1. Introduction
From 2004–2005, orthopedic trauma (fractures) accounted for 72% of musculoskeletal injury charges and it was the cause of almost one-half of all the disease or injury-related hospitalizations in the United States [1], [2], similar statistics were reported in 2012 with more than $59.5 million in total hospital charges in 2011 [3], [4]. For these reported charges, intervention is necessary to reconstruct a damaged skeleton and an effective fixation hardware is needed to support surgically set bones during the healing period. Internal fixation hardware (e.g., plates, screws, nails and wires) is placed over or within bones in order to hold opposing segments of fractured bone still during the healing period, without any deformation at the fracture site [5], [6]. In addition to trauma, internal fixation hardware, with or without bone grafts, is essential for skeletal reconstructive surgery [7]. While beneficial, in general, it is not practical to remove fixation hardware after the reconstructed bone has healed. However, the high stiffness of standard of care fixation hardware, relative to the stiffness of the host bone, may subsequently result in detrimental bone stress shielding and/or hardware stress concentrations [8], [9]. For children, teenagers and athletes, it is recommended to remove the hardware to avoid future bone fractures caused by unnatural loading patterns [10]. In addition, the fixation hardware may cause irritation in the adjacent soft tissue. Fixation hardware made of a biodegradable material that also offers the required stability during the healing period and subsequently degrades would mitigate stress shielding of the surrounding bone while avoiding any potential complications associated with a second fixation removal surgery [11].
Mg alloys are the most promising biodegradable materials for orthopedic internal fixation hardware [12], [13]. The Mg alloys of interest have a low specific density (1.74–2.0) and modulus of elasticity (41–45 GPa) closer to bone (5–23 GPa for cortical bone) [14], [15]. Currently used metallic implant materials have a high modulus of elasticity (e.g., 116 GPa for titanium Ti-6Al-4V) [14], [16]. The low modulus of elasticity of Mg alloys reduce the possibility of stress shielding associated with the use of stiffer metallic fixation hardware [17], [18], [19]. As a biocompatible material, Mg wires were used as a ligature for bleeding vessels > 100 years ago [20]. As metallic fixation hardware, an Mg-based skeletal fixation plate was first used by Lambotte [21] in 1907. That work was followed by several investigations of Mg and Mg alloy bone implants. These devices studies showed promising properties in stimulating bone ingrowth and healing. Mg alloys, however, were abandoned for decades due to their undesirable degradation rate and byproducts. The fast degradation rate of pure Mg in a physiological environment results in rapid loss of mechanical integrity and genesis of hydrogen gas [22], [23]. The premature loss of mechanical integrity diminishes the fixation's function. The release of hydrogen may also be detrimental to the healing process [24]. Moreover, the strength of pure Mg and the earliest studied alloys was not high enough and much lower when compared to other biocompatible metals such as stainless steel [16], [22].
During the last decade, the development of Mg alloys useful for resorbable skeletal fixation has received greater attention as new approaches to providing sufficient mechanical strength and useful corrosion (resorption) rates [25], [26]. Post-fabrication treatments of these alloys, such as coatings and mechanical treatments, have also been studied [27], [28]. Commercially available Mg alloys (e.g., WE43, AZ91 and AZ31) despite their higher mechanical strength and enhanced corrosion resistance, are generally not considered suitable for biomedical applications due to concerns regarding their biocompatibility [29], [30], [31], [32]. In order to achieve better biocompatibility and slower degradation, alloying with elements such as Al, Zn, Zr, Sr, Mn, Ca, and Rare earth (RE) elements (i.e. Gd, Y, La, Ce, Nd, Pr) has been studied [33], [34], [35], [36], [37], [38], [39], [40]. Among these alloys, Mg-Zn-Ca alloys have received the greatest interest because of their excellent biocompatibility, and the possibility to tailor the mechanical and corrosion properties by changing the Zn/Ca ratio and/or heat treatments [15], [39], [41], [42].
To achieve practical Mg-based implants, it is possible to apply a protective coating to prevent the biodegradation process until a desired time point. Coating resorption rate and byproduct safety are topics of recent investigations [12], [27].
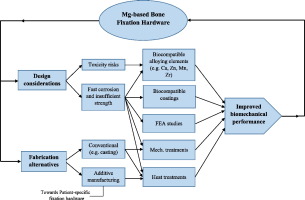
It would be a significant breakthrough if the needed fixation hardware properties and geometry can be tailored/designed to be patient-specific [1], [43]. Additive manufacturing (AM) or 3D printing of metals has received significant attention as a fabrication technique to produce highly accurate and complex-shaped structures such as patient-specific fixation hardware [44]. A number of tools can be considered for the improvement of resorbable implants' rendering. Finite element analysis (FEA) has been used in several studies to simulate and evaluate the performance of permanent fixation hardware [45], [46], [47], [48]. In Section 3.2, we will discuss different kinds of Mg coating. Also, more in-depth discussion of additive manufacturing of Mg alloys will be the subject of Section 4.5. The primary objective of this paper is to (1) present a crisp review of Mg-based alloys' design considerations for bone fixation applications based on the in vitro and in vivo performances, (2) discuss the emerging trends in the field of Mg fabrication, forming and post-fabrication treatments (e.g. coating and heat treatment) that can help to develop a Mg-based fixation hardware with enhanced biomechanical performance, (3) highlight the current challenges and strategies towards achieving Mg-based, resorbable, skeletal fixation devices.
2. Mg as a resorbable material
Bone implants have historically been made of metallic alloys such as stainless steel (316L SS), surgical grade titanium (Ti-6Al-4V) and CrCoMo due to their high strength, durability and biocompatibility [5]. The modulus of elasticity of these alloys (e.g., 116 GPa for Ti-6Al-4V [16]) is significantly higher than that for bones (5–23 GPa [12]). This mismatch in stiffness leads to the phenomenon known as stress shielding. The higher stiffness of the fixation device compared to bone causes the mechanical load to transfer away from the adjacent bone [49], [50]. The absence of mechanical loading leads to a reduction in the shielded bone mass and density and subsequently a loss of bone [8]. Also, in the areas of stress concentration where the stiffer fixation develops high stresses on the bone such as around screws, a bone fracture and subsequent screws pull-out is more likely. In addition to stress shielding, leaving metallic-based fixation inside the body after the healing period, causes other problems such as inflammatory local reactions, possible infection and the inability to adapt to bone growth near the fixation site [11], [51]. A new approach to address these issues is based on the use of biodegradable fixation. These materials should provide fixation only during the healing period and thereafter allowing the re-establishment a normal stress pattern [12]. Polymers such as polyglycolic acid (PGA), poly-l-Lactic acid (PLLA) and polycaprolactone (PCL) have been developed as biodegradable implants [38], [52], [53], [54], [55]. Biodegradable polymers have shown excellent biocompatibility and bioresorption properties, but their lower mechanical strength results in larger dimensions, which limits their use [53], [56], [57], [58].
Mg is a resorbable material in the body. This means that the corrosion byproducts are excreted or integrated through natural metabolic processes [59]. Mg-based orthopedic fixation hardware as a biodegradable material can overcome the limitation of polymers [14]. Due to Mg alloys advantages over other metallic and polymeric-based bone implants and its resorbable nature [60], there is a driving interest in the biomaterial community to develop Mg-based bone implants [61], [62], [63]. “Velox CD™” is a CE-approved bioresorbable Mg-based vascular closure device that was developed by Transluminal technologies [64]. Other bioresorbable Mg-based devices are currently in the final investigation stages before being available in the market for different biomedical applications such as ureteral stents [65], coronary scaffold (Biotronik Dream) [66], [67], and bone fixation hardware [68]. To date, the only commercially available Mg-based bone implant is the “MAGNEZIX™” screw fabricated by Syntellix [69], [70]. This implant is made of uncoated Mg-Y-RE-Zr alloy and it is approved for use in Europe for fixing small bones and bone fragments [71].
In comparison with the clinically in-use titanium-based and polymer-based fixation hardware, bone implant interface strength and osseointegration of Mg alloys have been evaluated through micro-focus computed tomography, push out force and extraction torque tests [72], [73]. Mg implants have shown increased bone-implant contact, higher implant stability and better osseointegration when compared with polymer-based and titanium-based implants especially at long implantation periods [72], [73]. Standard geometries of the currently available fixation hardware should be considered during in vivo evaluations since the difference in geometry may affect the load pattern following implantation. Two such studies of Mg-based fixation hardware were conducted by Chaya et al. [74], [75]. They implanted plates (20 × 4.5 × 1–1.5 mm) and screws (7 mm length and 1.75 mm shaft outer diameter) made of 99.9% pure Mg in a New Zealand White rabbit ulna fracture model, as seen in Fig. 1. Fracture healing and new bone formation were observed around the Mg-based fixations after 8 weeks. The hardware degraded at a rate of 0.4 ± 0.04 mm/year. After 16 weeks of implantation, bend test results showed similar flexural loads for the Mg-fixed and the healthy ulnae. An in vivo study in the rabbit conducted by Diekmann et al. [76] investigated the use of biodegradable Mg-Y-RE-Zr alloy “MAGNEZIX” interference screws for the reconstruction of the anterior cruciate ligament. Mg-based screws were implanted in the left tibiae of each of 18 rabbits for 4, 12 and 24 weeks. Maximum gas volume of 330.5 ± 83 mm3 was detected in the medullar cavity by the 4th week. The Mg-based screws showed an average degradation rate of 0.17 mm/year over the 24 weeks of implantation. Neither inflammatory reactions nor necrosis of the tendon were observed by histological evaluation.
 Fig. 1. Mg fixation plate and screws. Digital image showing devices prior to implantation (A). Schematic showing device placement with fractured ulna (B) (adapted from [75]).
Fig. 1. Mg fixation plate and screws. Digital image showing devices prior to implantation (A). Schematic showing device placement with fractured ulna (B) (adapted from [75]).2.1. FEA studies towards Mg-based fixation hardware
Finite element analysis can be used to calculate the state of stress and strain distribution through repaired bone and its fixation hardware. The model should include material and morphological properties of the implant, the cortical bone and the cancellous bone. Such analysis, in addition to predicting the safety of the implant, fatigue and fracture can be used to assess the possibility of bone fracture or resorption as the result of the implant [77]. This modeling approach is also used for design modification and optimization. It is desirable that the maximum Von Mises (VM) stress of cortical bone is lower than its maximum yield strength (108 MPa) for sufficient fixation hardware biomechanical stability [78], [79]. Mg-based fixation hardware should not mechanically fail while transferring compressive stress to the healing bone and should limit the interface displacement to < 300 μm. To date, one study has been conducted by Lee et al. [79] to compare the Mg-based resorbable screw system in a bilateral sagittal split ramus osteotomy (BSSO) with titanium-based and polymer (Inion CPS) IN-based systems. An occlusal load of 132 N was applied to the model on the lower first molar and different fixation geometries (number of screws) were studied. Generally, Mg-based screws had a similar pattern of the VM stress distribution compared to that of titanium-based screws more than to that of IN-based screws. For example, the fixation with 5 Mg-based screws showed better biomedical stability (i.e., Max. VM stress of 99.81 MPa in the cortical bone and 25.38 MPa in the cancellous bone) than the fixation with 5 IN-based screws (i.e., Max. VM stress of 109.02 MPa at cortical bone and 54.72 MPa at cancellous bone) for the advancement surgery. Also for setback surgery, the maximum VM stresses developed on the cancellous bone at all fixation geometries were lowest using Mg-based screws. This study showed promising results for the use of Mg-based fixation hardware just after implantation. However, the effect of the mechanical integrity loss of these devices due to degradation on bone immobilization during the healing period has never been investigated.
2.2. The need for patient-specific fixation hardware
Conventional fixation hardware systems have been in use for many years and they usually provide satisfactory results. However, several efforts have been being attempted to replace conventional fixation hardware with custom-designed, patient-specific, fixation hardware to overcome existing shortcomings of current designs such as their unsatisfactory results in case of severe injuries and abnormal anatomy, and the possibility of postoperative complications [80], [81]. In fixation hardware, the level and the distribution of stress depends on the fracture location as well as the geometry and the material properties of the hardware [1], [43]. The stress pattern in the hardware affects the stress profile in the surrounding bone and, as a result, the morphology and density of the bone. In addition, the patient's age, gender and health status all influence bone healing. Healing time can be divided to three phases; inflammatory phase (3–7 days), the strong healing union (3–4 months), and remodeling up to a year [25]. In healthy adults, the inflammatory phase usually takes from 3 to 4 months before the bone remodeling phase starts. Bone remodeling is primarily driven by the pattern of stress. In this period, therefore, it is necessary for the bone to receive a stress profile as close as possible to that of healthy bone.
The healing time is longer for older patients [25]. Therefore, degradation time of implant should take healing time into consideration. The geometry of the implant should be designed to deliver the desired pre- and post-degradation levels of stiffness. In the beginning, the implant should be stiff enough to immobilize the bone. After the initial healing is complete, the degradation should result in a reduced stiffness that allows the bone to receive enough mechanical load to recreate the original loading pattern. Personalized medicine that involves a custom-designed implant system based on patient-specific anatomy and patient's case could reduce operating time and result in better treatment outcomes.
The expected excellent outcomes of using patient-specific fixation hardware are represented in the reduced complication rates, and shortened operation time and hospital stay. All these advantages will result in lower overall treatment cost. Lethaus et al. [82] conducted a retrospective study based on 33 patients with skull bone defects, 17 of them underwent reconstruction surgery with patient-specific implants (titanium and polyether ether ketone, PEEK) and were compared to 16 patients treated with the conventional reconstructive surgery (re-implantation of autogenous bone specimen). They found that the complication rate, rate of reoperation, hospital stay, and the total treatment cost were significantly lower for patients with the patient-specific implants (15,532.08 €/patient) compared to the conventional treatment method (26,086.06 €/patient).
The development of patient-specific fixation hardware has historically been limited by the available tools and the cost of fabricating the custom-designed fixation. Additive manufacturing (3D printing) has made it possible to develop the needed patient-specific fixation system. Based on individual patient data (e.g. computed tomography scan), perfectly fitting customized fixations are created [17]. More details about the additive manufacturing of Mg are discussed in Section 4.5.
2.3. Corrosion of pure Mg
In addition to low yield strength (27.5 MPa) and hardness (28.9 HV) [38], Mg has a very fast corrosion rate (e.g., 2.89 mm/year in NaCl solution) [13] with a corrosion potential and hydrogen evolution rate of − 2.027 V [38] (− 1.906 V in another study) [42] and 56.5 mL/cm2/day [38] respectively in simulated body fluid (SBF). In other words, Mg is highly susceptible to galvanic corrosion and its corrosion reaction in an aqueous environment (physiological environment) produces Mg ions, hydrogen (H2) and hydroxyl group (OH). The hydroxyl groups quickly react with Mg ions and form a layer on the surface of magnesium hydroxide Mg(OH)2 that may convert into soluble magnesium chloride (MgCl2) as indicated in reactions 1 to 3 leading to an increase in the pH value [11], [83], [84], [85]. An increase in the pH (exceeding 7.8) is beneficial in slowing down the corrosion rate due to the formation of a stable magnesium hydroxide Mg(OH)2 surface layer. However, such an increase in the pH value may also cause the death of tissue cells with an increase in the inflammatory response [11].(1)(2)(3)
In addition, hydrogen pockets as shown in reaction 1 are formed during corrosion in the rate of 1 mL for every 1 mg of Mg. This may lead to separation of tissue and/or tissue layers, thus causing a delay in bone healing at the surgical site [24]. Also, the evolution of hydrogen may result in Mg hydrogen embitterment by the ingress of hydrogen into Mg, leading to reduction in its ductility and load bearing capacity. This reduction in ductility may cause a brittle fracture of the Mg-based fixation hardware [11]. Corrosion of Mg has a dynamic interface between Mg surface and the body fluids and cells [25]. This dynamic interface is more emerged in the early stage of corrosion due to the change in the pH to basification, hydrogen emission and Mg and/or Ca containing corrosive surface layers [25], [86]. All these changes during the corrosion process dynamically alter the interface between Mg and the physiological environment.
2.4. Cytotoxicity and animal testing
Although the short- and long-term effects of introducing Mg and Mg alloys into the human body and their biological response on cells and tissues is not the focus of this monograph, they present an important Mg-based fixation hardware design consideration. A quick screening of the short-term biocompatibility of Mg-based alloys can be evaluated by cytotoxicity testing while animal testing may serve as a key indicator for their both short- and long-term biocompatibility. However, the in vitro and in vivo toxicity effects have not been considered in many studies during the design of Mg-based fixation hardware. This leads to uncertainty and a lack of knowledge of the effects of several alloying and coating elements when implanted especially for their long-term effects.
The biocompatibility characteristics of Mg alloying elements are different. Some elements are known to be toxic alone or alloyed with Mg such as Cd and Pb, while the biocompatibility of other elements such as Al, Sr, Li, Zr, Si and RE-elements is uncertain and has not been proven yet. Although initial studies of such elements may show low toxicity [87], [88], their long-term effects on organs or general body health are not well known. For example, the addition of 0.5 wt.% Sr to a Mg-1 wt.%Ca alloy was found to have a low overall toxicity after an in vitro cytotoxicity testing with a mouse osteoblastic cell line [40]. Gu et al. [87] evaluated the in vitro and in vivo biocompatibility of as-rolled Mg-Sr (with a Sr content ranging from 1 to 4 wt.%) as an orthopedic biodegradable alloy. The as-rolled Mg-2Sr alloy showed grade I cytotoxicity from the in vitro cell experiment and an enhanced mineral density and thicker cortical bone around the alloy from the intramedullary implantation test. In addition to their role in enhancing Mg mechanical properties, the addition of 1–5 wt.% Zr and 2–5 wt.% Sr to Mg was found to be biocompatible in vitro and in vivo [89]. The in vitro biocompatibility assessment using osteoblast-like SaOS2 cells and MTS, and haemolysis tests. A higher bone mineral density (BMD) and bone mineral content (BMC) for the Mg-Zr alloy from the in vivo test suggest an improved osteointegrative properties. Also, the observed increase in serum ALP activity in the implanted animals reflected bone-forming ability [89]. In another study, Gu et al. [88] investigated the in vitro biocompatibility (cytotoxicity and hemocompatibility) of nine binary Mg-1X (wt.%) alloys with different alloying elements (Al, Ag, In, Mn, Si, Sn, Y, Zn and Zr). They found that pure Mg control was the only group that did not show reduced hemolysis and adhered platelets compared to all other prepared binary alloys. Also, Mg-1Al, Mg-1Sn and Mg-1Zn were the only alloys that had no significant reduced cell viability to fibroblasts (L-929 and NIH3T3) and osteoblasts (MC3T3-E1).
On the other hand, Mg. Ca, Zn, Mn are essential nutritive elements that show no cell toxicity [41], [90]. However, they may cause toxic effects if the body intake of such elements exceeds the recommended daily dosage. The daily allowance of Mg is 0.4–0.7 g and excessive Mg in the body may lead to nausea. Also, a higher level of Mg in serum (> 1.05 mmol/L) was found to cause muscular paralysis [16], [22], [35]. The highest daily intake that can be tolerated by the body is for Ca (0.8–1.4 g). However, inhibited intestinal adsorption of essential minerals may occur in case of excessive Ca dose [13], [91]. While, the allowable daily dose for Zn and Mn are 15–17 mg and 4 mg, respectively. Higher levels of Zn in the body may hinder bone development especially at higher concentration while excessive Mn may result in neurotoxicity [13], [22], [91]. The in vitro and in vivo biocompatibility of a binary Mg-1 wt.%Ca alloy was studied by Li et al. [35]. No toxicity to L-929 cells was observed and the viability of cells for the Mg-1 wt.%Ca alloy extraction medium was better than that of control. The in vivo test results showed high activity of osteocytes around the Mg-1 wt.%Ca pins implanted into rabbit femoral shafts with newly formed bone at the third month. Zhang et al. [37] studied the in vitro and in vivo biocompatibility of a high Zn content binary Mg-6 wt.%Zn alloy. The hot-extruded Mg-6 wt.%Zn alloy did not show any toxicity to L-929 cells. HE stained tissue (containing heart, liver, kidney and spleen tissues) and the biochemical measurements, including serum magnesium, serum creatinine (CREA), blood urea nitrogen (BUN), glutamic-pyruvic transaminase (GPT) and creatine kinase (CK) showed no harm to the vital organs and new formed bone surrounding the implanted rods into the femoral shaft of rabbits was confirmed.
As a functional way to delay the corrosion rate of Mg alloys for bone fixation applications, the assessment of the biocompatibility of a developed coating is as important as that for the coated alloy. Wang et al. [92] studied the in vivo bone response of Mg-Zn-Ca alloy rods coated with Ca-def HA implanted into rabbit femora. Good osteoconductivity and new bone formation were confirmed by histopathological examinations. Also, Wong, et al. [93] studied the in vitro and in vivo biocompatibility of a AZ91 alloy coated with polymeric membrane fabricated by polycaprolactone and dichloromethane. The in vitro studies indicated good cytocompatibility of eGFP and SaOS-2 osteoblasts with the polymer-coated alloy, while the in vivo test showed lower degradation rate for the polymer-coated alloy with higher volume of new bone.
It is important to mention that the release of elements and metal ions is related to the alloy corrosion rate in the physiological environment which leads to a change in the pH value around the implant. Any change in the pH value has a negative effect on surrounding cell viability. Hence, controlling the corrosion rate is a major priority not only to maintain the fixation hardware mechanical integrity, but also to insure biocompatibility. Also, the location of the implant plays a significant role in determining the allowable limits of released elements and corrosion products based on their local toxicity to the cells and tissues adjacent to the implant. For example, the diffusion and transfer of the released elements and corrosion products is influenced by the local blood supply, distance between tissue and the implant, and the implantation time [22].
2.5. Characteristics of successful Mg-based bone fixation hardware
In spite of the numerous advantages of Mg as a biomaterial, pure Mg cannot satisfy all the clinical requirements for internal fixation hardware due to its low mechanical properties and undesirable corrosion behavior. The required mechanical properties of Mg-based fixation hardware can be determined by combining finite element analysis (FEA) studies of the fixation procedure and static in vitro material testing. These studies then identify the required mechanical behavior of fixation to restore the normal stress-strain trajectories [77], [94]. Strength higher than 200 MPa, elongation higher than 10% and corrosion resistance < 0.5 mm/year (SBF at 37 °C) have been suggested to be the desired characteristics for resorbable bone fixture [95]. Fatigue, creep, and stress relaxation are other important mechanical parameters that should be considered during the material selection and design of Mg-based fixation hardware. Also, the degradation by-products (especially hydrogen) should be guaranteed that are removed by excretion without causing any toxicity to the body cells locally and/or systemically [94], [96]. A rate of 0.02 mL/cm2/h as a tolerated level of hydrogen evolution in the human body has been proposed [97]. To this end, suitable alloying and coating are required.
3. Mg alloying and fixation device coatings
Microstructure is the key factor that affects both mechanical and corrosion performance of Mg-based biomaterials. Microstructure can be altered by alloying (i.e., changing the chemical composition) as well as mechanical or heat treatment [11]. Furthermore, alloying can reduce corrosion rate and coating the surfaces of Mg-based fixation hardware delays corrosion. In this section, we will discuss Mg alloying and fixation device coating, while mechanical and heat treatments will be covered in Section 4.
3.1. Alloying
Solid solution strengthening, precipitate hardening and grain refinement strengthening are the main alloying mechanisms for improving the mechanical properties of Mg alloys [27]. Because of the hexagonal-closed-packed (HCP) crystal structure and the atomic diameter of Mg (0.320 nm), a wide range of elements such as Al, Zn, Ca, Zr, Si, and Rare Earth elements can form solid solutions with pure Mg [12], [13]. Numerous studies have been conducted on the feasibility of Mg alloying for orthopedic device applications by characterizing their microstructure, mechanical properties, corrosion behavior and biocompatibility using both in vitro and in vivo studies. Mg-Ca, Mg-RE, Mg-Zn and Mg-Zr are the most studied Mg-based alloying systems [14], [37], [38], [88], [98], [99]. Alloying elements have different toxicity (biocompatibility) characteristics. For example, Cd and Pb are well known to be toxic elements alone or alloyed with Mg [13]. However, Ca, Sr, Zr, Mn and Zn alone or alloyed with Mg are shown to be biocompatible [13], [35], [60]. Aluminum was initially considered but has been found to cause hepatotoxicity or allergic reactions [100], [101] with the potential of leading to Alzheimer's disease [102] and it may also cause muscle fiber damage [103]. The release of RE elements (e.g., yttrium) as an alloying element was found to cause severe hepatotoxicity [104], [105]. Mg-Ca, Mg-Zn and Mg-Zn-Ca have been found to be the most promising alloying systems because of their superior biocompatibility, moderate strength and corrosion characteristics. We will discuss more about these alloying systems here. For more details about other Mg-based alloying systems, the reader can be referred to [12], [13]. It is important to note that the presence of impurities such as Fe, Ni, Be and Cu reduces the strength of Mg alloys while accelerating their corrosion rate [106]. Impurity levels of these elements should be kept within the physiological tolerance limit (Fe: 30–50, Ni: 20–50, Be: 2–4 and Cu: 100–300 ppm by weight) [106], [107]. Adding certain alloying elements (e.g., Mn and Zn), or using the zone solidification method can eliminate the harmful effects of impurities [13].
3.1.1. Mg-Ca alloys
Mg-Ca alloys have received considerable attention for orthopedic fixation applications. They have low density (1.55 g/cm3), low cost, as well as the beneficial role of co-releasing of Mg and Ca ions during bone healing and remodeling [13]. The addition of Ca to pure Mg also protects the Mg alloy from oxidation during casting. This protection is due to the formation of a thin dense CaO film foam on the surface that could prevent ignition of Mg and reduce molten metal surface tension [108], [109]. Furthermore, the addition of Ca to Mg assists in grain refinement, strengthening the alloy over pure Mg [12]. In vitro and in vivo studies have revealed that a mixture of Mg(OH)2 and hydroxyapatite was formed on the surface of Mg-Ca alloys with new formation of bone on this surface after 3 months of implantation [35]. However, the rapid degradation in the physiological environment is the main obstacle in using uncoated Mg-Ca as bone fixation hardware [14]. Generally, an Mg-Ca alloy with Ca content of < 45 wt.% is composed of lamellar eutectic phases of α-Mg + Mg2Ca [39], [109], [110]. The Mg2Ca secondary phase has a high average melting temperature of 715 °C [111]. However, Li et al. found reduced corrosion resistance as the proportion of Mg2Ca intermetallic compound in the alloy microstructure increases [35]. This suggests that a Ca content above the solubility limit (1 wt.%) leads to increased formation of the Mg2Ca intermetallic compound, thus, the deterioration in the Mg-Ca alloy corrosion resistance [12], [35]. In other words, only the Mg-1 wt.%Ca alloy has no toxicity and degrades gradually enough to allow bone to heal fixated. Total absorption took place after 3 months of implantation in rabbit femoral shafts.
3.1.2. Mg-Zn alloys
Zinc is less corrosive than Mg and is known to support the immune system. It is also involved in various aspects of cellular metabolism [37], [91]. From a material viewpoint, the addition of Zn to pure Mg is known to improve strength due to the formation of the MgZn intermetallic compound and refining (reducing) the grain size [109], [112]. Also, the presence of Zn in Mg alloys mitigates the adverse corrosion effect of impurities such as Fe and Ni [38]. Koç et al. [113] studied the influence of content up to 3 wt.% on the mechanical and corrosion properties of the as-cast binary Mg-Zn alloy. Mechanical properties and corrosion resistance were found to increase by increasing the content of Zn% in the prepared Mg-Zn alloys. ZK30 and ZK60 are two examples of commercial Mg-Zn based alloys with a Zn content of < 5.5 wt.%. For example, the chemical composition of ZK30 is Mg-3 wt.%Zn-0.6 wt.%Zr-0.007 wt.%Fe. Zhang et al. [37] investigated a solution-treated and hot-extruded Mg-6 wt.%Zn alloy as a biodegradable material. The immersion test results showed that the hydroxyapatite (HA) and other Mg/Ca phosphates are the corrosion products of this alloy seen at the surface in SBF. The in vivo results showed that Mg-Zn implanted rods in the femoral shaft of rabbits were gradually absorbed at a rate of 2.32 mm/year without any harmful effects to vital organs.
In comparison to the Mg-rich alloys, Zn-rich Zn-Mg alloys offer lower corrosion rates and reduced hydrogen evolution [22]. The Zn-rich alloys are easier and safer to fabricate due to their lower reactivity and lower melting points [114]. Vojtěch et al. [114] investigated the effect of adding 1–3 wt.% Mg around the eutectic point. Zn-1 wt.%Mg alloy showed the maximum yield strength and elongation of 90 MPa and 2% respectively with a corrosion rate of < 0.145 mm/yr. This rate is significantly lower than any as-cast Mg-rich alloy. Murni et al. [115] evaluated the cytotoxicity of Zn-3 wt.%Mg alloy through osteoblast cell-material interaction. They found that the Zn-rich alloy was not cytotoxic. The addition of low amount of Mn to Zn-1–1.5 wt.%Mg alloys was found to enhance their corrosion and mechanical properties [116]. The disadvantages of Zn-Mg alloys are, the limited ductility (elongation 0.25–2%), higher density (~ 7.14 g/cm3) and higher stiffness (modulus of elasticity ~ 108 GPa). Table 1 presents a quantitative comparison between the as-cast Mg-1 wt.%Zn alloy and Zn-1 wt.%Mg alloy as two examples of the Mg-rich and Zn-rich alloys, respectively.
Table 1. The physical, mechanical and corrosion characteristics of the as-cast Mg-1 wt.%Zn and Zn-1 wt.%Mg alloys [88], [114].
| Property | Mg-rich (Mg-1 wt.%Zn) alloy | Zn-rich (Zn-1 wt.%Mg) alloy |
|---|---|---|
| Relative density | ~ 1.74 | ~ 7.14 |
| Modulus of elasticity (GPa) | ~ 45 | ~ 108 |
| Yield strength (MPa) | 25.5 | ~ 90 |
| Tensile strength (MPa) | 134 | 150 |
| Elongation (e%) | 18.2 | ~ 1.75 |
| Corrosion in SBF | Fast (1.52 mm/yr) | Slow (~ 0.06 mm/yr) |
| Melting temp. (°C) | ~ 650 | ~ 420 |
3.1.3. Mg-Zn-Ca alloys
Ternary Mg-Zn-Ca alloys have improved the biocompatibility and mechanical characteristics over pure Mg and binary Mg-Ca alloys, while lower density than Mg-Zn alloys and desirable anti-bacterial properties [117], [118]. In the Mg-rich alloy, various phases can be produced on the grain boundaries that result in different degradation and mechanical properties; the key differentiating factor to this end, is the Zn/Ca atomic ratio. The eutectic phase α-Mg + Mg2Ca + Ca2Mg6Zn3 form when the Zn/Ca atomic ratio is less than 1.1–1.2 [119], [120], [121] or 1.4 in another study [122]. Above these limits, in addition to the primary Mg, the lamellar eutectic phase α-Mg + Ca2Mg6Zn3 is observed [123]. However, only one study by Zander and Zumdick [124] reported presence of the presence of emblematic amount of Mg2Ca phase above these limits for the Mg-1.8 wt.%Zn-0.6 wt.%Ca alloy (Zn/Ca atomic ratio = 1.84). The presence of the Ca2Mg6Zn3 phase has a more rapid corrosion rate with better mechanical properties, and an increased level of the corrosion byproduct brucite (Mg(OH)2) and hydroxyapatite forms on the surface [35], [120]. This atomic ratio, as demonstrated by Larionova et al. [122], affects the interplanar distances in rapidly solidified alloys. An increased Zn/Ca atomic ratio leads to contraction of the phase lattice and a decreased value causes phase lattice expansion.
The high loading of alloying elements in binary systems (e.g., Ca in the Mg-Ca) leads to an increased percentage of the precipitated intermetallic compounds (e.g., Mg2Ca) [60]. Similarly, the percentage of such compounds for the Mg-Zn-Ca ternary alloys increases at a high loading of Zn and Ca (e.g., Mg2Ca, Ca2Mg6Zn3, MgZn and MgZn2). An increased percentage of these intermetallic compounds results in higher strength accompanied by more rapid corrosion rates. Ca content of < 0.5 wt.% was found as the optimum percentage for grain refinement of the Mg-rich Mg-Zn-Ca-Mn alloys [121]. It was also reported that the addition of > 1 wt.% Zn to the Mg-0.5 wt.%Ca and the Mg-1 wt.%Ca alloys significantly improves corrosion resistance [120], [125], [126]. However, a higher Zn loading above 2 to 3 wt.% causes a reduction in the corrosion resistance with significant deterioration above 5 wt.% [41], [60], [120], [125].
3.1.4. Mg-Zn-Ca bulk metallic glasses (BMGs)
Mg-Zn-Ca bulk metallic glasses (BMGs) have been studied as an alternative biodegradable fixation material with superior strength and corrosion resistance in comparison with traditional cast Mg-Zn-Ca alloys [118], [127]. Unlike the crystalline atomic structure of cast alloys, Mg-Zn-Ca glasses have disordered atomic (glass-like) structure [128]. The amorphous glassy structure is usually a result of a rapid cooling process where the molten alloy crystalline phases do not have enough time to nucleate and grow, as a results, the materials undergoes a glass transition and freeze in an amorphous glassy state [128], [129]. In vivo pig study conducted by Zberg et al. [128] indicated good tissue compatibility with wound-healing process signs and less hydrogen evolution for the amorphous Mg-Zn-Ca BMG than the crystalline alloy. The cast rods were melt-spun into glassy ribbons of approximately 50 μm thickness in a helium atmosphere. Subsequently, a copper mold injection casting system was used to produce 0.5 mm thick Mg-Zn-Ca BMG samples in an argon atmosphere. The glassy (60 + x)Mg-(35 − x)Zn-(5)Ca alloy was found to be the most promising Mg-Zn-Ca BMG with a range of tensile strength from 675 to 894 MPa and an elastic range of < 4%. The main limitations of Mg-Zn-Ca BMGs are the limited processing time available before the onset of crystallization, small critical casting thickness of approximately 3 mm, and the poor formability [118], [128], [130]. These limitations result in a difficulty in creating fixation devices of Mg-Zn-Ca BMGs.
3.1.5. Quaternary Mg-Zn-Ca-X alloys
To further tune the properties of the Mg-based biocompatible and resorbable alloys for bone fixation devices, a limited number of elements such as Mn and Zr can be added to Mg-Zn-Ca alloys. These alloying elements improve properties by incorporating essentially insoluble metallic impurities such as Fe and Ni into harmless intermetallic phases (e.g., AlMnFe phase in Mg-Al alloys) [12], [131]. In several studies, Mn (up to 1 wt.%) was added to different binary and ternary Mg alloys to enhance corrosion resistance. Bakhsheshi-Rad et al. [38] studied the mechanical and corrosion properties of various binary Mg-Ca and quaternary Mg-Ca-Zn-Mn alloying systems. They found that the addition of 0.5 wt.% Mn to the Mg-2 wt.%Ca-2 wt.%Zn and the Mg-2 wt.%Ca-4 wt.%Zn alloys enhances the mechanical properties and the corrosion resistance by decreasing the grain size to 78 μm and 59 μm, respectively. The small grain size provides a smoother surface, thus, fewer surface pits are present than in both Mg-Ca and Mg-Ca-Zn alloys. Also, it was found that the quaternary Mg-Ca-Zn-Mn alloys have reduced corrosion by the formation of a brucite (Mg(OH)2) protection than the other studied alloys. It has been shown that these quaternary alloys with 2 wt.% Zn have better mechanical and corrosion performance than those with 4 wt.% Zn.
The addition of Zr to Mg-Zn-Ca alloys improves corrosion resistance. This effect is observed at Zr content of < 0.42 wt.%. Higher concentrations of Zr, while improving strength due to grain refining, speeds corrosion [12], [60]. Qu et al. [132] developed a quaternary Mg-Zn-Ca-Y alloy for bone fixation. In their study, the alloy with Mg-2.0 wt.%Zn-0.5 wt.%Ca-1.0 wt.%Y chemical composition was implanted in rabbits to study biocompatibility for different durations (until 24 weeks). Low levels of Lymphocytes and macrophages were observed around the local muscle tissue during the first week revealing the presence of a mild inflammatory cells. After 2–4 weeks the inflammatory cells decreased and completely disappeared after 12 weeks. Also, a thin fibrous membrane was observed around these implants after 2 weeks. The fibrous membrane's thickness (15–25 μm) remained within the U.S. ASTM-F4 implant requirements (< 30 μm) for the 24-week implantation period. When compared with pure Mg, this alloy has a slower degradation rate with desirable biocompatibility. To this end, Mg-Zn-Ca alloys possess the best mechanical and corrosion properties in addition to being biocompatible. Also, the addition of Mn, Zr and/or Y to Mg-Zn-Ca alloys was found to significantly enhance the biomechanical performance. The mechanical and corrosion properties of as-cast binary, ternary and quaternary Mg-Zn-Ca alloys are listed in Table 2. The values of corrosion properties vary one study to another for similar alloy chemical compositions. This can be attributed to variation in alloy microstructure during preparation and the non-strict conditions of the conducted corrosion tests within each study.
Table 2. The tensile properties and the in vitro electrochemical corrosion characteristics for different Mg-Ca, Mg-Zn, Mg-Zn-Ca and Mg-Zn-Ca-Mn alloys.
| Alloy composition (%wt.) | Tensile properties | In vitro electrochemical corrosion characteristics (SBF) | Ref. | ||||
|---|---|---|---|---|---|---|---|
| 0.2% yield strength (MPa) | Tensile strength (MPa) | Elong. (%) | Corrosion potential Ecorr (μv vs. SCE) | Current density icorr(μA/cm2) | Calculated corrosion rate (mm/year) | ||
| Pure Mg | 27.5 | 97.5 | 7.31 | − 2027.4 | 370.7 | 8.47 | [38] |
| Mg-0.5%Caa | 51 | 91 | 5.0 | − 1876 | 186 | – | [98], [39] |
| Mg-2%Ca | 47.2 | 115.2 | 3.05 | − 1996.8 | 301.7 | 6.84 | [38] |
| Mg-4%Ca | 34.5 | 77.4 | 2.10 | − 2054.5 | 395.7 | 9.04 | [38] |
| Mg-1%Znb | 25.5 | 134 | 18.2 | − 1822 | 67.3 | 1.52 | [88] |
| Mg-1%Znc | 80 | 127 | 16 | − 1830 | 124 | – | [113] |
| Mg-3%Znc | 91 | 147 | 12 | − 1710 | 102 | – | [113] |
| Mg-2%Zn-3%Ca | 117 | 145 | 0.57 | − 1640 (Hank's) | 3.86 (Hank's) | – | [125] |
| Mg-4%Zn-0.2%Ca- | 60 | 185 | 12.5 | − 1700 | 267 | 2.05 | [42] |
| Mg-2%Zn-2%Ca-0.5%Mn | 78.3 | 168.5 | 7.83 | − 1616.6 | 78.3 | 1.78 | [38] |
| Mg-2%Zn-0.5%Ca-1.2%Mnd | 72 | 187 | 9.1 | − 1496 | 57.2 | – | [121] |
- a
-
The tensile properties were adopted from Ref. [98], while the corrosion properties were adopted from Ref. [39].
- b
-
The tensile properties were deduced from Fig. 2 in Ref. [88].
- c
-
The tensile properties were deduced from Fig. 4 in Ref. [113].
- d
-
The tensile properties were deduced from Fig. 5 in Ref. [121].
3.2. Coating
Coating Mg alloys improves their corrosion resistance in a physiological environment [12], [25], [133], [134]. Coating must be biocompatible and have high corrosion resistance to maintain the mechanical integrity of the fixation hardware during the initial bone healing period. Based on the interaction between the coating and the Mg alloy, coatings can be classified into three categories: substrate-involving coatings, non-substrate-involving coatings, and composite coatings [25]. Alkaline oxidation, fluoridation and micro-arc oxidation (MAO) are some examples of the substrate-involving coating techniques [25]. Gu et al. [135] performed an alkaline oxidation process by soaking a Mg-1.4 wt.%Ca alloy in three alkaline solutions (Na2HPO4, Na2CO3 and NaHCO3) for 24 h followed by annealing at 773 K for 12 h. The surface treated alloy showed an improved corrosion resistance and slower increase of the solution pH value, due to the formation of a magnesium oxide layer (MgO) of thickness of < 26 μm. Lei et al. [136] employed an anodic electrodeposition process in a concentrated potassium hydroxide (KOH) solution followed by heat treatment in air. In this process, a MgO coating was produced on an Mg-5.5–6.5 wt.%Zn-1.0–1.5 wt.%Ca alloy, thereby slowing the corrosion rate. Mousa et al. [137] used an anodization process with SBF as the electrolyte to deposit a protective apatite-like coating layer, mainly composed of MgO phase, on AZ31B Mg alloy. The anodizing voltage was found to have a significant effect on the coating hardness and corrosion resistance.
Micro-arc oxidation (MAO), also referred to as, plasma electrolytic oxidation (PEO) or micro plasma oxidation (MPO), is one of the most studied coating processes due to its simplicity and the formation of a hard protective oxide layer on the surface leading to enhanced corrosion resistance. During the MAO process, a highly adherent ceramic oxide coating is formed due to partial short-term melting of the oxide layer caused by a high voltage plasma discharges [138]. One of the remarkable features of MAO coatings is the presence of pores and cracks on the coating surface. This results in higher bond strength between the coating and the Mg alloy substrate [96]. MAO coating also enhances adhesion with subsequent organic or polymeric coatings [139].
While MAO coating usually shows good protection for a few weeks, the corrosion resistance quickly degrades thereafter due to the presence of surface pores [140], [141]. To address this limitation, one possible solution is controlling the process parameters and the electrolyte to change the distribution and interconnectivity of the pores [142], [143], [144]. For example, fine pores with better corrosion resistance are produced at lower voltages [145]. It is also possible to seal the outer layer pores and cracks with additional layers of ceramic coatings (e.g., hydroxyapatite [HA]) and/or polymeric coatings [96], [146]. Other solutions include the pretreatments using cerium conversion coating or increasing the thickness of the barrier layer [96], [147], [148].
Calcium phosphate (Ca/P) coatings such as hydroxyapatite (HA) and tricalcium phosphate (TCP) have been used in bone implants to improve the biocompatibility and biological response (bone ingrowth) as a non-substrate-involving coating. These ceramic coatings also offer a biocompatible platform for controlled drugs elution during the corrosion process such as antibiotics. The crystalline hydroxyapatite (HA), which is the most stable Ca/P compound in the human body, has been extensively studied as a promising Mg alloy coating [12]. Wang et al. [92] studied the effect of calcium-deficient hydroxyapatite (Ca-def HA) coating on the degradation behavior and bone response of a Mg-1 wt.%Ca-1 wt.%Zn alloy. The samples were coated using a pulse electrodeposition process. The coated Mg alloys had a significantly slower in vivo corrosion rate (0.15 mm/year) compared to the uncoated samples (0.8 mm/year). Wang et al. [149] used a similar approach and showed superior corrosion resistance and loss of mechanical properties in the coated samples. In a slow strain rate test in SBF with extension rate of 2.16 × 10− 5 mm/s until fracture, Ca-def HA coated samples had a higher ultimate strength of 152 MPa than that of the uncoated samples, 144 MPa. 10–30 μm brushite (CaHPO4-2H2O) Ca/P bioceramic coating of patented JDBM (Mg-Nd-Zn-Zr) alloy showed promising results with a bonding strength over 10 MPa [150]. Guan et al. [151]studied the in vitro and in vivo degradation of uncoated and brushite-coated Mg-based screws made of JDBM, i.e., Mg-3.1 wt.%Nd-0.2 wt.%Zn-0.4 wt.%Zr. The screws were implanted in the mandible of New Zealand White rabbits. Fig. 2shows that degradation through as visualized by synchrotron radiation X-ray microtomography. Both the in vitro and in vivo results showed that the uncoated and brushite-coated screws are biocompatible. The in vivo degradation rates of the coated screws after 1, 4, and 7 months of implantation were 0.161 ± 0.075, 0.097 ± 0.013, and 0.218 ± 0.030 mm/year, respectively. Lichen et al. [152] prepared a composite coating by using micro-arc oxidation (MAO) on Mg substrates in an aqueous solution that included hydroxyapatite (HA) powder. Potentiodynamic polarization tests and immersion tests in SBF indicated that the specimens with the composite coating that were anodized in the HA-containing electrolyte have a better corrosion resistance than those anodized in the HA-free electrolyte. In another work, Dou et al. [153] prepared a porous bioceramic containing tricalcium phosphate (TCP) coating by MAO with different voltages on Mg-4.75 wt.%Zn-0.55 wt.%Ca alloy. The results indicate that the voltage have a noticeable influence on the thickness and corrosion properties of the bioactive TCP-containing MAO coating.
 Fig. 2. Picture of original screw model (a) and 3D reconstruction images of C-JDBM screws after 1 (b), 4 (c), and 7 (d) months of implantation. No obvious degradation happened 1 month (b) postimplantation. Slight volume loss was found 4 months (c) postimplantation. Mg screw seriously degraded after 7 months (d) of implantation (adapted from [151]).
Fig. 2. Picture of original screw model (a) and 3D reconstruction images of C-JDBM screws after 1 (b), 4 (c), and 7 (d) months of implantation. No obvious degradation happened 1 month (b) postimplantation. Slight volume loss was found 4 months (c) postimplantation. Mg screw seriously degraded after 7 months (d) of implantation (adapted from [151]).Biodegradable polymers such as poly lactic-co-glycolic acid (PLGA), Polylactic acid (PLLA), dichloromethane (DCM), and polycaprolactone (PCL) have also been used as a non-substrate-involving coating [25], [154], [155], [156]. It is well known that a PLGA coating improves corrosion resistance and enables cell adhesion and organization of the attached cell's cytoskeleton [154]. Li et al. [157] investigated the effect of sealing the surface pores of MAO-treated pure Mg samples with a layer of PCL creating a composite coating. The PCL layer was deposited by dipping the MAO-treated samples in 4 and 7 wt.% PCL solution for 1 min. The samples were then pulled out slowly at a rate of 20 mm/min. The results obtained from the immersion test and the potentiodynamic polarization test revealed that the porous nature of MAO-treated Mg alloys did not by itself reduce corrosion resistance in Hanks' balanced salt solution (HBSS). However, the deposition of PCL onto the MAO-treated surfaces significantly increased the corrosion resistance. Zomorodian et al. [158] developed a composite coating on the AZ31 alloy by adding nanohydroxyapatite particles and an antibiotic, cephalexin, to PCL. Although the addition of the nanohydroxyapatite particles and antibiotic resulted in reduced corrosion protection, the composite coating has enhanced biocompatibility and anti-bacterial functionality.
Sheng et al. [159] prepared hexamethylenediaminetetrakis-(methylenephosphonic acid) (HDTMPA) surface-modified Mg alloy samples via a covalent immobilization process in conjunction with a sequential deposition process. HDTMPA is an organic phosphate that as a coating is biocompatible and provides corrosion resistance [159]. Electrochemical corrosion and immersion corrosion results reveal that the HDTMPA-coated Mg alloy samples provides reduced corrosion. Razavi et al. [160] show that a nanostructured akermanite (Ca2MgSi2O7) coating improves corrosion resistance and the surface bioactivity of the coated Mg alloys. These nanostructure coatings were grown on AZ91 Mg alloy samples through an electrophoretic deposition (EPD) process. Zhao et al. [161] used dual zirconium and oxygen ion implantation to create a rough, hydrophobic and ZrO2-containing surface film on the magnesium-calcium (Mg-Ca) and magnesium-strontium (Mg-Sr) alloys. Through in vitro weight loss measurements and electrochemical corrosion testing of the coated alloy samples, it was shown that the coating increased corrosion resistance.
A compact fluoride conversion coating composed of MgO and MgF2 was applied to AZ31B alloy samples (Mg-3 wt.%Al-1.1 wt.%Zn-0.70 wt.%Mn) by Yan et al. [162]. Immersion and electrochemical test results showed an improved corrosion resistance for the coated alloy in SBF. Razavi et al. [163] combined micro arc oxidation (MAO) and electrophoretic deposition (EPD) to coat AZ91 alloy samples with CaMgSi2O6 nanostructured diopside. Electrochemical corrosion, immersion and compression test results showed that the diopside coating not only slowed down the corrosion rate, but also enhanced in vitro bioactivity, mechanical stability and cytocompatibility of the alloy. Zomorodian et al. [164] created a composite coating composed of a thin inner layer of polyetherimide (PEI), as an adhesion promoter between Mg substrate and a nanohydroxyapatite-modified PCL outer layer to provide corrosion protection on AZ31 alloy samples. Increasing the PCL concentration results in a better corrosion protection of the Mg alloy, while the presence of nanohydroxyapatite particles enhances the cellular response over the coating and reduces the coating's corrosion resistance.
While a coating can control degradation timing, it should not undermine the stabilization function of the resulting implant. Tan et al. [73] used the extraction torque test to compare the interface strength between AZ31B screws and the surrounding host tissues of uncoated samples and samples with a Si-containing coating. The results were compared with those of surgical grade titanium (Ti6Al4V) and biodegradable PLLA bone fixation screws. After 4 weeks of implantation, the extraction torque was similar for all of the implanted screws (15–17 N·cm). The only exception was for the uncoated AZ31B screws with a torque of 5 N·cm. After 21 weeks of implantation, the extraction torque for the coated AZ31B was the highest (about 22 N·cm) while the PLLA screws had the lowest extraction torque (about 11 N·cm).
Sanchez et al. [165] is the only study we are aware of that attempts to correlate in vitro and in vivo results obtained from different Mg alloy coating studies. We recommend efforts be made to set standards for in vitro and in vivo coated alloy sample corrosion testing. Some of the discussed in vitro and in vivo work results in this section are listed in Table 3.
Table 3. The in-vitro and in-vivo corrosion characteristics and biocompatibility for different coatings created on Mg alloys.