1. Introduction
Dental implants are surgically fixed substitutes for roots of lost or non-functional teeth. Restorative dental materials help to repair damage to teeth caused by gum disease, poor bone density or trauma. The restoration of teeth is very important because tooth damage, loss or dysfunction may cause speech disorders and may even lead to deterioration of the temporomandibular joint with severe pain [1]. Although bridgework and dentures address the cosmetic problem of missing teeth, they fail to restore proper chewing functions. Permanent implants, on the other hand, exert appropriate force on the jawbone and keep them functional and healthy [2]. Due to further developments in dental care technologies, a tremendous growth in dental implants and prosthetics market has been observed during the past few years. Hence, there is an extensive need of better quality dental materials, which not only helps to restore the functions of teeth but also causes less harm to the human body.
The choice of the dental implant materials, their manufacturing processes, biocompatibility and long-term stability in line with medical ethics and professional codes of practice are therefore crucial. Materials such as ceramics and metal alloys are extensively used for the fabrication of dental implants. Ceramics either make up the entire implants or can be applied in the form of a coating onto the metallic core. An ideal implant should be biocompatible, possess higher strength, fatigue and fracture toughness behavior and should be able to withstand the reactive environment it is exposed to inside the human body [4]. In addition, the stiffness/modulus of elasticity should be as close to that of bone to prevent stress-shielding effect. Stress-shielding effect arises when there is a mismatch between the strength of two materials [3]. Among the different metallic materials employed as implants, the use of stainless steel has been restricted as an internal fixation device because of its poor fatigue strength and high susceptibility to pitting and galvanic corrosion. Similarly, cobalt–based alloys suffer from poor fatigue strength and possess comparatively high modulus of elasticity than other implant materials used. In addition, the ion release from these materials has been found to have carcinogenic effect [4]. Thus, the above mentioned reasons make titanium and its alloys the ultimate choice as dental implants because in addition to exhibiting superior biocompatibility and high strength to weight ratio, Ti-alloys also exhibit low modulus of elasticity and enhanced mechanical properties such as high fatigue strength (140–1160 MPa) and fracture toughness. Various titanium dental implants are available commercially and are classified into groups based on their shape (cylindrical, conical, hybrid) and the type of connection to the prosthetic component such as Morse taper, internal hexagon, external hexagon and dodecagon [5]. Remarkably, their corrosion resistancestems from the high affinity of Ti towards oxygen which results in the formation of a thin and stable passive oxide layer that protects the bulk material from reactive species [6]. The oxide layer formed on Ti alloys typically consists of TiO2 but may coexist along with other titanium oxides such as TiO and Ti2O3 [7]. The thickness of the oxide film formed are < 10 nm and offer high resistance to a variety of chemical attack thereby making Ti-based implants highly corrosion resistant in the oral environment.
The main aim of this review is to focus on the degradation mechanism of titanium dental implants along with the various surface treatments utilized to improve the performance and service life of dental implants. Based on an extensive review performed, the future challenges in the field of implant dentistry are also identified and presented.
2. Materials and methods
The electronic search was performed using Medline (PubMed), Embase, Google scholar, book chapters and Prorequest dissertations and theses database. Grey literature such as reports were also used to analyze the current status and future scope of dental implant market. The MESH term used were: dental implants AND market, wear and dental implants, corrosion and oral environment, tribocorrosion and artificial saliva, toxicity and debris, surface modification and alloying, new dental alloys and corrosion resistance, anodization AND titanium plasma spray (TPS), nitriding, cryogenic treatment, coating AND titanium. Languages were limited to French and English. The time period of the literature search was between March – September 2016 and there were no exclusions made based on the year of published paper. Mendeley V.1.15 was used to manage the references. Important criteria's for inclusion of articles were: Fig. 1 shows the flowchart illustrating the inclusion and exclusion criteria and study flow for the systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
 Fig. 1. Inclusion and exclusion criteria and study flow for the systematic review.
Fig. 1. Inclusion and exclusion criteria and study flow for the systematic review.3. Discussion
3.1. Clinical aspects
Osseointegration is a term that refers to the formation of a direct functional and structural connection between living bone and an artificial implant and is governed by a variety of surface properties such as composition, roughness, wettability, surface energy, surface tension, orientation and texture [8]. In particular, the important factor that determines osteogenesis is the adsorption of specific proteins onto the metal surface. The adsorption of surface proteins should be such that the bioactive peptides which helps cell-binding is available for the incoming proteins. The initial process involving the complex interplay of various signaling molecules and inflammatory mediators which leads to osteoconduction and osteoinduction takes place a period of about 21 days [9]. It has been found that there exists a critical level of micromotion (50 and 150 μm) above which fibrous encapsulation prevails over osseointegration [10].
Widely accepted protocol for the successful placement of endosteal implants involves a traumatic surgical technique and healing period of at least 3 months with no-load to promote regeneration and prevent fibrous encapsulation. In contrast, another method which ensures quicker recovery is by adopting for immediate loading involving a single-stage treatment method where the crown and abutment are placed on the implant right after surgery. Advantages of this method are quicker recovery and less post-surgical care. However, the chances of implant failure are high due to overloading [8], [10], [11], [12], [13].
This paper aims to focus on the degradation mechanism of titanium dental implants along with the various surface treatments to improve the performance and service life of dental implants and the future challenges in the field of implant dentistry.
4. Degradation mechanisms
The reasons for implant failure can be categorized according to patient-related, biological and mechanical factors which are given in Fig. 2. Patient-related factors such as smoking, metabolic diseases (diabetes mellitus) and disorders (anorexia nervosa) increases the risk of implant failure when compared to healthy individuals. Other factors that lead to implant failure include infection/peri-implantitis, aseptic loosening, osteomyelitis and improper bone-bonding due to over-load. In addition, there are certain electrochemical and tribological factors such as corrosion and wear that accelerates the aforementioned reasons thereby, leading to implant-failure [14], [15], [16].
 Fig. 2. Factors affecting implant stability.
Fig. 2. Factors affecting implant stability.As described earlier, the presence of protective oxide layer keeps the current flow and the release of corrosion products at very low level. However, no metallic material is completely resistant to corrosion or ionization within living tissues. Mechanical disruption of the oxide layer leads to deterioration of metal surface by processes such corrosion and wear. Many types of electrochemical corrosion are possible in the oral environment because saliva contains aggressive anions such as chlorides which causes dissolution of the oxide layer and leads to release of metal ions into the surrounding tissues. The electrochemical behavior of Ti-based implants is dependent on various factors such as composition, concentration of anions, pH, buffering capacity and surface-related properties of the implant (Fig. 3) [17].
 Fig. 3. Factors influencing the failure of dental implant.
Fig. 3. Factors influencing the failure of dental implant.Wear, another degradation mechanism, refers to the deformation of the surface of materials as a result of mechanical interaction between two opposite surfaces [18]. The wear resistance of artificial dental materials is essential for long-term stability of the implant. Generally, wear resistance is dependent upon the hardness, roughness, fracture toughness and the Young's modulus of the interacting materials. As wear measurements in vivo is highly complex and time-consuming, wear analysis is usually performed in simulators in the presence of artificial saliva.
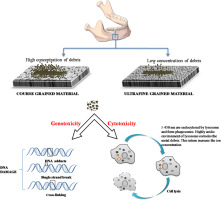
Similarly, fretting corrosion is another degradation mechanism which refers to the small oscillating movements between two interacting materials (bone-implant, plates-screws) in presence of corrosive oral environment. Simultaneous action of electrochemical and mechanical interaction occurring on materials subjected to relative movement, i.e. wear and corrosion occurring simultaneously is referred to as tribocorrosion. Above mentioned degradation mechanism (wear, corrosion, tribocorrosion) causes the release of metal ions/debris into the peri-implant region. As illustrated in Fig. 4, metal debris activates a cascade of signaling molecules that result in the activation and differentiation of osteoclast cells and leads to bone resorption/osteolysis of the peri-implant region. As a result, the bonding between the bone and implant is lost and results in implant loosening. Occurrence of implant loosening in the absence of bacterial infection is referred to as aseptic loosening. Smaller sized wear debris combine with biomolecules and elicit Type IV immunogenic response and causes ezhema, prolongs bone healing and is also accompanied with pain. Also, it has been found that the titanium implanted region turns black. This is because the surface of titanium undergoes repassivation when the oxide layer is disrupted. In certain conditions, the repassivation process forms so much TiO2 oxide layer that the region turns blacker. This process is referred to as metallosis and has been considered to be harmful [19]. It is therefore important to study the dental implants in-vitro under physiological condition to understand their behavior in the oral environment and develop strategies to combat the issues that lead to implant failure.
 Fig. 4. a) Release of wear debris from the dental implant leads to the activation of immune cells. Macrophages are one of the first cells to act 48 h after exposure to debris. Debris causes both cytotoxity and genotoxity. Macrophage takes up these debris via phagocytosis and secretes cytokines and chemokines, which enables to further activate the differentiation of osteoclast from the precursor cells. b) Upon exposure to macrophage colony-stimulating factor (MCSF) and RANKL synthesized by the T cells and synovial fibroblasts, osteoclasts fuse to polykaryons termed preosteoclasts, which then undergo further differentiation into mature osteoclasts leading to bone- resorption and osteolysis.
Fig. 4. a) Release of wear debris from the dental implant leads to the activation of immune cells. Macrophages are one of the first cells to act 48 h after exposure to debris. Debris causes both cytotoxity and genotoxity. Macrophage takes up these debris via phagocytosis and secretes cytokines and chemokines, which enables to further activate the differentiation of osteoclast from the precursor cells. b) Upon exposure to macrophage colony-stimulating factor (MCSF) and RANKL synthesized by the T cells and synovial fibroblasts, osteoclasts fuse to polykaryons termed preosteoclasts, which then undergo further differentiation into mature osteoclasts leading to bone- resorption and osteolysis.4.1. Corrosion of titanium and titanium alloy dental implants
The rate of a corrosion process depends on (i) oxide layer formed; (ii) pH; (iii) Concentration and composition of the electrolyte; and (iv) transport of oxygen vacancy across the film [20]. Different types of corrosion the materials are prone to, are represented in Fig. 5.
 Fig. 5. Types of corrosion taking place in an implant.
Fig. 5. Types of corrosion taking place in an implant.The TiO2/Ti has an O to Ti concentration that varies gradually from TiO2 film to a much lower ratio in the bulk [21]. Pan et al. [22] suggested that duplex layers formed on the surface of Ti alloys comprise of an inner barrier layer and an outer porous and less stable layer.
Fluorides are usually present in toothpastes and mouthwashes to prevent the development of dental caries. However, exposure of higher concentration of fluorides exerts detrimental effect on the implant as the oxide films of dental implants react with fluoride solutions and forms titanium-fluoride molecules such as titanium fluoride or sodium titanium fluoride. The lattice parameters of these compounds induce many structural defects in the oxide layer and hence, the bulk material becomes more prone to electrochemical attack [23].
In order to determine the concentration of fluorides that causes the disruption of the protective oxide layer of dental implants various studies have been carried out with varied concentration of fluoride ions. Alves-Rezende et al. investigated the electrochemical behavior of commercially-pure Ti and Ti–10% Molybdenum. The electrolyte consisted of 0.12% chlorhexidinedigluconate, 0.03% triclosan along with 0.05% sodium fluoride and 0.5 g/L cetylpyridinium chloride with 0.05% sodium fluoride. However, these concentrations exhibited negligible effect on the stability of the oxide layer [24].
D. Mareci et al. monitored the electrochemical behavior of surface modified Zr5Ti, Zr25Ti, Zr45Ti in artificial saliva containing sodium fluoride concentration of about 0.2, 0.5 and 1 wt% simulating the fluoride concentrations in dental rinses and showed that thermal oxidation of ZrTi alloys in air at 500C for 2 h drastically minimized the effect of fluorides on the corrosion behavior of ZrTi alloys due to the formation of a stable passive oxide layers of TiO2 and ZrO2. The outermost layer was found to be enriched with TiO2which reduced the dissolution of oxide film induced by the presence of fluoride ions [25].
Analogously to the fluoride effect, there are also other compounds that affect the corrosion process of titanium alloys. L P Faverani et al. reported the corrosion kinetics of Ti-6Al-4V in different mouthwash solutions consisting of 0.053% cetylpyridinium chloride and 3% hydrogen peroxide, with only artificial saliva as control. It was found that while 3% H2O2 had adverse effect, under other conditions Ti-6Al-4V was stable and showed low susceptibility to corrosion [22].
Overall, these reports substantiate the role of oxide layer in providing corrosion resistance in most adverse oral environments which consists of varying pH, acid attack and the presence of chemical compound such as cetylpyridinium chloride, sodium fluoride and hydrogen peroxide.
4.2. Wear of titanium and titanium alloys dental implants
The fretting behavior of cortical bone at the bone-implant interface was investigated using commercial titanium grade 2 and TC4 alloy against cortical bone. The wear depth and coefficient of friction were found to be higher in case of bone-TC4 than bone-commercially pure titanium. Abrasive wear and delamination were characterized as the wear mechanism of the cortical bone [26]. Hence, it is of utmost importance to develop highly wear resistant material or employ surface modification of titanium alloys to minimize its potential against natural cortical bone at bone-implant interface. Cast Ti-6Al-7Nb was found to exhibit better wear resistance than CP-Ti (grade 2 and 3) because of higher hardness and the presence of two-phase microstructure consisting of acicular ά phase in prior β grain [5]. Similarly, C. Ohkubo conducted in-vitrostudy to evaluate the wear behavior of cast CP Ti titanium along with 3 and 5 wt% copper and Ti-6Al-4V with 1 and 5 wt% copper. The unmodified Ti and Ti6-Al-4V alloys were used as control. Compared to other concentrations of Cu, 4 wt% Cu was found to exhibit highest wear by introducing α-Ti/Ti2Cu eutectoid which helped to improve resistance to plastic deformation and enhance wear resistance [27].
In addition to usage of alloying elements to improve the wear characteristics, coatings could also be performed on the implant surface. Poly-ether-ether-ketone (PEEK) was coated on Ti-6Al-4V due to its higher mechanical propertiesand wear resistance. The samples were synthesized by hot-pressing the PEEK veneer onto Ti-6Al-4V cylinders. Wear tests were carried out at 30 N load, frequency of 1 Hz with stroke length of 3 mm in artificial saliva using Al2O3 as the counter body. As expected, the coefficient of friction and specific wear rate was less than the uncoated Ti-6Al-4V sample. Hence, this shows that PEEK/TI-6Al-4V hybrid could be used to support masticatory loads, improve biocompatibility and wear resistance [28].
Similarly, the effect of three different surface treatments such as plasma nitriding, TiAlN thin film deposition by closed field unbalanced magnetron sputtering (CFUBMS) and Al2O3 coating using plasma spray method on improving the wear resistance of Ti-6Al-4V was evaluated by F. Yildiz et al. Wear tests were performed using pin-on-disc tribotester in physiological solution at 37 °C. Although, all surface treatments improved the wear resistance of Ti-6Al-4V alloy, Al2O3 coating exhibited highest wear resistance while lowest was obtained in case of nitrided samples [29].
4.3. Tribocorrosion of titanium and titanium alloys
Tribocorrosion, which occurs under the dual action of wear and corrosion, may occur under a variety of conditions such as i.e. sliding, fretting, rolling, impingement in a corrosive medium. The schematic representation of tribocorrosion mechanism is given in Fig. 6.
 Fig. 6. Schematic representation of tribocorrosion mechanism.
Fig. 6. Schematic representation of tribocorrosion mechanism.4.3.1. Tribocorrosion under sliding conditions
Tribocorrosion studies of titanium and its alloys under sliding conditions with and without surface treatment are shown in Table 1, Table 3 respectively. Similarly, Table 2, Table 4 enlists the tribocorrosion study of untreated and surface modified titanium alloys under fretting condition. In general, the tribocorrosion works are focused on the evaluation of surface treatments, which improve the wear and corrosion performance of bulk materials (i.e. CP-Ti and its alloys) and on the analysis of environmental variables (pH, fluoride content in the artificial saliva, temperature) on the tribocorrosion behavior of dental implants [30], [31], [32], [33], [34], [35].
Table 1. Tribocorrosion studies under sliding conditions of Ti alloys for dental implants.
| Ref. |
Titanium alloy (surface treatment) |
Counterpart (diameter) [mm] | Motion Contact geometry | Solutiona |
Load [N] (Avg press) [MPa] |
Avg speed [mm/s] (duration) [s] |
Electrochem. control | Friction range | Wear coefficient [mm3/Nm] |
|---|---|---|---|---|---|---|---|---|---|
| [36] | Ti6Al4V | Al2O3 ball (10) |
Reciprocating Ball on plate |
NaCl | 1 (264) | 6 (1800) | OCP | 0.4 |
2.0 · 10− 4 5.0 · 10− 4 |
| [37] | Ti6Al4V | Al2O3 ball (6) |
Unidirectional Ball on plate |
AS, AS + fluorides | 5 (960) | 19 (3600) | OCP, applied potential |
2.5 · 10− 4 5.8 · 10− 4 |
|
| [38] | Ti6Al4V | Al2O3 ball (6) |
Unidirectional Ball on plate |
AS, AS + pHs | 5 (960) | 19 (3600) | OCP, applied potential |
1.3 · 10− 4 1.7 · 10− 6 |
|
| [39] | Ti-G2, Ti-G5, Ti6Al4V, Ti6Al4V ELI | Al2O3 ball (6) |
Unidirectional Ball on plate |
PBS, PBS + BSA | 5 (960) | 19 (3600) | Applied potential |
2.9 · 10− 4 7.3 · 10− 4 |
|
| [40] | Ti29Nb13Ta4.6Zr | UHMWPE (12) |
Reciprocating Ball on plate |
HBSS | 6.5 (23) | 10 (3600) | OCP, Applied potential | 0.15–0.3 |
6.8 · 10− 7 1.7 · 10− 6 |
| [41] | Cp Ti, Ti6Al4V | Al2O3 ball (28) | Ball on disc | AS, AS + lipopolysaccharide | 20 (372) | (2400) | OCP, Applied potential | 0.4 | |
| [46] | Cp Ti | Al2O3 ball (28) | Ball on disc | AS, AS + pHs | 20 (372) | (2400) | Applied potential | ||
| [45] | Cp Ti | Al2O3 ball (10) |
Reciprocating Ball on plate |
MF, MF + fluorides | 3 (370) | 4 (1200) | OCP | 0.35–0.5 | |
| [89] | Ti6Al4V ELI, Ti13Nb13Zr | 30CrNiMo8 (35) | Block on disc | SBF | 40 | 260, 500, 1000 (600) | |||
| [35] | Ti35Nb7.2Zr5.7Ta, Ti35Nb7.2Zr5.7Ta0.5b | Chromium steel | Pin on disc | Hank's, BS | 50 (1) | 1000 (2) | 0.4–0.65 | ||
| [42] | Ti12.5Mo, Ti13Nb13Zr, Ti29Nb13Ta4.6Zr | UHMWPE ball (12) |
Reciprocating Ball on plate |
HBSS + HA, HBSS + BSA, HBSS + DPPC | 6.5 (34–35) | 10 (3600) | OCP, applied potential | 0.15–0.39 |
Ball 2.1 · 10− 7 7.7 · 10− 6 |
| [48] | Ti13Nb13Zr0.5B | Chromium steel | Pin on disc | Hank's, BS | 50 (1) | 1000 (2) | 0.4–0.5 | ||
| [90] | Ti13Nb13Zr | Chromium steel | Pin on disc | Hank's, BS | 50 (1) | 1000 (2) | 0.22–0.5 | ||
| [43] | Ti6Al4V | Al2O3 ball (2) |
Reciprocating Ball on plate |
SBF | 0.098 (356) | 0.1, 1, 10, 20 (3600) | OCP, applied potential, potential sweep |
- a
-
AS: artificial saliva, BS: bovine serum, BSA: bovine serum albumin, DPPC: dipalmitoylphospatidylcholine, HBSS: Hank's balance salt solution, MF: modified Fusayama, PBS: phosphate buffered solution, SBF: simulated body fluid.
Table 2. Tribocorrosion studies under fretting conditions of Ti alloys for dental implants.
| Ref. |
Titanium alloy (surface treatment) |
Counterpart (diameter) [mm] |
Motion Contact geometry (frequency, amplitude) [Hz, m] |
Solutiona |
Load [N] (Avg press) [MPa] |
Avg speed [mm/s] (duration) [s] |
Electroch. Control | Friction range | Wear coefficient [mm3/Nm] |
|---|---|---|---|---|---|---|---|---|---|
| [40] | Ti29Nb13Ta4.6Zr | Al2O3 ball (10) |
Reciprocating Ball on plate (1, 100) |
HBSS | 10 (400) | 0.2 (1800) | OCP, applied potential | 0.2–0.6 |
5.9 · 10− 5 1.4 · 10− 4 |
| [91] | Ti6Al4V | Ti6Al4V, CoCrMo cone-shaped flat bottom pin (0.35–0.8) |
Reciprocating Pin on plate (1.25, 50) |
PBS | 0.5–50 | 0.125 (125) | Applied | 0.3–0.7 | |
| [92] | Ti6Al4Fe, Ti12.5Mo, Ti13Nb13Zr, Ti29Nb13Ta4.6Zr | Al2O3 ball (10) |
Reciprocating Ball on plate (1, 100) |
HBSS, HBSS + BSA, HBSS + HA, HBSS + DPPC | 10 (400) | 0.2 (3600) | OCP, applied potential | 0.4–1 |
1.7 · 10− 4 4.9 · 10− 3 |
| [93] | Ti6Al4V | Al2O3 ball (8) |
Reciprocating Ball on plate (5, 180) |
AS, AS + fluorides | 3 (440) | 1.8 (3600) | OCP |
1.0 · 10− 8 1.5 · 10− 8 |
|
| [94] | Cp Ti | Al2O3 ball (8) |
Reciprocating Ball on plate (5–10, 180) |
HBSS | 3, 5, 7, 10 (500–1200) | 1.8 (150–7200) | OCP | ||
| [95] | Ti6Al4V | Si3N4 ball (4) |
Reciprocating Ball on plate (40, 100) |
BS | 49.6 (2680) | 8 (90000) | – | 0.05–0.08 |
8.4 · 10− 10 1.6 · 10− 9 |
| [50] | Ti6Al4V | Al2O3 ball (10) |
Reciprocating Ball on plate (1, 100) |
PBS, PBS + collagen, PBS + BSA, HEPES, HEPES + BSA | 10–30 (607–876) | 0.2 (3600) | Applied potential | 0.5–0.8 | |
| [54] | Ti G2 | Al2O3 ball (10) |
Reciprocating Ball on plate (1, 200) |
AS, AS + citric acid, AS + cathodic inhibitor, AS + anodic inhibitor, AS + organic inhibitor | 2 (333) | 0.4 (5000, 10,000) | OCP, applied potential | 0.7–0.9 |
1.0 · 10− 5 1.5 · 10− 5 |
- a
-
AS: artificial saliva, BS: bovine serum, BSA: bovine serum albumin, DPPC: dipalmitoylphospatidylcholine, HBSS: Hank's balance salt solution, MF: modified Fusayama, PBS: phosphate buffered solution, SBF: simulated body fluid.
Table 3. Tribocorrosion studies of surface treated Ti alloys under fretting condition.
| Ref. |
Titanium alloy (surface treatment) |
Counterpart (diameter) [mm] |
Motion Contact geometry (frequency, amplitude) [Hz, m] |
Solutiona |
Load [N] (Avg press) [MPa] |
Avg speed [mm/s] (duration) [s] |
Electroch. control |
|---|---|---|---|---|---|---|---|
| [96] | Ti6Al7Nb (N implantation) | Zr2O (25.2) |
Torsional Ball on plate |
20% BS | 100 | – | – |
| [51] | Cp Ti (anodized) | Al2O3 ball (8) |
Reciprocating Ball on plate (5, 180) |
Ringers | 3 (500) | 1.8 (3600) | OCP |
- a
-
AS: artificial saliva, BS: bovine serum, BSA: bovine serum albumin, DPPC: dipalmitoylphospatidylcholine, HBSS: Hank's balance salt solution, HEPES: 2-[4-(2-hydroxyethyl)-1-piperazinyl]-ethanesulfonic acid, PBS: phosphate buffered solution.
Table 4. Efficiency of the common surface treatments for the Bone-to-Implant Contact (BIC) [97], [98].
| Surfaces | % BIC |
|---|---|
| Electro-polished | 20 to 25 |
| Acid-etched (unmeasured roughness) | 20 to 25 |
| Sandblasted (with low roughness Sa < 1 μm) | 20 to 25 |
| Sandblasted (with high roughness Sa: 1 to 3 μm) | 30 to 40 |
| SLA | 50 to 60 |
| TPS | 30 to 40 |
Tribocorrosion studies are carried out under different mechanical conditions due to the flexibility given by the experimental set-ups, tribometers in lubricated conditions. The used average speed is between 0.1 and up to 1000 mm/s, while average contact pressure is maintained between 23 MPa and 3.15 GPa. Vast varieties of electrolytes are used, from very simple solutions (sodium chloride) to more complex formulations. Although independent of the pH, tribocorrosion damage is mainly due to the mechanical action in the simulated body fluids. Addition of fluorides to acidified artificial saliva accelerates the corrosion process and increase ion release into the surrounding. Hence, pH of the electrolyte is not the only criteria determining the tribocorrosive behavior of dental implants [27].
Among the 23 papers testing titanium alloys under sliding conditions, 13 were carried out under electrochemical control of the system by monitoring the OCP (open-circuit potential) or by imposing an applied passive potential [36], [37], [38], [39], [40], [41], [42], [43]. Under OCP conditions, a potential dissolution of oxide layer is observed when rubbing starts due to the galvanic coupling formed between the worn area/anode and the unworn zone/cathode, which has been described by Vieira et al. [44]. As discussed earlier, the extent of the dissolution of the protective oxide layer depends on the surface treatment of the tested material, electrolyte and the contact pressure, load and frequency [32], [35], [45].
4.3.2. Tribocorrosion under fretting conditions
In dental implants, fretting can occur at the interface between the implant and alveolar bone during occlusal movements. In the fretting-corrosion studies the average sliding speed used are in a narrow range, between 0.2 and 1.8 mm/s, while the average contact pressures vary from very low values (333 MPa), to very high ones (12 GPa). The selected reciprocating amplitudes range between 50 and 180 μm at a typical frequency of 1 Hz, but usually lies between 1 and 40 Hz [36], [37], [38], [39], [40], [41], [42], [46], [47], [48].
Diomidis et al. demonstrated that Ti–29Nb–13Ta–4.6Zr has the ability to repassivate both under sliding and fretting corrosion even after depassivation. The influence of proteins and/or other inorganic compounds on the passivation behavior during fretting-corrosion has also been studied widely [49]. Viera et al. described an increase in the wear volume and corrosion rate in the presence of cathodic or organic inhibitor. Hiromoto and Mischler [50] also could not find any effect of bovine serum albumin on either on the wear rate or the wear accelerated corrosion of the Ti6Al4V alloy. Similarly, Diomidis et al. [49]observed that the presence of synovial constituents lubricated the contact making slip easier but could not find any appreciable effect on the electrochemical response of beta-titanium alloys.