1. Biological role of Sr
Sr is a chemical element with atomic number 38, which had been discovered in lead mines [73]. Food is the main source for Sr uptake for humans, and some types of food like grains and seafood may contain up to 25 mg/kg of Sr. Also small amounts of Sr enter the human body through the skin and lungs. The common daily Sr balance has been established as follows: intake with food/fluid – 1.9 mg, secretion with urine – 0.34 mg, excretion with faeces – 1.5 mg, secretion via sweat glands – 0.02 mg and loss with hair – 0.2 × 10− 3 [73].
The major chemical element found in the bone is calcium (Ca), but there are actually at least a dozen of other chemical elements in the bone and one of them is Sr. Although Sr is a bone-seeking element, most of it is absorbed in bones and teeth, thus demonstrating its excellent ability to bind with the mineral phase of bone – biological hydroxyapatite (HAp), which made this divalent cation interesting for the application in medicine [63], [69], [83]. 99% of the total amount of Sr in the body is deposited in the bone (36–140 mg/kg), preferably in the new trabecular bone and in “favourite” sites such as diaphysis of the femur, lumbar vertebra, and the iliac crest [33], [97]. However, the total amount of Sr in the skeleton is small compared with that of Ca and displays only 3.5% of the Ca molar content [73] or accounts for 0.035% of the Ca content [15].
The Sr content in a new compact bone is calculated to be three to four times higher than that in an old compact bone, and approximately 2.5 times higher in a new cancellous bone than in an old one. This proportion is kept throughout the lifetime, partly because the bone turnover is higher in cancellous bone than in cortical bone, and the newly formed bone is more abundant in cancellous bone than in cortical bone [102]. It is well known that Sr has a unique mechanism of action increasing pre-osteoblast proliferation, osteoblast differentiation, type I collagen synthesis and bone matrix mineralization, while osteoclast differentiation and activation are inhibited [16], [20].
2. Sr/Sr RAN and osteoporosis
In the last decade, extensive research has been done on the effects of Sr on bone, due to the development of the antiosteoporosis drug Sr RAN, which has attracted the most attention due to its promising physicochemical and pharmacokinetic characteristics [86]. Sr RAN is registered as a medication in over than 70 countries and has been used for post-menopausal osteoporosis in women and men for more than 10 years. Sr RAN as dual action agent simultaneously increases the osteoblast differentiation while osteoclast formation is inhibited [14]. Clinical trials have shown that SrR significantly improves bone mass and quality, and increases bone strength through changes in bone matrix properties and bone mineral density (BMD) in patients [68], [100]. Moreover, Sr RAN remarkably reduces the risk of spine or hip fractures [84].
In spite of the unique mode of action and impressive clinical trials, Sr Ran is licensed for use in Europe, but is not approved by the US Food and Drug Administration (FDA) [19], [30], [63]. Taking into account the fact that Sr and Sr RAN possess a unique and similar mode of action, this review will mainly focus on the common description of Sr and Sr Ran functions as well as possible pathophysiological mechanisms (unless an exception is indicated further in the text).
Osteoporosis is a metabolic bone disease characterized by a high bone turnover, low bone mass, destruction of bone microstructure, and increased risk of bone fragility and fractures [32]. In patients with osteoporosis, osteogenesis regression and osteoclasts enhancement occur, also weakening the functionality of osteoblasts, resulting in affected bone spicules and decreased bone volume, and leading to the increased risk of fractures of supportive tissue. It is mainly believed to be an oestrogen-deficiency disease because the oestrogen deficiency increases the rate of bone remodelling which, in association with a negative remodelling balance (resorption exceeding formation), results in impaired bone architecture, mass and strength [68]. In addition, other genetic and epigenetic factors may play an important role in triggering of osteoporosis. Fracture prevention is the main goal of any therapy for osteoporosis. Sr Ran as an antiosteoporotic agent has the theoretical premises to promote fracture healing and osseointegration. Numerous clinical studies have demonstrated that the systemic Sr Ran treatment minimizes the risk of vertebral, nonvertebral and hip fractures in a wide range of post-menopausal women with documented osteoporosis [57], [85], [90].
3. General effects of Sr/Sr RAN on bone
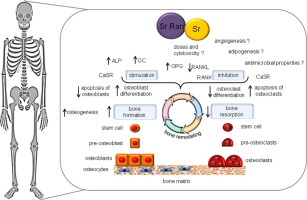
The dual action of Sr Ran and Sr, promoting osteoblast-mediated bone formation and inhibiting osteoclast-mediated bone resorption, has been under extensive research both in vitro and in vivo [30], [63]. The main effects of Sr/Sr Ran from in vitro and in vivo studies at the cellular and tissue level are summarized in Fig. 1.
 Fig. 1. In vivo and in vitro effects of Sr/Sr RAN. See the text for details.
Fig. 1. In vivo and in vitro effects of Sr/Sr RAN. See the text for details.Both Sr and Sr Ran enhance bone formation and slow down bone resorption. The resulting increase in bone mineral density (BMD) seems to be associated with the improved mechanical properties of the bone. Sr is generally believed to have a dual action of promoting osteogenic bone formation and inhibiting osteoclastic bone resorption [63]. Sr induces the higher expression of the osteoblastic genes – alkaline phosphatase (ALP), osteocalcin (OC) and bone sialoprotein, combined with increased bone nodules, and a reduction in the number of mature osteoclasts in vitro [14]. Several other authors also support the effects of Sr to induce pre-osteoblast proliferation and enhance osteoblast activity, as demonstrated by the increase in the expression of several early and late osteoblastic markers, such as type I collagen, ALP, bone sialoprotein, OC and, ultimately, bone matrix mineralization and nodule formation in bone marrow stromal cell cultures and immature osteoblasts [9], [18], [27]. Exactly the same – the presence of dual functions – is described for Sr RAN in the bone remodelling process, during which the bone is constantly being renewed [4]. Sr Ran is suggested to inhibit the bone resorption induced by osteoclasts as well as osteoclast differentiation/activation [10], and to stimulate the bone-forming function of the osteoblast and pre-osteoblast proliferation/differentiation.
Recently, Almeida et al. have demonstrated that Sr RAN enhances the expression of Type I Collagen and osteopontin (OPN), which are important components of the organic bone matrix [107]. Moreover, Sr RAN is able to promote the formation of large bone-like nodules in osteogenic cultures and accelerate the acquisition of the osteoblastic phenotype in pre-osteoblastic cells. This effect can explain the benefits of Sr RAN in reducing the risk of nonvertebral, hip, and vertebral bone fractures in post-menopausal women.
4. Mechanism of action of Sr/Sr RAN at the cellular& molecular level
Despite the notably positive effects of Sr/Sr RAN on the bone, mechanisms underlying the cellular effects triggered by it are not completely known. Some mechanisms have been suggested only in the last decade [24], [67]. At first, bone cells (osteoblasts, osteoclasts and osteocytes) harbour the Ca-sensing receptor (CaSR), a G-protein coupled receptor that is activated by extracellular divalent cations such as Ca and, with lower affinity, Sr. CaSR has been shown to mediate, at least in part, Sr induced osteoblast proliferation [23], [25], and osteoclast apoptosis [54]. Sr, like Ca, can activate the Ca-sensing receptor (CaSR), resulting in the activation of inositol-1,4,5-trisphosphate (IP3) production and MAPK signalling. These findings suggest that Sr can activate the osteoblastic cell replication through CaSR.
The second mechanism is related to the ability of Sr to activate the extracellular signal-regulated kinase (ERK) 1/2 phosphorylation, indicating that, in addition to CaSR, another receptor could mediate the effect of Sr on osteoblastic cell replication, so one of the suggestions indicates that Sr induces the expression of cyclooxygenase (COX)-2 and prostaglandin E2 (PGE2) via the activation of the ERK signalling pathway [28].
The third hypothesis states that Sr may inhibit bone resorption, increasing the cellular activity marker osteoprotegerin and decreasing the receptor activator of the nuclear factor kappa B ligand (RANKL) expression by osteoblasts [6], [18]. OPG is a protein that inhibits the RANKL-induced osteoclastogenesis by operating as a decoy receptor for RANKL [61]. This is a mechanism to be studied more intensively by scientists in biomaterial experiments, especially when new combinations between Sr and biomaterials should be defined.
The OPG/RANKL system plays a key role in osteoclast differentiation. The effect of Sr RAN on bone formation and resorption is blunted in mice devoid of OPG [81]. Thus, the enhancement of OPG expression and the inhibition of RANKL production by osteoblasts at various differentiation stages impair the osteoclast differentiation and function. Interestingly, CaSR signalling has been shown to play a key role in mediating the modulation of the OPG/RANKL system by Sr. However, although osteoblasts devoid CaSR, they can still respond to Sr in terms of proliferation and apoptosis. Other cation-sensing molecules and different mechanisms can be involved in mediating the effects of Sr on bone cells. In this respect, it has been recently shown that the Sr-mediated activation of the calcineurin/nuclear factor of the activated Tc pathway induces osteoblastogenesis via the stimulation of both canonical and non-canonical pathway [39].
There is a system of factors enrolling OPG/RANK/RANKL, which has been described as a critical system for controlling osteoclast biology. RANKL, which is synthesized by osteoblasts, is an essential factor for osteoclast differentiation and bone resorption. The latter process actually is initiated and continued by RANKL. On the other hand, osteoblasts as the most important cell type of the bone secrete the soluble OPG with high affinity for RANKL. Bone resorption is refused due to the OPG/RANKL complex suppression to connect RANK [59]. In such a way, the primary detectable factor for the evaluation of bone resorption is OPG; the higher it is, the more believable is the RANKL binding and the decrease of resorption. And here the role of Sr Ran is verified with a significant increase of the OPG expression level for both normal and osteoarthritis affected supportive tissue cells; data are reflected by the increase of OPG production. The increased OPG levels in the case of Sr Ran are in accordance with other in vitro studies demonstrating that Sr Ran increases the OPG mRNA and protein levels in human trabecular osteoblasts [6]. However, Sr Ran has been observed to have no true effect on the intracellular RANKL protein, but to have an indirect influence via the significant decrease of the membranous localization of RANKL [98].
Human RANKL is known to exist as three isoforms: RANKL1, 2, and 3. RANKL1 and 2 encode for transmembrane forms of RANKL. RANKL3 lacks intracellular and transmembrane domains and is released in a soluble form. However, when RANKL1 or 2 is co-transfected with RANKL3, a reduced level of membranous localization is observed, indicating that RANKL3 prevents the membranous localization of RANKL. Thus, RANKL3 acts as an inhibitor of osteoclastogenesis [55]. Sr Ran decreases the membranous RANKL level and is associated with a decreased resorption activity of the cells. Furthermore, data showing a decreased membrane-associated RANKL level along with the higher production of OPG protein suggest a higher OPG/RANKL protein ratio, thus, a decreased resorptive activity of these cells [98].
The above mentioned are not the only possible mechanisms for the explanation of Sr effects in the bone. In the research on osteoblasts derived from CaSR knockout [CaSR(−/−)] and wild-type [CaSR(+/+)] mice, it has been shown that Sr Ran increases the cell replication in both [CaSR(−/−)] and [CaSR(+/+)] cells. This indicates that Sr Ran can act independently of the CaSR/ERK1/2 cascade to promote the osteoblast replication and thus CaSR is not the only receptor involved in the protective effect of Sr RAN on osteoblast apoptosis. The effects of Sr RAN on the osteoblast replication and survival involve ERK1/2 and serine/threonine-specific protein kinase (Akt) signalling and PGE2 production, characterized as an independent way of CaSR expression. These findings confirm two – CaSR-dependent and CaSR-independent – pathways of the Sr influence on osteoblasts, showing another possibility to modulate experimental/clinical osteoblastogenesis [39].
The fifth possible mechanism is related to the Sr modulatory effects on the selective osteogenesis inducing genes/their induction products. Recently, Geoffroy et al. [43] have demonstrated that Sr RAN effectively increases bone volume, cortical thickness and the number of trabeculae, while decreasing trabecular separation with an amelioration of bone microarchitecture leading to the improved bone strength and a reduced vertebral fracture risk, if applied on the mice overexpressing runt-related transcription factor 2 (Runx2), which leads to an increased RANKL expression, accelerated bone loss and spontaneous fractures.
Finally, one of the significant factors which are still under discussion is the influence of biomechanical strength on bone cells. During the physical activity, osteocytes undergo the activation by a fluid flow and they no longer stimulate osteoclastogenesis and produce factors (bone morphogenetic protein-2 (BMP2), Wnt3a, NO, and PGE2), which stimulate the osteoblast activity [51], [92]. Thus, osteocytes can contribute to the regulation of bone mass and structure by dictating the balance between bone formation and resorption in response to the mechanical stimulation. The combination of Sr Ran and osteocytes (mechanically stimulated by the pulsating fluid flow (PFF)) releases the signalling molecules involved in the regulation of bone turnover such as NO, PGE2 and OPG simultaneously with the inhibition of osteoclast number. Additionally, osteoblasts, stimulated only by PFF, increase the expression of proliferation marker Ki67, but decrease the secretion of Runx2 and the bone mineralization factor osteocalcin, at the same time decreasing the osteoclastogenesis 1.9 times. These findings highlighted the existing paracrine signal transduction way between osteocytes and osteoblasts, positively affected by Sr ions [8]. The morphopathogenetical mechanisms of the Sr RAN influence are not fully clear up to now and are found to be similar to those of Sr ions. As in the case of Sr ions, the main cell type influenced by Sr RAN is osteoblasts and their precursors whose mechanism of action is mediated by CaSR and the RANK/RANKL/OPG pathway [14], [18], [21], [23], [24], [27], [37], [41]. The activation of CaSR by Sr Ran activates Akt, and both Akt and canonical Wnts lead to the nuclear translocation of beta-catenin. Beta-catenin signalling in bone is then further enhanced by Sr RAN through down-regulation of the Wnt inhibitor sclerostin [91]. In addition, Sr RAN may signal through the fibroblast growth factor receptor in osteoblasts, lacking the Ca sensing receptor, which has been found in in vitro experiments with MC3T3-E1 human osteoblasts [26], [82].
Regarding the genes, in vitro research on bone marrow-derived stromal cells cultured for 21 days and exposed to Sr RAN, a significant time- and concentration-dependent increase in the expression of the osteogenesis gene, Runx2, and bone sialoprotein, but not osteocalcin, was detected. This indicates the Sr RAN cell type-specific gene expression on the background of common osteoblast differentiation. The biomechanical influence, in combination with Sr RAN, also mediates the response of osteocytes (osteocyte-depending osteoblast differentiation) with still not completely elucidated mechanisms. Thus, the PFF and Sr RAN combination is thought to alter osteocytes via a change in the cytoskeletal architecture, or a change in the expression of Cx43-containing hemichannels [42].
5. Sr/Sr RAN and osteoclasts
Osteoclasts are known to be activated by various factors, among which the most common are: pro- and inflammatory cytokines, glycation end-products, stress, ageing, and also biomaterial implantation with the subsequent first acute stage of the tissue response. Thus, from local inflammatory cytokines, produced mainly by macrophages, leading to the recruitment and activation of osteoclasts at the bone implant interface, interleukin-1 (IL-1), IL-6, tumour necrosis factor (TNF-α) are primarily mentioned [71]. Later data were focused only on TNFa and IL-1b as a factor that is able to induce the osteoclastic development and the decrease of osteoblast activity simultaneously [101]. Both inflammation and ageing released cytokines stimulate the expression of RANKL by osteoblasts, which contributes to the recruitment and activation of osteoclasts with the following bone loss [94].
Sr RAN belongs to the inhibitors of osteoclastogenesis; thus, Sr Ran treated osteocytes produce soluble factors for osteoclastogenesis suppression [8]. Despite the unknown mechanisms, nitric oxide (NO) is supposed to be involved in this osteoclastogenesis suppression [108]. There is another soluble product produced by Sr Ran treated osteocytes – osteoprotegerin (OPG). The combination of 3 mM Sr Ran and mechanical stimulation moves the RANKL/OPG ratio in osteocytes towards OPG, followed by the inhibition of osteoclastogenesis, especially in the areas subjected to high mechanical stress [8].
6. Sr/Sr RAN and apoptosis
Apoptosis is a programmed cell death which is thought to affect all types of supportive tissue cells. Sr inhibits osteoblast apoptosis and induces the terminal differentiation of osteoblasts into osteocytes, as demonstrated by the increase of sclerostin expression. This effect could play a pivotal role in the uncoupling of bone turnover induced by Sr RAN, since osteocytes can influence both osteoblast and osteoclast functions by producing paracrine signals [6].
When a person is not physically active, there will be less interstitial fluid flow around the osteocytes, and therefore osteocytes will either undergo the apoptosis or produce factors that activate bone resorption by osteoclasts, e.g.RANKL [1]. On the other hand, osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss, and/or inhibits the activity of bone forming osteoblasts (e.g. sclerostin) [89]. A special role in mediating of apoptosis is played by NO (Tan et al., 2008). Soluble NO expressed by the Sr Ran activated osteocyte could be involved in the osteocyte apoptosis regulation. At the same time, apoptotic osteocyte remnants likely trigger the osteoclastic bone resorption [56].
Of special interest in implantology are osteoclast apoptosis and Sr as its potential inducer. Since Sr ions have been found to control the osteoclast programmed death, a description of variable mechanisms for Sr raised apoptosis has also appeared. The mechanisms include protein kinase C signalling, an effect that is possibly mediated by CaSR, which is expressed in osteoclasts. Bone cells (osteoblasts, osteoclasts and osteocytes) harbour CaSR, a G-protein coupled receptor that is activated by extracellular divalent cations such as Ca and, with lower affinity, Sr. CaSR has been shown to induce osteoclast apoptosis [54].
Data obtained from the osteoarthritis (OA) animal model with an articular cartilage and subchondral bone research have shown that the high dose of Sr significantly decreases the chondrocyte apoptosis, and improves the expression of SOX9, a critical transcription factor responsible for the expression of anabolic genes type II collagen and aggrecan, after both 3 and 6 weeks. In contrast, the low-dose Sr does not significantly change the microarchitecture indices, mineral-to-collagen ratio and intrinsic mechanical properties of cartilage and bone after both 3 and 6 weeks. Summarizing these findings, it is obvious that the high dose Sr treatment can reduce the articular cartilage degeneration and subchondral bone remodelling in the rat OA model, but further research is still necessary in humans [104].
7. Sr/Sr RAN and stem cells
In the last decade, an increased number of studies on the Sr effect on the different types of stem cells have been performed. It has been observed that Sr increases the osteogenic potential and decreases the adipogenic potential of bone marrow stromal cells (BMSCs) in vitro [80].
Besides the osteogenic development from BMSCs, also the chondrogenic one is available from another pool of stem cells. Thus, supplementation of Sr ions in a chondrogenic medium (CM) promotes the early chondrogenic differentiation of dedifferentiated fat cells (DFAT cells) in vitro. The mRNA expression analysis has revealed that CM with 1.5 mM Sr increases the expression of chondrogenic marker, collagen type 2 alpha 1, whereas there is no significant change in osteogenic markers, collagen type 1 alpha 1, Runx-2, osteocalcin, hypertrophic chondrogenic marker and collagen type 10 alpha 1. Inhibitors for ERK1/2, Akt, and CaSR pathways significantly diminish the alcian blue staining intensity, providing the evidence that these signal pathways are associated with chondrogenic differentiation of DFAT cells. Thus, CaSR and ERK1/2 pathways independently induce the Sr-mediated early chondrogenic differentiation and all together these findings suggest that the Sr supplementation into CM may provide a powerful platform for preparing chondrogenically differentiated DFAT cells for cartilage regeneration [77].
Several studies have dealt with the search for the best concentrations of Sr and Sr Ran to induce the differentiation into the supportive tissue progenitors. A common view suggests that the minimal presence of Sr in the medium induces the differentiation of stem cells. 105–104 M SrCl2 supplementation has been described to enhance the proliferation of human primary bone cells [16]. Similar results are related also to animal cells. Bone marrow mesenchymal stem cells (BMSCs), isolated from 4-week-old rats and cultured in vitro within the Sr concentration range of 0.1–3.0 mmol/L, showed the dose-dependent increase of ALP activity in cells. ALP activity reached the highest level after the treatment with 3 mmol/L Sr, which also significantly promoted the formation of Ca nodules [66], indicating the best stimulating influence of Sr on the stem cells with an increase of concentration.
From the mechanisms to be involved in the osteogenic differentiation of MSCs, the upregulation of extracellular matrix (ECM) gene expression and the activation of the Wnt/β-catenin pathway are mentioned. Furthermore, a decreased expression of carbonic anhydrase and vitronectin receptor, two markers for osteoclastic bone resorption, was observed in chicken bone marrow cell cultures and they were supposed to play a role in suppression of osteoclastogenesis [10]. Finally, as an additional mechanism, also the involvement of the BMP-2/Smad pathway in Sr-induced osteoblast differentiation of rat BMSCs is mentioned.
Besides Sr, also Sr Ran alone strongly affects the differentiation of naïve stem cells [27], [103], even more notably than the pre-osteoblast differentiation. Thus, in vitro studies with human mesenchymal stem cells (hMSCs) if Sr Ran was used revealed the early expression of core binding factor alpha one (Cbfa1) gene (on the 4th day of cell culture; the control group showed the same on the 14th day) and osteonectin on the 11th day (control on the 21st day). These data indicate the enhanced osteoblastic differentiation (Cbfa 1) and bone remodelling (osteonectin). Moreover, the concentration of Sr Ran was recommended to be optimized between 0.2107 and 21.07 μg/ml, whereas the concentrations up to 210.7 μg/ml were found to delay the effect on osteoblastic differentiation with delayed expression on Cbfa1 and osteonectin, as well as the inhibitory effect on bone sialoprotein expression [96].
8. Sr/Sr RAN and in vivo animal research
There are numerous in vivo animal experiments showing the positive treatment effect of Sr therapeutic agents, especially Sr Ran, on the bone. In mice experiments, Sr is reflected by an increase in vertebral bone mass. Studies in rats have demonstrated that in addition to the increased bone mass, there is an increase in cortical thickness and an improvement in trabecular and cortical microarchitecture, leading to an increased bone quality and strength [2], [3], [63], [106]. In a model of hind limb immobilization, where bone resorption is high and bone formation is low, Sr RAN has been shown to “conserve” the bone mass [53]. A similar effect of Sr has been observed also in male mice, where the drug increases the vertebral body mass due to the stimulated bone formation and reduced resorption, as well as in adult primate species (Macaca fascicularis) where the resorption of alveolar bone is diminished [21]. However, there is no strict evidence of the assumption that Sr RAN directly impairs the mineralization of supportive tissue. In vivo, in rat calvarial cultures, Sr Ran has been shown to enhance the replication of pre-osteoblasts and collagen synthesis [22]. The effect of Sr ions has been also observed in ovariectomized (OVX) goats, receiving a low Ca diet and a supplement of Ca and Sr in different concentrations. The results revealed that the goats receiving a dose of 40 mg/day Sr with 100 mg/kg/day Ca had the highest number of insulin like growth factor (IGF) and osteogenesis stimulating gene Runx2, but the smallest inflammatory cytokine TNF-α expression. These findings confirm that the simultaneous administration of Sr-Ca ions improves the expression of osteogenic genes and new bone formation [65]. The osteoporotic rat model after 6 weeks treated with Sr Ran showed higher mechanical strength and fracture stiffness of tibia in comparison with the control group. Furthermore, callus maturity of the Sr Ran treated group was significantly higher than in the case of the control group [78]. A similar effect of Sr Ran was observed also in ovariectomized (OVX) rats with osteoporotic fractures. A study performed by Habermann et al. has demonstrated that only the treatment with Sr Ran leads to a significant increase in callus resistance compared to the OVX control rats, whereas Sr Ran increases the bone volume/tissue volume ratio of the callus [48]. Other studies, done by Bruel et al., have shown that the Sr Ran treated group of rats demonstrates a significantly higher callus volume in comparison with control animals. It has been also observed that Sr Ran enhances the callus formation but has no impact on the callus remodelling process [17]. The systemic administration of Sr RAN accelerates the healing of a bone defect created in rat proximal tibiae, with a significant effect on cortical thickness and on trabecular microarchitecture [105]. Sr is integrated both in cortical and in trabecular bone, and healing the defect. However, the effect of Sr RAN on fracture healing is dependent on bone quality. These results open up new perspectives for the use of Sr RAN in clinical studies as a pharmacologic agent with a potential beneficial effect on bone defect repair. Despite the desired effect of Sr RAN on fracture healing of animal models [64], [105], there are still large gaps in the understanding of the potential effect of Sr RAN on fragility fractures in osteoporotic patients [93].
9. Sr/Sr RAN and clinical research
The first clinical studies on the efficacy of Sr RAN in preventing osteoporosis-related fracture have been demonstrated in two randomized placebo-controlled phase 3 studies [70], [85]. Accordingly, a large prospective research study in the clinical field titled Spinal Osteoporosis Therapeutic Intervention (SOTI) and Treatment of Peripheral Osteoporosis study (TROPOS), involving 10,491 people in 75 centres in 12 countries, was carried out. Results obtained have verified that Sr Ran plays an important role in the gradual BMD increase as well as in the decrease of both vertebral and non-vertebral fracture risk after the Sr Ran (2 g/day) application within the period of 3 years [69], [70], [85]. The largest European observational studies ever of patients treated with Sr RAN have been described by [7]. An observational registry involved 32,446 women with post-menopausal osteoporosis in 7 countries. Follow-up over 32 months confirmed the good efficiency and safety profile of Sr RAN in the management of post-menopausal osteoporosis [7]. The clinical study of the higher-risk subgroup of osteoporotic women over the age of 80, who received the 2 g/day therapy of Sr Ran, showed that the treatment reduced the non-vertebral fracture risk in the elderly population preserving this benefit during the next 5 years [95]. In addition, the effect of Sr RAN on BMD in osteoporotic men was similar to those in post-menopausal osteoporotic women, supporting its use in the treatment of osteoporosis in men [58].Thirty three osteoporotic post-menopausal women were treated with 2 g/day Sr RAN for 6 months. The serum IGF-1, leptin and osteocalcin levels were measured before and after the treatment. The results revealed that the Sr Ran treatment significantly increased the level of IGF-1 (which is one of the most powerful growth inducers) [47], indicating the metabolic stimulation of tissues, including supportive tissue. There is little published preclinical data, which are supported by recent clinical case studies [63] indicate that there might be a clinical benefit of using Sr RAN to induce fracture healing in humans. The small case series of two and four patients with delayed union or non-union of long bone fractures reported radiographic signs of healing and clinical improvement following Sr RAN therapy [52]. However, Scaglione et al. reported that Sr RAN administered in acute cases of 40 patients older than 60 years does not improve and/or accelerate wrist fracture healing [93]. There is still no clear understanding of how Sr RAN acts to improve the bone strength in humans [68].
10. Dose-dependent effect of Sr/Sr RAN
It should be taken into account that while low doses of Sr have been shown to stimulate bone formation, high doses have deleterious effects on bone mineralization [44].
It has been shown by several authors that the content of Sr in the newly formed bone is strongly dose- dependent and a plateau in the global bone Sr content was reached after 3 years of treatment with Sr RAN [11]. The effect of Sr on the bone after the Sr RAN administration has been shown to decrease dramatically (almost by 50% within 10 weeks) [37], indicating that the Sr incorporation into the newly formed bone occurs mainly onto the biological HAp crystal surface by ionic substitution between Sr and Ca (Fig. 2) [33]. Summarizing these findings, it could be concluded that after the end of the Sr Ran treatment, the release of Sr by the bone could occur, thus the subsequent decrease of the bone mechanical properties in long term could be foreseen.
 Fig. 2. Sr Ran content in the newly formed bone as a function of time.
Fig. 2. Sr Ran content in the newly formed bone as a function of time.The common view of scientists suggests that while low doses of Sr stimulate bone formation, high doses have a deleterious effect on bone mineralization, through the decrease in Ca absorption and possible alterations of the bone mineral properties [44]. The changes in the bone mineral content vary especially if the Ca intake is reduced [45]. This effect seems to be caused by a combination of the impaired intestinal absorption of Ca and the reduced renal production of 1,25-dihydroxy cholecalciferol and was approved in the experiments with rats using variable peroral Sr doses [73]. Thus, if a constant Ca level in the diet was maintained, a significant increase in the bone Ca content was observed for the animals receiving 87.5 μmol/day Sr, but increasing the Sr dose up to 875 μmol/day, reduced bone Ca content and hypocalcaemia were observed.
Both Ca and Sr ions show equally intensive reabsorption from the intestine [72], but not if used simultaneously. If this is not considered, less Sr is reabsorbed and even its smaller amount reaches the bone. Thus, the best choice is the separate use of both Ca and Sr, [44], avoiding the “competition” between Sr and Ca for the reabsorption into the intestine and providing adequate Ca intake [40].
After the reabsorption, Sr affects the osteoblasts. In vitro studies have indicated that the amount of Sr is very important for bone producing cells [102]. If the cell culture medium is supplemented with 2–5 μg/ml of Sr, normal nodule formation and mineralization were observed, while other doses were not effective or, if exceeded, even demonstrated the inhibition of HAp formation. The significance of the dose was described well by Paracelsus – “Everything is poisonous and nothing is non-toxic, only the dose makes something not poisonous” and used regarding the Sr influence by Habibovic and Barralet [49].
The therapeutic dose of Sr Ran in humans is 2 g/day, which is suggested to appear safe and efficient for osteoporosis treatment as well as vertebral and non-vertebral fractures risk reduction. Even after a long-term treatment (up to 5 years), the quality of the bone mineralization (degree of mineralization and heterogeneity index) [12] is maintained at the tissue level [36].
On the other hand, the term “the optimal dose” seems to be individual for the species proven in animal experiments. For example, if 25 and 150 mg/kg/day of Sr Ran were used in OVX rats, the anabolic bone response was not stimulated, moreover, failed to improve the bone biomechanical properties. The serum levels of Sr produced by these dose levels in rats were similar to the levels produced in subjects treated with the clinical dose of 2 g/day [70]. More and more studies on the selective effect of the Sr ion concentration elucidated other possible selective pathways to improve the bone health. Thus, the combination of 3 mM Sr Ran and PFF has been shown to enhance the NO and PGE2 release from osteocytes (NO: 1.3-fold; PGE 2: 6-fold) almost equally to the static osteocytes factor expression (NO: 1.6-fold; PGE2: 2.8-fold) and to reduce osteocyte-stimulated osteoclastogenesis 4.3-fold (by PFF treated osteocytes – 1.9-fold) [8].