Abstract
Tissue-engineered constructs can replicate the structural and physiological properties of natural tissues. The constructs can be designed to address the transplantation issues affected by the shortage of donor tissues and organs. One of the major concerns in tissue engineering is the design and development of structures that improve the interaction between materials and cells and provide an ideal platform for cells to form functional tissue. Several contributing factors need to be considered to design and fabricate the constructs, including biomaterials, biological, topographical, biophysical, and morphological factors either alone or in combination. Here, we review the application, advancement, and future directions of these essential factors in designing and developing constructs for tissue regeneration. In particular, we focus on original approaches and engineering tools to design construct parameters in tissue engineering.
Graphical abstract
Keywords
Tissue engineering
Regenerative therapy
Biomimetic construct
Scaffolds
1. Introduction
In recent years, tissue engineering constructs have been considered a promising approach for the functional engineering of tissues due to the limited capabilities of the human body to regenerate its organs [[1], [2], [3]]. Tissue regeneration approaches for replacing or repairing damaged tissues consist of cell therapy, growth factors, and biomaterial scaffolds, either alone or in combination. The term “tissue engineering” as recognized today, was first introduced at a National Science Foundation panel meetingn in 1987. Tissue engineering as a medical field started in the early nineties when tissue engineering was defined as “an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain or improve tissue function” [4]. Whereas cell therapy involves the delivery of sole cells through systemic or local injection into the bloodstream or via direct transplantation io a local tissue, the tissue engineering paradigm broadly intersects the “regenerative medicine” as later defined in 1992 [5], but it is inherently founded upon three pillarsso-calleded “Tissue engineering triad” namely, cells, biomaterial scaffolds, and stimuli. Each of the three is fundamental. The matrix or scaffold helps the living cells and guides the development and regeneration of new living tissue. The scaffold can be a natural or synthetic origin, composite, or a mixture of both. The living cells can be seeded on the scaffold before implantation or can migrate into the acellular scaffold after implantation in the body. In any case, the cell behavior will be influenced by the microenvironmental stimuli induced by the scaffold.
The simplest strategy is to replace tissue with an analogous counterpart of identical or different origin. Natural tissue scaffolds, includinge autograft, allograft, xenograft, and decellularized extracellular matrix (ECM), are considered current standard treatments for healing large and massive injuries. Autograft is to engraft healthy autologous tissue from an uninjured site, reducing immunological rejection and transmission problems compared with allograft and xenograft [6]. Apart from the success of autograft transplantation, almost 10% of these procedures fail due to infection and necrosis [7,8]. Moreover, autografts present limitations such as donor morbidity and limited availability for severe injuries [6]. Decellularized ECM tissue has been explored as an alternative. However, these matrices often have lower mechanical properties than those observed in native tissue [9]. Also, limited vascularization of ECM matrices can limit the diffusion of nutrients and oxygen across the matrices and lead to fibrotic tissue deposition [9,10]. The application of the native ECM in the field of tissue regeneration has been discussed elsewhere [11,12].
Since current strategies cannot achieve satisfactory results for tissue regeneration, novel strategies are being developed to address current challenges. Several animal studies demonstrated the feasibility of developing a biomimetic scaffold to support the regeneration of damaged tissue [[13], [14], [15], [16]].
The engineered scaffold should provide appropriate functions for the tissue and restore the tissue with cellular phenotypic expression. Moreover, unlike bulk prostheses, scaffold, under an engineered porosity, counteracts the foreign body reaction (FBR), which occurs by the recruitment of M1 macrophages as a consequence of an inflammatory immune response and the possible formation of foreign body giant cells. In fact, if the biomaterial is bioinert, it will be surrounded by a fibrotic scar; if it is not, it will be degraded in time, which is not desirable using replacement prostheses [17]. Differently from bulk prostheses, the tissue engineering scaffolds are designed to be populated by cells and to degrade in a controlled manner, thus promoting the formation of a functional tissue.
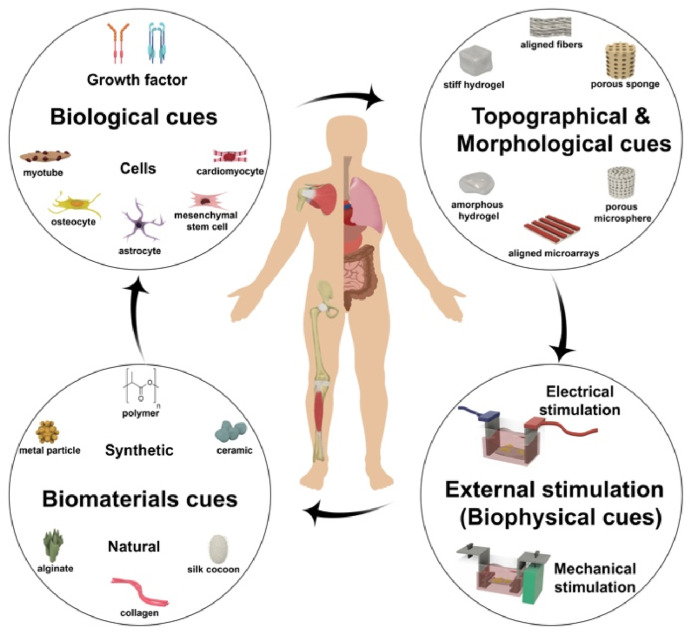
Along with genetic characters, the cell microenvironment inside the body determines cells’ function. The insight about the role of the microenvironment is essential to design and fabricate a scaffold mimicking the native ECM. The main function of the scaffold is to enable cell growth in the third dimension (3D), which is relevant in all tissues and organs. There are different approaches to fabricating a 3D biomimetic construct. In the first approach, the scaffold seeded with cells provides a template to direct host cells to create their own ECM in vitro, which can be performed in static or dynamic (i.e., in a bioreactor) culture conditions before being implanted [18,19]. In another approach, the acellular scaffold is fabricated as a medical device and implanted into the body, and then the living cells will be recruited from the body after implantation, which is called in vivo approach [[20], [21], [22]]. To engineer a functional scaffold that mimics the characteristic of native tissue, some essential physical or biological factors need to be incorporated into the scaffold to create a healing environment. Fig. 1shows a schematic illustration of the essential factors in the design and development of tissue engineering substitutes. The bioreactors can provide a controlled chemical, electrical, or mechanical environment for cell-seeded scaffolds or cells alone to grow and undergo the remodeling phase for in vitro studies or before implantation to enhance the characteristics of the construct and achieve success after implantation.
 Fig. 1
Fig. 1Designing scaffolds that can regulate cell behavior, including proliferation and differentiation, is a crucial aspect of tissue engineering scaffold fabrication. This review paper emphasizes the critical role of stimuli delivered by scaffolds to cells and focuses on recent advances in the development of artificial scaffolds for tissue engineering. By highlighting these key developments, this review underscores the importance of scaffold design in promoting optimal cellular behavior and advancing the field of tissue engineering.
2. Biomaterials
Engineered scaffolds must possess suitable physical properties, biodegradability, and biocompatibility to function as a biomimetic substrate for cell proliferation, differentiation, and migration. The scaffold's degradation behavior determines the biochemical factors released during the degradation process, which can impact tissue regeneration and inflammatory responses post-implantation. The scaffold's performance in supporting tissue regeneration is dependent on its biomechanical properties during this phase. Several studies investigated appropriate biomaterials that can provide mechanical, biological, topographical, and morphological properties to stimulate tissue regeneration [1,23,24]. The efficacy of an enormous variety of natural, synthetic, and hybrid biomaterials has been demonstrated for tissue regeneration [23,[25], [26], [27], [28], [29], [30], [31], [32]]. Fig. 2 shows the advantages and disadvantages of different biomaterials for tissue engineering.
 Fig. 2
Fig. 2As the main component of ECM (i.e., about 25% of total body protein in vertebrates), collagen has been the most popular biomaterial to be explored in tissue engineering. The majority of medical-grade collagen and gelatin, a denaturized form of collagen, are from animal origin, namely bovine and porcine tissue from controlled origins. Human collagen is obtained by biotechnology, isolated from human tissues (e.g., lung and placenta), or from human cell culture [50]. In addition, collagen can be modified and crosslinked to control biodegradation. Originally, collagen-based biomaterials have been extensively investigated for skin regeneration [51]. There are different types of collagen-based materials for wound healing that have been translated into clinical trials, such as injectable Woun’Dres Collagen Hydrogel [52], collagen sponges [53], Matriderm [54], DenovoSkin, Transcyte [55], and Engineered Skin Substitute [56]. Boyce et al. reported the efficacy of collagen-glycosaminoglycan-based scaffolds seeded with autologous keratinocytes and fibroblasts to reduce the need for donor skin and decrease mortality in patients with more than 50% burn of the total body surface area [57]. The scaffolds were prepared from split-thickness skin biopsies collected from 16 pediatric burn patients and applied to 15 patients enrolled in the study during 2007–2010 and were monitored for twelve months for data collection. The results demonstrated that this treatment provides available autologous skin substitutes for closure and grafting full-thickness burns while reducing donor morbidity and mortality in pediatric burn patients [57].
Studies exhibited that the combination of synthetic, natural, and other biocompatible materials could be considered as an ideal approach to overcome the current limitations of a single material and improve the behavior of the scaffold in a biological environment [[58], [59], [60], [61], [62], [63], [64]]. For example, Danti et al. investigated the possible advantages of poly(ethylene oxide terephthalate)/poly(butylene terephthalate) (PEOT/PBT) electrospun fibers loaded with chitin nanofibril (CN), in tympanic membrane scaffolds application. The scaffold was then coated with CNs via electrospray technique. Biological results showed good cytocompatibility and anti-inflammatory property which make these scaffolds a promising material for tympanoplasty [65]. De la Ossa et al. developed different types of polyhydroxyalkanoate (PHA) electrospun fibrous scaffold loaded with olive leaf extract (OLE) as anti-inflammatory and anti-bacterial agents for wound healing and tissue skin regeneration applications. The results demonstrated that PHA fiber meshes reduced pro-inflammatory cytokines and the presence of OLE may enable indirect antibacterial properties, which is necessary for wound healing and tissue regeneration [66].
Rothrauff et al. [58] reported the regenerative potential of PLLA and PCL fibrous scaffolds upon seeding the scaffolds with stem cells for ligament and tendon regeneration [58]. They showed the ability of the multilayered scaffolds in the forms of stacked or braided to induce tenogenic gene expression, improve mechanical properties, and increase suture retention strength [58]. Moreover, studies showed the promising approach of using advanced conductive materials combined with synthetic or natural polymers to mimic the cellular properties and enhance tissue regeneration of excitable tissues such as cardiac, nerve, and skeletal muscle [[67], [68], [69], [70], [71], [72], [73], [74], [75]]. For example, Patel et al. conducted a research where they engineered polymeric scaffolds to simultaneously administer topographical and electrical cues to mouse neural stem cells (mNSCs). They utilized a straightforward method employing aligned electrospun fibers as templates to generate microgroove patterns, a process termed electrospun fiber-template lithography. Polyvinylpyrrolidone fibers were prepared initially, and subsequently, poly(lactic-co-glycolic acid) (PLGA) was deposited onto them. The removal of the fiber template resulted in the production of freestanding PLGA scaffolds with microgrooves measuring 1.72 ± 0.24 μm in width. Subsequently, they applied polypyrrole (PPy) through chemical oxidative polymerization, imparting conductivity to the microgrooved PLGA scaffolds. Their findings suggest that the combined influence of electrical stimulation and topographical guidance provided by PPy-coated microgroove PLGA scaffolds holds promise for neural tissue applications [68].
The new approach in tissue engineering is based on the application of smart and bioactive scaffolds, since different tissues of the human body, such as bone, muscles, and nerves, have been shown to possess electrical activity and even piezoelectricity [76].
The application of smart piezoelectric biomaterials may be considered a promising route to promote tissue regeneration in a biomimetic way [77]. Indeed, due to body movements or cell attachment and migration, such devices are able to produce transient surface charge variations and subsequently electrical potential variations to the material with no need for extra energy sources or wired electrodes. Piezoelectric polymers such as PVDF and its copolymers have demonstrated a high potential for novel tissue engineering strategies and have been used in different piezoelectric tissues [78,79]. Several studies have been conducted to fabricate a composite scaffold for tissue regeneration, presented in Table 1.
| Tissue | Morphology | Biomaterials | Model | Biological factors | Topographical factors | External stimulation factors | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell | Growth factor/Cytokine | Roughness | Pores and spheres | Alignment | Electrical stimulation | Mechanical stimulation | |||||
| Tendon/ligament | Fibroblast-like | Fibroblasts that secrete collagen proteins | In vitro & In vivo, Rat | hBMSC Secretome | [80] | ||||||
| Nanofibers | PLGA | In vivo, Rat | ASCs | BMP-2 | Aligned nanofibers | [81] | |||||
| Nanofibers | Polycaprolactone (PCL)/silk fibroin | In vivo, Rabbit | Rabbit Dermal Fibroblasts | Aligned nanofibers | [62] | ||||||
| Hydrogel/Nanofibers | Polycaprolactone (PCL)/nylon-6 (PA6)/alginate (Alg)/GelMA | In vitro, MSCs | BMP-12 | Dynamic stretch | [82] | ||||||
| Hydrogel yarns | Alginate/Gelatin methacryloyl (GelMA) | In vitro (hBM‐MSCs) | BMP‐12 | Aligned filaments | Static Stretch | [83] | |||||
| Nanofibers | Trichostatin A/Poly(L-lactic) acid (PLLA) | In vitro (Mouse tail tendon cells)/In vivo, Rat | Aligned nanofibers | [84] | |||||||
| Nano/microfibers | Polycaprolactone (PCL)/PLA | In vitro (HADMSC/HT/HUVEC) | Transforming growth factor beta (TGFβ)3 | 12.2 ± 1.1 μm | Aligned structure | Dynamic stretch | [85] | ||||
| Nanofibers | PEU | In vivo, rat | Platelet-rich plasma (PRP) | [86] | |||||||
| Nanotopographic | Polycaprolactone (PCL) | In vitro & In vivo, Rbbits | Aligned Nanotopography | [87] | |||||||
| Braided fibers | Polyethylene terephthalate (PET)/Poly(L-lactic) acid (PLLA) | In vivo, rabbit | BMP-2, FGF-2, and FGF-8 | [88] | |||||||
| Sponge | Polycaprolactone (PCL) | In vivo, rabbit | MSCs | 215 μm | [89] | ||||||
| 3D printed | Polycaprolactone (PCL)/PLGA | In vitro, Rabbit BMSC | IGF-1 | 507–523 μm | [90] | ||||||
| Hydrogel | Gelatin | In vivo, Rat | FGF-2 | [91] | |||||||
| sponge | Alginate | In vivo, rabbit | TGF-β1 | [92] | |||||||
| Bone | Microsphere | chitosan/PLAGA | In vitro (MC3T3) | 170–200 μm | [93] | ||||||
| 3D porous | Polycaprolactone (PCL)/Bioceramic | In vitro (MG63 osteoblast-like cells) | pore size 250–400 μm | [94] | |||||||
| sponge | atelocollagen | In vivo, Rat | hMSC Secretome | [95] | |||||||
| Membrane | collagen | In vivo, Rat calvaria defect model | MSCs | BMSCs Secretome | [96] | ||||||
| 3D printed | Poly(lactic) acid (PLA), polyethyleneimine | In vivo, Rat | human gingival mesenchymal stem cells | [97] | |||||||
| 3D printed | Polycaprolactone (PCL)/β-tricalcium phosphate (β-TCP) | In vitro (hMSCs) | 350–400 μm | Intermittent Hydrostatic Pressure | [25] | ||||||
| 3D printed | Gelatin methacrylate (GelMA)/polycaprolactone and hydroxyapatite (PCL-HA) | In vitro (C3H cells), In vivo, Rabbit | interleukin-4 (IL-4) | 100–200 μm | [98] | ||||||
| composite | HA/β-TCP microsphere/poloxamer 407 hydrogel | In vitro (enzyme-linked immunosorbent assay (ELISA))/In vivo, rat | MC3T3-E1 | rhBMP‐2 | [99] | ||||||
| sponge | PPY/Polycaprolactone (PCL) | In vitro (AD-MSCs) | DC, 0–250 μA | [100] | |||||||
| Membrane | Hydroxyapatite | In vitro (BMSCs) | 0.2–1.65 μm | [101] | |||||||
| Nanofibers | Poly(Lactic) acid (PLA)/PANi | In vitro (BMSCs) | 91%–93% | [102] | |||||||
| Granules | β-TCP | In vivo, Rat | ADMSC | 0.1–0.2 μA of DC | [103] | ||||||
| 3D printed | methacrylated‐oligocaprolactone‐poloxamer/calcium‐phosphates | In vivo, horses | 40.03%–51.14% | [104] | |||||||
| Nanofibers | Poly(L-lactic) acid (PLLA), Polydopamine, Bio-minerals |
In vitro) hADSCs) In vivo, Mouse |
hADSCs | PDGF | [105] | ||||||
| Muscle | Nanofibers | Poly(L-lactic) acid (PLLA) and Graphene |
In vitro (C2C12, ADSCS) In vivo, Rat |
[74] | |||||||
| Membrane | poly (citric acid-octanediol-polyethylene glycol)(PCE)/reduced graphene oxide (RGO) | In vitro ((C2C12)/In vivo, Rat | [106] | ||||||||
| 3D fascicle-inspired muscle construct | Myoblasts/fibrinogen/Matrigel | In vitro (C2C12) | Aligned fibers | 0.1–0.5 Hz, 2.5 V/mm | Mechanical stretching | [107] | |||||
| Membrane | sodium alginate (SA)/polycaprolactone (PCL)/reduced graphene oxide (rGO) | In vitro (C2C12) | [75] | ||||||||
| Nanofibers | Collagen/hyaluronic acid/PANI | In vitro (H9c2 and C2C12 myoblast) | Aligned nanofibers | [108] | |||||||
| Nanofibers | Polycaprolactone (PCL)/PEDOT:PSS | In vitro (C2C12) | Aligned nanofibers | [23] | |||||||
| Nanofibers | multiwalled carbon nanotubes/Polycaprolactone (PCL)/polyvinyl alcohol (PVA)/polyacrylic acid (PAA) | In vitro (rat muscle cells) | Core-shell nanofibers | [72] | |||||||
| Nanofibers | Poly (ethylene oxide terephthalate)/poly (butylene terephthalate) | In vitro (hMSCs) | 14.3 ± 2.5 nm_71.0 ± 11.0 nm | 27.2–230.5 nm | [109] | ||||||
| sponge | Alginate | In vivo, Rat | BM-MSCs | 70–150 μm | [110] | ||||||
| Cardiac | microparticles | PLGA | In vivo, Mouse | hMSC secretome | [111] | ||||||
| Nanofibers | rGO/silk fibroin | In vitro (rat cardiomyocytes) | Aligned nanofibers | 5 V/cm | [29] | ||||||
| Injectable hydrogel | Gelatin and Laponite | In vivo, Rat | hASCs secretome | [112] | |||||||
| Sponge/Membrane | Collagen/Alginate | In vitro (Rat Cardiac stem cells) | HGF/IGF-1 | mean pore size >100 μm | [113] | ||||||
| Microspheres | Gellan/gelatin |
In vitro (rat cardiac progenitor cells) In vivo, Rat |
rat cardiac progenitor cells | IGF-1 | 66 ± 17 μm (dry conditions) & 123 ± 24 μm (wet conditions) | [114] | |||||
| Smooth muscle | Sponge/Nanofiber | cellulose acetate/chitosan/silk fibroin | In vitro (smooth muscle cells (SMCs)) | 263.1 ± 75.7 μm | [115] | ||||||
| Sponge | DegraPol | In vitro (human smooth muscle cells) | 100–300 μm | [116] | |||||||
| Sponge | PPy/poly (trimethylene carbonate) | In vitro (ASC) | 38–250 μm | 1 ms, 10–60 mV | [117] | ||||||
| Vascular | Membrane | Poly(ethylene glycol)(PEG)/decellularized scaffold/heparin–chitosan polyelectrolyte | In vitro, In vivo (Pig) | [118] | |||||||
| Porous | Titanium | In vivo, rabbits | 261, 480 and 668 μm | Mechanical Traction | [119] | ||||||
| Sponge | Poly(ethylene glycol) diacrylate/Pluronic F-127 | In vitro (Human umbilical vein endothelial cells) | VEGF and bFGF | 51 ± 19–56 ± 17 μm | [120] | ||||||
| Breast cancer | 3D printed | dopamine-modified alginate and polydopamine | In vitro (human normal breast cells)/In vivo, Mouse | [121] | |||||||
| Nerve | Nanofiber | polylactic acid (PLA)/polyaniline (PAni) | In vitro (pheochromocytoma (PC-12)) | [122] | |||||||
| Nanofiber yarns | poly(p-dioxanone) (PPDO) biopolymer and carbon nanotubes (CNTs) | In vitro (rabbit Schwann cells (rSCs) and human adipose-derived mesenchymal stem cells (hADMSCs)) | 1 h per day, 0–500 mV/mm | [123] | |||||||
| Cytodex 3 microcarriers | Dextran and denatured porcine-skin collagen | In vivo, Rat | hMSC secretome | [124] | |||||||
| Hydrogel | collagen | In vitro, dorsal root ganglia (DRG) | beta-nerve growth factor (βNGF) | [125] | |||||||
| Nanofibers | PLGA/multiwalled carbon nanotubes (MWCNTs) | In vitro (S42 and PC12 cells) | nerve growth factor (NGF) | Aligned nanofibers | 40 mV for 30 min | [126] | |||||
| Nanofibers | PPy-Graphene/PLGA | In vitro (retinal ganglion cells) | Aligned nanofibers | 0.1–1 V/cm | [69] | ||||||
| Nanofibers | Poly(Lactic) acid (PLA)/PPy | In vitro (PC12) | Aligned nanofibers | 40 mV for 30 min | [67] | ||||||
| Nanofibers | Poly(Lactic) acid (PLA)/PPy | In vitro (human umbilical cord MSCs) | Aligned nanofibers | 100 mV/mm | [127] | ||||||
| Skin | Composite | Chitosan/graphene | In vitro (NIH3T3 cells), in vivo (rat skin wound model) | basic fibroblast growth factor (bFGF) and ponericin G1 (PonG1) | 100 mV/cm | [128] | |||||
| Sponge | Collagen/gelatin | In vivo, Mouse | bFGF/hepatocyte growth factor (HGF) | [129] | |||||||
| Nanofiber | poly(ethylene argininyl aspartate diglyceride)/PLGA |
In vitro (CCL-64, fibroblast, HUVEC), In vivo (mouse) |
VEGF and TGF-β3 | [130] | |||||||
| Lipis nanoparticles | Lipids/Poloxamer 188 |
In vivo (diabetic rat), In vitro |
NIH 3T3 fibroblasts and HaCaT keratinocytes | Epidermal growth factor (EGF)/curcumin (Cur) | [131] | ||||||
| Fabric |
In vitro: polypyrrole/polyethylene terephthalate in vivo: collagen matrix (Zimmer) |
In vivo (mouse), In vitro (primary skin fibroblasts) |
primary skin fibroblasts | pulsed EF, 100 mV mm− 1, 10 s within 1200 s or a 300 s within 600 s for 24 h. | [132] | ||||||
| Sponge | cellulose nanofiber/poly (vinyl) alcohol (CNF/PVA) | In vitro (fibroblast cells) | 88–95% and 20–90 μm | [133] | |||||||
| Lung | Sponge | Bovine dermal collagen | In vitro (A549 and RLE-6TN), in vivo (Rat) | hepatocyte growth factor (HGF) | 79.84% and 52.6 μm | [134] | |||||
| Nanofiber | poly(vinylidene fluoride-co-trifluoroethylene)/zinc oxide | In vitro (A549) | 85%–94% | [135] | |||||||
2.1. Biological factors
One of the main considerations for engineering a scaffold is to decide whether the scaffold is supposed to be a vehicle to deliver cells, growth factors, and biological factors or whether the scaffold alone will promote the tissue regeneration process. It is essential to consider the clinical demands when designing delivery-based scaffolds. To regenerate functional tissue, a similar cellular environment needs to be created for the cells [136]. Several studies reported the efficacy of bioactive materials for tissue regeneration and host integration. The biological factors can be incorporated into the engineered scaffolds through several physical and chemical techniques. They can be blended/encapsulated into the scaffold adsorbed/bound to the surface, and covalently conjugated to it.
In this section, the importance and critical requirements of biological factors for tissue regeneration, such as growth factors, and cell-based therapy, will be discussed.
2.1.1. Growth factors
Studies reported that the incorporation of growth factors could promote the therapeutic effects of the scaffolds [[137], [138], [139], [140], [141]]. The tissue regeneration process is regulated by complex signaling networks, including different growth factors, cytokines, and chemokines. Among them, some factors showed a significant role in regenerative engineering, including the transforming growth factor-β (TGF-β) [[85], [92], [130]], fibroblast growth factor (FGF) [91,120], hepatocyte growth factor (HGF) [[113], [129], [134]], vascular endothelial growth factor (VEGF) [130,120], insulin-like growth factor (IGF) [114], platelet-derived growth factor (PDGF) [105], nerve growth factor (NGF) [142,125], connective tissue growth factor (CTGF) [143,144], and interleukins (ILs) [98].
Besides the regenerative ability of growth factors, some of the critical parameters need to be controlled, such as the type of growth factor, dosage, and release profile at the repair site (Fig. 3).
 Fig. 3
Fig. 3It is essential to select the appropriate growth factors for a specific tissue. For example, angiogenesis is mainly regulated by the tissue oxygen level and tissue growth factors like hypoxic and chemokines [145,146]. The type of growth factor has a critical role during the tissue regenerative process. Several studies demonstrated the efficacy of using growth factors such as connective tissue growth factor (CTGF), TGF, and stromal-derived factor (SDF) to promote cell activity and angiogenesis In particular, a number of studies have highlighted the significant effects of CTGFs on endothelial cell proliferation, adhesion, migration, and vascularization [147]. Research showed a promoted viability of the endothelial cells in CTGFs-loaded scaffolds compared with scaffolds alone [143,144].
The osteoinductive effects of IGF-1, EGF, and VEGF on human Mesenchymal Stem Cells (hMSCs) by increasing the alkaline phosphatase (ALP) activity and mineralization have been shown in vitro [148]. In addition, these growth factors can show significant effects on other tissues. For example, IGF-1 has a significant role in osteogenesis, cardiogenesis, and tenogenesis [114,90]. Rosellini et al. formulated injectable microspheres using a combination of two natural polymers (gellan and gelatin) for cardiac repair. The microspheres were produced via water-in-oil emulsion and were further functionalized by loading with IGF-1. In vitro cell culture experiments demonstrated that these microspheres facilitated the adhesion of rat cardiac progenitor cells and the formation of clusters, indicating promising attributes for potential utilization as injectable scaffolds in cardiac tissue engineering endeavors [114].
Moreover, studies suggested the regenerative potential of multiple growth factors for tissue regeneration [130,120,129,[88], [131], [149]]. Ogino et al. investigated the efficacy of the dual-controlled release of hepatocyte growth factor (HGF) and the basic FGF (bFGF) infused with a collagen/gelatin scaffold using a mouse model of skin defects. Based on the results, the neo-epithelium length and wound closure were improved in the HGF-low dosage group compared with the normal saline solution group one week after the surgery. Two weeks after surgery, the bFGF and HGF + bFGF groups promoted wound closure, dermis-like tissue formation, neo-epithelium length, and newly formed capillary formation compared with the normal saline solution and HGF-high dosage groups. Also, the results showed the significant formation of the newly formed capillary in the HGF + bFGF group compared with the bFGF group [129].
The appropriate dosage and the release profile of the growth factors in the body are other important factors in controlling tissue regeneration during angiogenesis, myogenesis, osteogenesis, chondrogenesis, and neurogenesis [129,150]. For instance, appropriate dosages of BMP-2 and VEGF can change their efficacy during osteogenesis and angiogenesis [150]. Studies showed that using a smaller dosage of BMP-2 to induce osteoinductivity recruits stem cells via chemotaxis, while the higher dosage in the range of microgram has a differentiation stimuli role that has a direct effect on endochondral ossification [150]. Besides the dose dependency, the release profile of the growth factors is a major concern in tissue regeneration. Lipner et al. demonstrated that long-time presence and sustained release of BMP-2 in ASCs-seeded scaffold reduced the mechanical properties of the tendon-bone interface and caused bone resorption [81].
It has also been revealed that functional blood vessels can be regenerated through the slow and sustained release of VEGF, while an uncontrolled release showed a reverse effect [150].
Despite all the conducted studies to improve the efficacy of scaffolds by growth factors, the accessibility and cost of growth factors are still controversial and need to be addressed.
2.2. Cell-based therapy
Cell-based therapies have been considered a promising strategy for tissue regeneration. The therapeutic benefits of cell therapy have been demonstrated in various organs and tissues, such as the heart [[111], [151], [152], [153]], lungs [[154], [155], [156], [157]], intestine [[158], [159], [160]], liver [161], and the musculoskeletal system [80,162]. One of the critical challenges in these strategies is to choose the right cell source among autologous, allogeneic, or xenogeneic cells. Although allogeneic or xenogeneic are cost-effective and more efficient for the fabrication of an off-the-shelf scaffold, using autologous cells avoids immune rejection and consequently the need for immunosuppressive medicine. To address the current challenges in cell-based therapies, stem cells have attracted attention to be considered the primary cell source.
Pumberger et al. reported the regenerative ability of the rat bone marrow-derived MSCs (BM-hMSCs) for muscle regeneration in a rat model of traumas to the left soleus muscle [110]. Bone marrows were isolated from rat tibia and seeded onto the porous alginate scaffold fabricated by lyophilization with pore sizes ranging in 70–150 μm. The porous scaffold was utilized as the cell delivery vehicle to ensure the local delivery of hMSCs at the injury site. Based on the results, muscle treatment with hMSCs improved muscle fiber density and the number of regenerated fibers compared with control groups without cells at 4 weeks post-implantation [110].
Several studies show that the therapeutic effects of stem cells could be attributed to their secretome. For example, it has been reported that the therapeutic reason for cell-based strategy for neurological and brain disorders, such as multiple sclerosis (MS) and Parkinson's disease (PD), is attributed to the secretome secretion of stem cells [[124], [163], [164], [165], [166], [167]].
Also, it has been shown that MSCs can significantly reduce muscle degeneration in tendon-muscle injuries by promoting cell proliferation, reducing inflammation, and modulating the immune system [162]. Flück et al. examined the ability of injected autologous MSCs to prevent muscle degeneration via the stimulation of myogenesis and suppressing adipogenesis in the ISP muscle following delayed tendon repair. As the result, they observed that injecting autologous MSCs into degenerated rotator cuff muscles could stop the conversion of muscle to fat during the recuperation from tendon repair. This preservation of fat-free mass was achieved through extracellular reactions, which halted adjuvant-induced muscle fiber hypertrophy [162].
The potential of several synthetic and natural biomaterial-based scaffolds has been investigated as secretome delivery vehicles [80,[96], [97], [168], [169]]. Chen et al. have found that secretome of human bone marrow-derived stem cells (hBMSC) could improve tendon–bone healing through regulating macrophage polarization in a rat model of rotator cuff repair which was relied on its immune-regulative property [80]. In another study, Diomede et al. reported the beneficial effects of 3D-printed PLA and polyethyleneimine (PEI) scaffold incorporated with human gingival stem cell-derived extracellular vesicles on bone tissue regeneration in a rat calvarial bone defect [97]. The results demonstrated new bone formation, vascularization, and the potential of the construct to repair the defect [97].
Moreover, studies have also reported the efficacy of secretome for soft tissue regeneration. Recently Luo et al. investigated the therapeutic potential of the secreted secretome from the human bone marrow-derived mesenchymal stem cell in the mice model of myocardial infarction [111]. Injection of two-layer microparticles, including MSC-conditioned media/PLGA as the core and MSC cell membrane as the shell in the mice model with acute myocardial infarction demonstrated enhanced angiogenesis and myocardial functions [111].
Despite all promising outcomes, some challenges need to be addressed to translate cell-free-based therapies to the clinic. There is a need to investigate the ability to scale up the manufacture of this strategy at a commercial scale. Moreover,the standard procedures for cell isolation, culture, and purifying need to be developed.
2.3. Topographical factors
The surface topography of the engineered scaffold is another critical factor that can modulate the scaffold's biological response and clinical success. The topography of implant materials plays an important role in directing stem cell fate. In 1996, Recum et al. initially defined topography as the morphology of the surface and categorized it to surface roughness and texture [170]. Random size and distribution of surface hills and pits change the surface topography through roughness. However, changing the surface morphology through grooves, pores, ridges, porosity, and other configurations, can create surface texture [170].
Studies reported the roughness quantifications by measuring the protrusions or depressions at the surface [[101], [109], [171], [172], [173]]. The surface roughness can have significant effects on cell adhesion, migration, proliferation, and differentiation. For example, the microscale surface roughness has been recognized to alter the osteogenesis of stem cells [101,109]. By optimizing the roughness scale of the material surface to roughness (Ra) ≈ 0.77–1.09 μm, bone formation can be highly induced [101].
Besides different types of surface configurations, the orientation of surface texture can also be categorized as isotropic (no orientation, for example, randomly distributed fibers) or anisotropic (a clear orientation such as ridges, aligned fibers, and grooves). The anisotropic surface has been studied in terms of a tool to direct cell alignment, which often influences stem cell fate. The isotropic surface is not determined to influence cell alignment; instead, it is proved to control cell function. The surface texture can be created as either an anisotropic or an isotropic surface and modulate stem cell differentiation to target phenotype. Besides, it has been reported that the distribution of topographical features also influences cell response [[101], [109], [172], [173], [174]]. In this section, the most common types of surface texture that have been shown to affect cellular behavior in vitro and tissue response in vivo will be discussed.
3. Pores and porosity
Pore size, porosity, and pore interconnectivity are critical structural properties of the scaffold and significantly affect its function [134,175,176]. The smaller pores can create a higher density that helps intracellular signaling, cell attachment, and structural strength. In comparison, larger pores help dispose of waste, gas diffusion, and supply of nutrients, resulting in tissue ingrowth and vascularization [85,109,119,94]. Therefore, an optimum size needs to be selected to provide nutrient transfer and mechanical strength during the regeneration phase of new tissue [150,177,178]. Similarly, higher porosity facilitates the nutrient exchange and release of bio-factors like genes and proteins. However, high porosity sometimes reduces the structural stability of the scaffold [179,109,94]. For efficient cell proliferation, infiltration, migration, and providing nutrients, pores need to be available and interconnected at the same time. A porous surface provides better mechanical integrity between tissue and the scaffold. Moreover, interconnected pore networks help with the stimulation and growth of new tissues [85,134,179].
Some tissues, such as bone and skin, show a gradient porous structure (GPS) that provides optimum performance [104]. For example, GPS in bone tissue helps its physical strength and loading capability [104]. This gradient structure results in mechanical stability and optimum porosity at the same time and is considered one of the ideal designs for tissue engineering [1,179]. GPS facilitates the migration of cells during regeneration, and it is one of the major factors in osteochondral tissue engineering for articular cartilage defects treatment [180].
Studies recommended wide ranges of pore sizes for different types of tissues. For example, a wide range of 20–1500 μm is used for osteoblast activity in bone tissue-engineered scaffolds [94,93]. The minimum required pore size in blood vessels is about 30–40 μm [120]. This range of sizes facilitates endothelial cell entrance and creates a path for metabolic components [120,179,119,94,116]. Studies reported novel strategies to predict, measure, and adjust the pore size distribution within the scaffolds [3,181,182].
3.1. Structure alignment
Tissues with highly aligned structures such as tendons, ligaments, skeletal, cardiac, and blood vessels show the alignment and migration of the cells along the direction of collagen fibers [1,23,172,174,183]. Studies demonstrated that the aligned topography of the engineered scaffolds can significantly induce cellular elongation, alignment, and differentiation and guide the cell migration along the direction of the scaffold [1,23,85,172,173,174,184,108]. The scaffold can be fabricated with highly aligned structures through topographical patterns such as ridges/grooves, wrinkles, pillars, and fibers in nano- or micro-scale [172]. Based on the literature, the micro-scale crinkles show more effects on cell attachment and migration, while the nano-scale crinkles significantly induce cell alignment. Moreover, it has been demonstrated that cell alignment can be affected by changing the width, depth, and geometries of grooves, pillars, and fibers [184,185,186].
The efficacy of multilayered scaffolds composed of highly aligned poly(l-lactic acid) (PLLA) nanofiber yarns was investigated by Li et al. [174]. According to the results, the engineered scaffolds with topographic factors showed higher mechanical properties, promoted H9C2 cell proliferation, and guided the cell orientation. Also, the controllable pore structure of the scaffolds allowed the cell to penetrate through the thickness of the 3D scaffold and distribute uniformly in each layer [174].
Liu et al. investigated the efficacy of controllable surface topography by fabricating a core-shell fibrous scaffold [172]. The scaffold was fabricated by electrospinning polycaprolactone (PCL) nanofibers onto polyglycolic acid (PGA) microfibers. Based on the results, A-PCLs group (a shell of aligned PCL nanofibers on the core of PGA yarn) with appropriate surface topography of PCL nanofibers and mechanical properties of PGA microfibers showed higher structural properties and promoted the adhesion and proliferation of BALB/3T3 (mouse embryonic fibroblast cell line). The cells presented an irregular oval form on the R-PCL scaffolds while significantly higher aspect ratio, attachment, and proliferation were observed along the A-PCLs [172].
The aligned structure as a topographical cue can be applied to the scaffold combined with other stimulation factors such as electroconductive biomaterials. Roshanbinfar et al. designed a composite fibrous mesh intended to mimic the cardiac ECM in microstructure and electrical conductivity. To achieve this goal, they fabricated electrospun fiber mats using collagen, hyaluronic acid and PANi as protein, polysaccharide, and a synthetic polymer respectively, at varying concentrations. These fiber mats demonstrated electrical conductivity similar to and superior mechanical properties compared to the native human myocardium. Analysis of cell-matrix interaction using postnatal rat cardiomyocytes indicates that the fiber mats are non-cytotoxic, facilitating cell attachment and contraction. Their findings proved the capability to tune various characteristics of the fiber mats, such as morphology, fiber alignment and diameter, mechanical properties, and electrical conductivity, by adjusting the composition of the fiber mats [108]. The significant effects of fiber alignment have also been reported on human adipose-derived mesenchymal stem cells (HADMSC) and human tenocytes (HT) differentiation and tendon-specific gene expression [85].
3.1.1. External stimulation factors (biophysical factors)
Tissue engineering aimsto mimic the cellular microenvironment to provide essential factors and guide the cells to regenerate into functional tissue. The cellular environment in the body provides critical biophysical signals to modulate cell activity, such as electrical signals, fluid flow, and dynamic biomechanical signals.
The human body generates endogenous electrical potential that plays an important role in wound healing and tissue regeneration. This electrical potential guides cell migration and supports tissue healing. Several strategies have been conducted to provide these stimulation factors and enhance cell growth for tissue regeneration (Table 1).
Besides electrical stimulation, it has been shown that mechanical stimulation through bioreactors can significantly enhance tissue formation, especially for the regeneration of bone, cartilage, blood vessels, skeletal and cardiac muscle [25,85,[82], [83], [187]].
3.2. Electrical stimulation
External electrical stimulation is an approach that induces an electrical charge in cells to mimic the natural endogenous electrical potential [188]. External electrical stimulation has extensively been used in both clinics and research labs to treat neuronal diseases, muscular diseases, and wound healing [5]. Several studies investigated the effects of external electrical stimulation on different cell types such as, mesenchymal stem cells (MSC), myoblasts, fibroblasts, osteoblasts, and neural cells [29,67,69,139,[100], [103], [117], [126], [127], [128], [132], [189]]. Their results showed the regenerative effects of electrical stimulation on cell adhesion, proliferation, differentiation, elongation, and migration.
Tissues and organs in the body show different electrical resistivity due to the differences in tissue composition, tissue type, tissue density, cell membrane permeability, and electrolyte content. Owing to these variations in electrical resistivity, the method by which the electrical stimulation is applied must be optimized and adjusted to achieve the desired therapeutic effects [190]. There are various types of electrical stimulation strategies. According to the literature, electrical stimulation strategies can be divided into three categories, including direct current/alternating current electric field (DC/AC EF), capacitive coupling electric field (CCEF), and inductive coupling electric field (ICEF) [190]. Various cell types have been studied and found to be responsive to these ES methods under different experimental settings [29,67,69,139,[100], [103], [117], [126], [127], [128], [132], [189]].
Electrical stimulation is one of the promising approaches for cardiac regeneration to promote the differentiation of cardiac myocytes and induce contractions [29,139,117]. Studies evaluated the efficacy of external electrical stimulation alone or in combination with electroactive materials [29,67,69,[126], [127], [128],100]. For example, Zhao et al. reported the regenerative potential of aligned and random rGO/silk fibroin nanofibrous scaffolds with and without external electrical stimulation for cardiac regeneration [29]. The rGO/silk scaffold showed a high conductivity while maintaining the topological factors and flexibility of nanofibrous scaffolds. Upon seeding the scaffolds with rat cardiomyocytes and applying external electrical stimulation (1Hz, 5 V/cm for 4 days), the rGO/silk scaffold with aligned orientation promoted cell spreading, cardiac-specific protein expression, the formation of sarcomeric structure, the formation of cell-cell gap junctions, and spontaneous beating of regenerated cardiac tissues. Using the rGO layer enhanced the effect of electrical stimulation on cardiac tissues [29].
3.3. Mechanical stimulation
Mechanical stimulation as a biophysical cue has been applied to 3D engineered scaffolds to enhance, regulate, and accelerate the regeneration of functional tissue. Its role is to stimulate the synthesis of ECM, guide cell differentiation, enhance biomimetic construct organization, and improve the function of cells or tissues. This approach is one of the most effective factors for engineering a functional skeletal tissue, including bone [25], cartilage [191], ligament, tendon [85,83], and skeletal muscle [192] that significantly affects the development, rehabilitation, and healing of the tissues. It has been reported that mechanical stimulation induces MSCs differentiation even without biological factors [25,193,194].
Applying mechanical loading is a promising strategy to promote cartilage regeneration by improving chondrocytes' metabolic activity, composition, and biomechanical properties. Ouyang et al. reported the efficacy of dynamic tensile mechanical stimulation on the co-cultured rabbit chondrocytes and bone-derived MSCs for cartilage regeneration [191]. The results suggested the regenerative ability of this combination to promote cell proliferation and cartilage phenotype. The significance of mechanical stimulation has also been reported for skeletal muscle regeneration by providing similar mechanical loads to those seen in the body [107]. The study exhibited the effects of mechanical stimulation on cell proliferation, myofiber organization, myofiber diameter, ECM composition, and muscle elasticity [107].
The potential of mechanical stimulation to induce differentiation of stem cells and stimulate tendon/ligament regeneration has also been reported [85,82,83]. The use of mechanical stimulation aligns the cells along with the force direction resulting in the generation of aligned collagen fibers and upregulation of specific genes (Collagen I, Collagen III, tenascin C). Wu et al. reported the efficacy of dynamic stretch on human adipose-derived mesenchymal stem cells (HADMSC)/Human tenocytes (HT)/Human umbilical vein endothelial cells (HUVEC) tri-culture for promoting collagen secretion and tenogenic differentiation [85]. HADMSC/HT/HUVEC were seeded on the woven fabric scaffold and cultured under both static and dynamic environments for two weeks. The in vitro results showed the effects of the dynamic environment on enhancing the expression of collagen and tenogenic differentiation markers (SCX, TNC, COL1, COL3, TNMD, and VEGFA genes) compared to the static condition [85].
3.4. Morphological factors
The final step for designing scaffolds is to define the required fabrication technique. The morphology of the engineered scaffold is a critical consideration that can modulate the biological response and clinical success of the scaffold [15,25,85,134,83,132,[121], [123], [195]]. During the past decade, several strategies for scaffold fabrication have been reported (Fig. 4). They include hydrogel-based scaffolds [13,101,[196], [197], [198]], fibrous scaffolds [15,68,108,199,200], microsphere, 3D porous or spongy scaffolds [120,129,134,119,94,93].
 Fig. 4
Fig. 43.5. Hydrogel
Hydrogels are among the most studied biomaterials in drug delivery, regenerative medicine, and tissue engineering [82,83,112,201]. Luo et al. developed a 3D porous hydrogel-based scaffold to heal breast cancer recurrence and fill the cavity to repair the tissue after surgery [121]. The 3D-printed scaffold was fabricated using dopamine-modified alginate and polydopamine (PDA). The in vitro results showed scaffold flexibility, similar to Young's modulus with normal breast tissues, and higher human normal breast cell adhesion and proliferation. In vitro and in vivo data exhibited an appropriate photothermal effect that can inhibit the local recurrence of breast cancer. The regenerative ability of the scaffold was further evaluated in a mouse subcutaneous tumor model. The in vivo results showed the structural stability of the scaffold one month after implantation. Moreover, the photothermal therapy of 3D printed hydrogel had no systemic toxicity to the major organs [121].
The efficacy of hydrogel-based scaffolds has also been reported for hard tissue regeneration. For example, Tateiwa et al. assessed the bone-regenerating potential of a new composite composed of hydroxyapatite (HA), beta-tricalcium phosphate (β-TCP) microsphere/poloxamer 407-based hydrogel, and recombinant human (rh) BMP-2. Their findings demonstrated that this innovative composite outperformed collagen sponge in terms of bone regeneration capacity. This superiority was attributed to the sustained release of rhBMP-2 and the superior quality of BMP-induced bone, as confirmed through both in vitro and in vivo evaluations [99].
Also, due to hydrogels' ability to absorb and release water, the use of polymeric hydrogels provides a moist environment for wound healing while regulating excessive wound exudates [45,149].
Finally, hydrogels derived from decellularized tissue-specific ECM can offer functionalities similar to those of naturally existing ECM. For instance, Dong-Woo Cho et al. demonstrated that ECM-based hydrogels obtained through decellularization hold significant promise for tissue and organ regeneration, including the kidney [218]. Furthermore, in another study, they formulated hydrogels incorporating decellularized ECM sourced from the endometrium-specific layer and the entire uterus, demonstrating their potential as efficient organ-specific biomaterials. Their findings indicated that intrauterine administration of UdECM prompts endometrial regeneration and enhances fertility, highlighting the therapeutic potential of such biomaterials [219].
3.6. Fiber-based scaffolds
Among biomaterial scaffolds, fibrous structures have the most similarity to most tissue organizations by providing similar structures with similar mechanical properties [1,21,23,171,[220], [221], [222], [223]]. Wu et al. evaluated the regenerative ability of a woven fibrous scaffold containing nanofiber PCL yarns and PLA multifilaments for tendon regeneration [85]. The woven scaffold showed appropriate topographical factors, including large pore size and aligned microstructures that significantly improved the mechanical properties of the construct compared with the control groups. Upon seeding the scaffold with (HADMSC)/(HT)/(HUVEC) tri-culture with dynamic conditioning, the results showed the highest upregulation of collagen secretion and tenogenic differentiation [85]. Mengsteab et al. reported the efficacy of fibrous braided structures in enhancing the osteointegration and regeneration of ACL tissue in a rabbit model [88]. Based on the results, using non-degradable polyethylene terephthalate (PET) fibers in the PLLA bioengineered ACL matrix improved strength retention after surgery. They also demonstrated that the application of BMP-2, FGF-2, and FGF-8 combined with fibrous scaffold enhanced regeneration and suppressed inflammation in the knee joint [88].
3.6.1. Microsphere
Microsphere-based scaffolds have some remarkable properties for tissue regeneration applications, such as their packed and porous structures that act as an excellent template for cell growth and proliferation. These scaffolds can control the release of bioactive molecules and provide unique mechanical properties [202,203]. A desirable approach for controlling the local concentration of biological agents is to use biodegradable microspheres capable of releasing biological agents at an appropriate rate. Table 1 present some conducted studies that developed injectable or implantable microsphere-based scaffolds either alone or as vehicles of biological agents.
3.6.2. Spongy
Besides the discussed strategies to fabricate a porous structure, other techniques can be considered to fabricate spongy scaffolds. These spongy structures have unique morphological properties that can be adjusted to show similar morphology to natural ECM depending on the application in the body [120,129,125,94,133,115].
Ghafari et al. reported the efficacy of a bilayer scaffold fabricated with cellulose nanofiber and poly (vinyl) alcohol (CNF/PVA) using an emulsion freeze-drying technique [133]. The bilayer structure was fabricated by changing the concentration of CNF/PVA for each layer. The layers had interconnected pores with two different porosities and pore sizes that replicated the structure of Epidermis and Dermis layers of skin tissue. Based on the results, mechanical properties and pore size showed a strong correlation when a low value of modulus was observed for a larger pore diameter. Besides morphological similarity, the study showed the biocompatibility of the scaffold for skin tissue regeneration [133].
4. Conclusions and future directions
Recently, several studies showed significant developments in the field of regenerative tissue engineering, especially in the design and fabrication of biomimetic scaffolds for repairing and regenerating tissues in laboratory conditions. One of the technical hurdles in tissue engineering is developing a complex 2D/3D construct that can transport sufficient oxygen and nutrients to the scaffold to promote tissue formation in the body. Studies reported various methods to address this issue, including biological factors, novel materials, the scaffold's engineered architecture, and the in vitro maturation of the cell-seeded scaffold before implantation. This review aimed to demonstrate the recent development and essential factors in designing and fabrication of biomimetic scaffolds. The focus of the review was mainly on biomaterials, biological, physical, topographical, and morphological factors, with an emphasis on the advantages and disadvantages of different fabrication techniques.
The discussed knowledge in this review on the design and fabrication of laboratory-scale scaffolds provides a better understanding for prospective scientists and ongoing studies in the field of tissue regenerative engineering. Biomimetic scaffolds are the principal structure that supports mechanical properties, cell attachment, proliferation, and organ regeneration, which can be achieved by manipulating the discussed essential factors. According to the literature, the factors showed significant effects on the final properties of the scaffolds, their regenerative potential, biocompatibility, immunogenicity and cytotoxicity, cell proliferation/differentiation, mechanical strength, electrical behavior, and other structural features. Considering each cue, it is concluded that there is no one magic cue that is ideal in every scenario. Indeed, even for a specific application, rarely one cue can provide all of the appropriate properties. Although several studies evaluated the efficacy of combined factors, there is still a pressing need to develop ideal scaffolds for different tissues and organs for clinical applications. So far, most of the studies have been conducted in small animal models. It is crucial to evaluate the performance of factors in larger animals before clinical trials. Also, the ability to scale up the scaffolds’ fabrication is still an important consideration that needs to be addressed.
CRediT authorship contribution statement
Shiva Norouzi: Writing – original draft, Visualization, Investigation, Data curation. Nikoo Saveh Shemshaki: Writing – original draft, Visualization, Investigation, Data curation, Conceptualization. Ehsan Norouzi: Visualization, Validation, Software, Investigation, Formal analysis. Masoud Latifi: Supervision, Methodology, Conceptualization. Bahareh Azimi: Writing – review & editing, Methodology. Serena Danti: Writing – review & editing, Methodology. Xiaolan Qiao: Methodology, Funding acquisition. Yuee Miao: Writing – review & editing, Supervision, Conceptualization. Shengyuan Yang: Writing – review & editing, Supervision, Conceptualization. Mohsen Gorji: Supervision, Conceptualization. Vasilije Petrovic: Supervision, Conceptualization. M. Ali Aboudzadeh: Writing – review & editing, Validation, Supervision, Conceptualization, Investigation, Visualization. Roohollah Bagherzadeh: Validation, Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The support provided by Institute for Advanced Textile Materials and Technologies (ATMT), and the INTERNATIONAL COOPERATION Fund of the Science and Technology Commission of Shanghai Municipality (No. 20520741500) are highly appreciated.
M.A.A acknowledges his contract financing through the Ministry of Universities of Spain and the European Union - Next Generation EU.
Data availability
No data was used for the research described in the article.
- 1
-
These authors contributed equally.
© 2024 The Authors. Published by Elsevier Ltd.