| IVUS-FLIm | + | + | + | – | + | ++ | ++ | +++ | – | ++ | ++ |
| IVUS-IVPA | ++ | + | + | + | + | +++ | + | +++ | – | ++a | ++ |
| OCT-NIRF | +++ | +++ | +++ | ++ | +++a | ++ | ++ | + | + | +++a | ++ |
| OCT-NIRAF | +++ | +++ | +++ | ++ | ++ | ++ | ++ | + | + | ++ | ++ |
- a
-
With contrast agent.
- b
-
Non-standard analysis.
Image validation based on histology comes with two limitations for translation of technology beyond the preclinical phase. First, the observation of TCFA-like non-culprit lesions appears to have little predictive value in clinical usage, based on more than a decade of plaque imaging with IVUS and OCT. This may be a corollary of the truism that “the pathologist is always right, but s/he is always too late.” Second, representation of complex unstable atherosclerotic plaques in today's high information density imaging techniques leads to the interesting conundrum that providing more detail is helpful for experts, but confusing for general users. Image interpretation and quantification in OCT still is a topic of scientific discussion and study 48, 92, 93, 94.
Confounders in interpretation have driven the development of multimodal catheters. There is a large potential as well, relatively underexplored for intravascular imaging, for image interpretation supported by automatic analysis. For example, algorithms for OCT-based automatic cap thickness measurements 95, 96, tissue optical properties 97, 98, and plaque size [99] can support objective image reading. Quantitative parametrization can also assist in clinical validation, which is critical for large-scale acceptance of a technology. Imaging impact on treatment strategy depends on the validation of dichotomous criteria that should be accessible to the operator in real time: “is there a thin-cap lesion?”, “is there a large lipid core?”, with validated thresholds for what quantitatively constitutes a “thin” cap or a “large” plaque. The chemogram created by NIRS 35, 36 is such a powerful representation of coronary atherosclerosis because it allows the extraction of threshold values 38, 39, 41 of the lipid-core burden index (LCBI) that lend themselves to rigorous validation in a setting of PCI. Recent OCT analyses follow this analogy [100].
Plaque erosion, the presence of intracoronary thrombus without the presence of a ruptured plaque [101], is emerging as a frequent substrate of ACS 102, 103. It occurs at sites with impaired endothelium, rich in smooth muscle cells and proteoglycans [104]. For technologies that target inflammation or lipid-rich plaque, erosion is an important confounder in the prediction of ACS. It is impossible to identify erosion sites prospectively. Their pathological characteristics have negligible contrast on existing imaging tools, and present an enormous opportunity for innovation.
This review has sketched the rapid evolution and diversification of intravascular imaging technology in the past five years, building on a growing clinical evidence base for real-world utility. New technologies first prove their value as research tools, and if particularly successful may advance to general clinical adoption. This is not a rapid process: The validation of IVUS in therapy guidance is only reaching definitive answers after 25 years of clinical imaging ••9, 10. For OCT, the first data appeared after a decade [4] but randomized trials are still scarce [13]. NIRS/IVUS appears to reach this milestone faster, with the completion of currently ongoing trials expected in 2018. The global burden of coronary artery disease, and its complex pathophysiology that is changing under the influence of lipid-lowering medication, necessitates the continuing hunt for thrombogenic plaque.
Conflict of interest
Erasmus MC has a patent licensing agreement with Terumo Corporation. Dr. Van Soest has the right to receive royalties as part of this agreement. Prof. Bouma is an inventor on patents, assigned to Massachusetts General Hospital and licensed to Terumo Corporation, and also has sponsored research from Terumo Corporation. The other authors have no disclosures to make.
Acknowledgments
Laura Marcu acknowledges funding support from NIH Grant Number: R01 HL067377. Brett Bouma is supported in part by grants from the National Institutes of Health grants P41EB-015903 and R01HL-119065.