1. Introduction
Chitosan nanocomposite is a term that refers to a chitosan polymer that contains distributed nanofillers with an average particle size of less than 100 nm that are spread throughout the polymer. Rouget discovered this natural polymer chitin derivative in 1859 as a deacetylated form of the naturally occurring polymer [1]. Chitosan made its way into the pharmaceutical industry in the 1990s, which piqued the attention of academics and industrialists who sought to develop more effective and innovative treatment systems based on it. Chitosan is versatile because of its dynamic protein nature, which serves as a reaction means for a wide range of novel group strands under moderate conditions. Furthermore, the amino nature of the Chitosan gives it positive charges (polysaccharides). Chitosan exists as copolymer structures consisting of repeated glycosidic units that include a single NH2 and two O.H. groups per repeating glycosidic unit [2]. A low pH affects the stability of the chitosan's polycationic character. A lower pH facilitates protonation of the amino group, thus maintaining the polycationic of Chitosan. This results in increased solubility qualities. The anionic nature of Chitosan makes them water-soluble, increasing paste fluidity and possessing anticoagulant effects due to the sulfonation of the cationic Chitosan. The degree deacetylation ranges from 30% to 95% (Table 1) depending on the production method and employed sources [3].
Table 1. Preparation of N-carboxyethyl chitosan 1 from chitosan of various degrees of deacetylation (DDA) and molecular weight.
| Code | Chitosan | Product 1a) | ||||
|---|---|---|---|---|---|---|
| DDA | Mn | Mw | Ds | Mn | Mw | |
| % | kg-mol− | Kg-mol−1 | kg-mol−1 | kg-mol−1 | ||
| D60 | 60 | 73,000 | 130,000 | 0.20 | 78,000 | 160,000 |
| M30 | 70 | 270,000 | 410,000 | 0.23 | 330,000 | 480,000 |
| D80 | 85 | 154,000 | 530,000 | 0.28 | 180,000 | 600,000 |
| SK10 | 85 | 42,000 | 100,000 | 0.44 | 42,000 | 110,000 |
| 01P | 85 | 24,000 | 68,000 | 0.45 | 21,000 | 92,000 |
| D95 | 95 | 110,000 | 390,000 | 0.28 | 90,000 | 470,000 |
As a result of chitosan's antibacterial properties, low immunogenicity, biocompatibility, and biodegradability, it has great promise for use in various sectors, including food processing. This functional group is responsible for most of the chitosan's biological activities, including transfection, controlled drug delivery, efflux pump inhibition, in situ gelations, permeability augmentation, colon targeting, and mucoadhesion [4]. In addition, bio adhesiveness, a polymer feature that allows Chitosan to adhere to soft and hard tissues, is helpful in various medical fields, including orthopaedics, dentistry, ophthalmology, and surgery [5]. This research aims to derive chitosan composites and nanoparticlesfrom crustaceans that may be used in Nanomedicine. This paper discusses the crustacean's source of Chitosan, nanomedicine uses of chitosan nanocomposites, chitosan nanoparticles, and the rationale for developing synthetic chitosan nanocomposites, among other topics. In addition, the study reviews synthetic chitosan nanocomposites, describe the mechanical properties of chitosan nanocomposites, discusses the process techniques for nano chitosan composites, and discuss the effects of microstructures on the mechanical characteristics of nano chitosan composites, among other things.
1.1. Chemical structure of chitosan and chitin
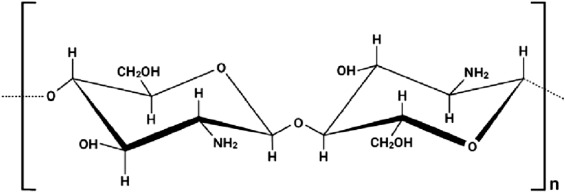
Fig. 1 describes the linear-chain polysaccharide chitosan, which consists of the N-acetyl-2-amino-2-deoxy-d-glucopyranose, which is the acetylated unit and 2-amino 2-deoxy-glucopyranose which is the deacetylated unit. This is where the repeating units are linked by a beta (1–4) glycosidic bond, shown in Fig. 1 [6]. However, Fig. 2 represents the chemical structure of Chitin, which shows the cellulose-like biopolymer found widely distributed in nature, especially among the marine animals or invertebrates, insects, fungi and yeasts [7]. This chemical structure is abundant in nature and its linear polymeric made of repeating beta (1, 4)- N-acetylglucosamine units [8].
 Fig. 1. Chemical structure of Chitosan.
Fig. 1. Chemical structure of Chitosan. Fig. 2. Chemical structure of Chitin.
Fig. 2. Chemical structure of Chitin.2. Crustacean's source of chitosan
A large percentage of Chitosan is found naturally in several biomasses, including cellulose [6], as a partially deacetylated source of Chitin. Another source of Chitosan is fungi, whose cell walls contain Chitosan at different lifecycle phases. The class of Zygomycetes has explicitly been identified as a good source of Chitosan [9], with the class of Zygomycetes being the most well-known. As much as 94% deacetylation has been recorded, with chitosan concentrations in the range of 1–10% on a dry biomass basis. The enzyme deacetylase converts Chitin to Chitosan (Fig. 3), not the other way around [10]. The deacetylation enzymes are assumed near the plasma membrane where the Chitin transverses. While the Chitin is generated, the deacetylase enzyme turns it into Chitosan. Fusarium might become an intriguing source of Chitosan in the future because Chitosan can be separated using less invasive processes. In addition to fungus, bacteria have been shown to convert Chitin into Chitosan by deacetylase [11]. However, because of the insolubility of Chitin, the efficacy of this process is restricted.
 Fig. 3. Deacetylation of chitin to chitosan [10].
Fig. 3. Deacetylation of chitin to chitosan [10].Regarding the similarities, there are structural and compositional similarities to the glycosaminoglycans (GAGs). Those types usually elicit a minimal immune response in terms of antimicrobial responses when used for biomedical applications. Those types also demonstrate the effective chelating abilities with the metal ions and the swelling properties. Chitin sources, their isolation, and the breakdown of Chitin in nature are all helped. Chitosan may also be made from these fast-proliferating microorganisms or isolated enzymes.
Chitin has been discovered in various animals since the beginning of the twentieth century. When zoologists started calling any complex yellow-brownish structures chitin, they didn't do a chemical investigation, which led to several misinterpretations of their results. The presence of Chitin is detected by chemical testing, as Hyman proved this when he used iodine-based colour teststo examine Chitin's existence in diverse marine organisms [12]. With time and technological advancement, newer spectroscopy technologies, such as Fourier transforms infrared spectroscopy, became more famous for chitin detection. Chitin quantification is a difficult task that has just recently been published in a few papers. At this time, quantitative data on chitin contents is still insufficient, and the provided values must be treated with caution, as previously stated.
According to current estimations, a considerable amount of the Chitin generated by the biosphere is now considered present in the seas [13]. This organism may be found in many aquatic species, including corals, horseshoe worms, Ectoprocta, lamp shells, Bryozoa, sponges, and Mollusca, including cuttlefish and clams, among other species. Additionally, Chitin has been identified in fungi, algae, velvet worms, and protozoa [14]. Arachnids, arachnid-like creatures like spiders, and crustaceans are also suppliers of Chitin.
Chitin may be found in the exoskeletons of Arthropoda (insects and worms). The skeleton is a sturdy and stiff material that serves as a support system for the body and defence against predators. They may be explained by the presence of crystallinity that results in high stiffness and stiffness retention when chitin is combined with proteins. This Chitin–protein complex generate a stable fibrous structure [12]. Crustacean shells are majorly made of chitin, minerals, pigments, proteins, and in some instances, lipids [12]. Crustacean shells also include minerals, proteins, and pigments [13]. The chitin concentration in crab shells varies from 6 per cent to 72 per cent, depending on the species [14]. This significant difference may be attributed to several factors, including the source of the biomass, the portion of the biomass evaluated for chitin analysis, and the analysis technique utilized. Crabs and shrimp contain 10 and 72 per cent chitin [14]. There are variances in the chitin content across the different species; for instance, the Carcinus crab contains 64 per cent chitin while the Cancer crab includes 72 per cent chitin, and the king crab contains 35 per cent chitin, respectively [14].
Insects' internal systems, like the chitinous liners of the alimentary tract and the trachea, may also include chitin. Insect cuticles comprise catecholamines, Chitin, proteins and fats, and other components such as chitosan [6]. Attributed to the prevalence of quinones, proteins, and Chitin may be cross-linked with catecholamines [15]. Insects and crustaceans both use Chitin as a structural component in their exoskeletons. Physical and chemical damage is prevented, and infectious illnesses are protected against because it strengthens the strength of the skeleton and provides structure. The amount of Chitin found in various insect species varies greatly. The amount of Chitin in an insect's body differs greatly depending on its life stage. When it comes to chitin content in wasp larvae, they have a lower percentage than the pupae and adults [16], with higher rates of 6.2% and 10.3%, respectively.
Spiders, like other arthropods, have Chitin with a high level of acetyl (5–8.5%). Geolycosa vultures and Hogna radiata chitin structures were analysed physicochemically by Kaya et al. [16], and acetylation degrees of 97% and 99%, respectively, were discovered in the Chitin of the two spider species (Geolycosa, vultures and Hogna radiata). Squids are Mollusca's prototype for the protein chitin; hence, many studies target them. There has been some evidence that squid pen may become a more prominent chitin source in the future because of the high levels of chitin concentration (up to 49%) discovered in the animal [17]. It is important to remember that the chitin-rich cell only accounts for a tiny fraction of the whole squid; therefore, these high proportions are linked to the cell's makeup. According to new studies, chitin may contribute to skeletons in calcification marine sponges [17]. In addition to Chitin, the Cnidarian taxa's structures frequently include calcium-based minerals. An antipathy-like protein is found in black coral, making them stand out from the rest. Exoskeletons of Lophophorates (octopuses, phoronids, and brachiopods) form Chitin and tubes. In fungal cell walls, Chitin is the most crucial ultrastructural component, entrenched in the amorphous matrix and serving as the underpinning for the cell wall form. Fungal cell walls are composed mostly of chitin [18]. Chitin has been detected in the spore and mycelia but not in any other state. The dry cell wall base is found in concentrations ranging from two per cent (w/w) to 50% (w/w), with the lowest value matching yeasts and the highest corresponding to Euascomycetes. According to the kind of fungal cell wall being researched, glucans, condensed tannins, and Chitosan may also be present. Chitin (plus chitosan) output is lower because fungus cell walls make up a small part of total biomass. Mycelia from Aspergillus Niger, Mucor rouxii, and Agaricus bisporus yielded 8–16% glucosamine concentrations on the dry weight.
When it comes to the algae, it has been shown since 1965 that Thalassiosira and other types of diatoms use chitin ropes to connect two newly split daughter cells, creating cell strands that float in water [18]. It has been revealed that Chitin exists in diatoms in different outlays, like the siliceous shell diatom. Clathromorphum compactum, calcified coralline algae, may contain the protein chitin, which helps create the algae's skeleton and protects it against acidification and nibbling in shallow waters, according to researchers. Furthermore, laboratory investigations have shown that Chlorella Vulgaris and Pithophora oogonia have chitin in their cell walls. However, quantifiable data on algae's chitin content is hard to get. Algae containing Chitin derived from plants may be advantageous in some applications.
3. Global distribution of chitin and chitosan in nature
Chitin production throughout the globe is predicted to equal 1011 tons per year [19]. This polymer is present in various creatures, such as diatomaceous, arthropods, and fungi. Apart from cellulose, Chitin is also the most common polysaccharide. However, until recently, most of Chitin's commercial use has been restricted to seafood, such as crabs, shrimp, lobster, and squid [20].
However, research shows that millions of tons of shells from crabs, lobsters, and shrimp are created each year across the globe [20]. Chitin, protein, and minerals, for instance, calcium carbonate, are the main valuable components in commercial crustacean wastes, computed on a dry weight basis. The leading global producers of Chitin include Poland, India, China, Japan, the USA, and Norway [21]. However, the synthesis of Chitosan from fungi has expanded dramatically in recent years [19]. The process of chitin- chitosan production from crustaceans’ shells is illustrated in Fig. 4 while some of the sources of chitosan with the respective DDA is shown in Table 2
 Fig. 4. Production of Chitosan from crustaceans wastes.
Fig. 4. Production of Chitosan from crustaceans wastes.Table 2. Sources and content of Chitin.
| Source | Chitin (%) (DDA) |
|---|---|
| Insect cuticle | 5–25 |
| Krill cuticle | 20–30 |
| Fungi cell wall | 10–25 |
| Clam/oyster shell | 3–6 |
| Shrimp cuticle | 30–40 |
| Crab cuticle | 15–30 |
| Squid pen | 20–40 |
4. Functionalization of chitosan
Chitosan is a biomaterial that, when chemically changed, can be applied in the biomedical field. Amino and hydroxyl groups, when present, have been shown to have fascinating effects on desired properties [19]. Chitosan's most common alterations are graft copolymerization, cross-linking, esterification, carboxymethylation, and etherification [22].
4.1. Cross-linking
Polymers in a hydrogel are cross-linked through non-covalent and covalent linkages, leading to three-dimensional systems of polymer molecules in a hydrogel system. These systems, among other things, can inflate and hydrate quickly and be stable in terms of shape. With external factors, e.g., cation strength or pH levels, it is possible to develop Chitosan hydrogels that are physically and chemically sensitive. Electrostatic repulsion between the free chitosan amino in an acidic condition causes the polymer material to expand [23]. Chemical Chitosan can be extracted using both hydroxyl and amino. As a result of their excellent reactivity with the cross-linking agent, amino groups are often used in cross-linking procedures. Dialdehydes, such as glyoxal and glutaraldehyde [24], organic acids, are often cross-linked with chitosan chains. Biopolymer membranes based on Chitosan with high proton conductivity have been made feasible by combining glutaraldehyde and sulfosuccinate acid.
The microstructures had a significant contributory effect on the mechanical properties exhibited by the chitosan. In addition, the microstructure develops special physical and chemical sensitivities. Those effects exhibited due to the microstructure have been investigated by different researchers. Findings indicate that those nanoparticles are widely dispersed uniformly in a matrix form up to 40 wt% with a high-speed homogenizer. There is also an elastic modulus increment achieved monotonically when nanoparticles are added. However, there is a fracture strength drop; due to the defect introduced by the nanoparticles [25].
Chitosan-based hydrogels also have the potential for swelling and dehydrating depending on the environment. The addition of zincs, an important trace element found in bone with special regulatory cellular function, ensures osteoblastogenesis and suppresses osteoclastogenesis. Zinc also possesses special antimicrobial properties, utilized as a feature to tackle implant-associated microbial infections [26].
4.2. Carboxymethylation
Because Chitosan's limited solubility makes it difficult to dissolve, it has several potential applications. Consequently, chitosan derivatives have been produced to facilitate chitosan water solubility. Carboxymethylation is a widely used method of chemical synthesis. Isopropanol and sodium hydroxide dissolves Chitosan in an aqueous solution while using a magnetic stirrer at room temperature [27]. The suspension is then diluted with monochloroacetic acid and isopropanol. Amphoteric polymer Chitosan's solubility is affected by the pH of the solution. However, although the reaction may co-occur with both O- and N-carboxymethylation, it is possible to acquire just one of the two products [27].
4.3. Esterification
Esterification is another chemical modification that has been well studied in the literature. Using acetyl chloride in MeSO3H as a solvent revealed that when compared to N-acetylated Chitosan, O-acetylated Chitosan is the primary result under these conditions [19]. Acetylation of Chitosan increases its antifungal efficacy significantly.
4.4. Phosphorylation
Chitosan derivatives that have been phosphorylated may be made in various ways. Antimicrobial and osteoinductive characteristics make these derivatives of great importance. Heat treatment with orthophosphoric acid results in the phosphorylation of Chitosan, which may be employed in several applications. Additionally, it is feasible to generate highly efficient phosphorylated Chitosan by reacting phosphorous pentoxide with methanesulphonic acid [28]. The cell wall improves when the phosphorylation of the hydroxyl is enhanced on the carbons 3 and 6 of Chitosan.
5. Preparation of chitosan nanoparticles
Chitosan nanoparticles are effective transporters for medicines and active substances because of their small size. The characteristics of chitosan may be modified by various structural alterations [19]. The methods used to make chitosan-based nanoparticles include complex coacervation approach, ionotropic gelation approach, micro-emulsion approach, coprecipitationapproach, and emulsification solvent diffusion approach. In addition to using less solvent and requiring less work, these methods provide several other advantages. Using these approaches, researchers have shown that the molecular weight and extent to which acetylated determine nanoparticle surface charge and size. In the polymeric matrix, drugs are held by pharmacologic connections such as electrostatic contact, h - bonding, and hydrophobic interactions. It is important to remember that medication loading, and release aren't the only factors to consider. Examine ionic strength and salt, enzyme, protein, and pH stability when administering nanoparticles to determine their intended use and the physiological environment in which they will be issued. We now have a wide range of options for formulating our products, which is a positive development.
5.1. Ionotropic gelation
Passively charged chitosan solution is mixed with a negatively charged polyanionic solution to create a gel. The process involves the use of stabilizers, for instance, poloxamer. The complexation of the charged molecules occurs readily in mechanical agitation at room temperature. We can see the chitosan being separated into spherical particles with various sizes and surface charges in Fig. 5. These range from 20 to 200 nm in diameter and 560 to 930 nm in diameter; in general, Chitosan-TPP/vitamin C nanoparticles were created under one hour at room temperature with continuous stirring using ionotropic gelation [20]. Many advantages of ionotropic gelation include moderate processing conditions, low toxicities, slight changes to the chemical composition of the drug to be encapsulated, and the ability to perform the technique in an aqueous environment and low toxicity. The method's significant drawbacks are its low durability in acidic environments and difficulties in entrapping medicines with large molecular weights [19].
 Fig. 5. Preparation of chitosan nanoparticles via Ionotropic Gelation technique [29]
Fig. 5. Preparation of chitosan nanoparticles via Ionotropic Gelation technique [29]5.2. Complex coacervation method
In coacervation, spherical particles are separated by electrostatically mixing solutions as shown in Fig. 6. Combining the positively charged chitosan amino with the negative phosphate groups of DNAs leads to creating the chitosan nanoparticles [21]. The molecular weight of the two polymers affects the efficacy of entrapment and the release of the medication [19]. The process is facilitated by an aqueous solution and a lower temperature. This increases the likelihood of the encapsulated chemicals being active longer in the environment. The technique's principal limitations include the NPs' poor stability, limited drug load, and the need to cross-link the complex using an unrecommended chemical reagent such as glutaraldehyde [21]. Dextran sulfate DNA is introduced to a cationic polymer (for instance, gelatin, dextran sulfate, and polyethyleneimine) swirled at room temperature while an anionic solution is added. After charge neutralization, the mixture is allowed to rest for some time. The following are some advantages: the procedure is straightforward, there are no severe conditions, and the nanoparticles develop spontaneously [20].
 Fig. 6. preparation of chitosan nanoparticles through complex coacervationtechnique [30]
Fig. 6. preparation of chitosan nanoparticles through complex coacervationtechnique [30]5.3. Coprecipitation method
An acidic solution, such as ammonium hydroxide, was shown to produce coprecipitation and create a highly monodisperse nanoparticle population when chitosan solution, manufactured in an acetic acid solution of medium acidity, was added. Nanoparticles with diameters as tiny as 10 nm may be effectively encapsulated. Using this method, it is possible to view a wide range of particle sizes, either an advantage or a problem. Coacervate droplets were formed with ammonium hydroxide and then added to the solution to create nanoparticles of chitosan (LA-g-chitosan). This method resulted in spherical and uniformly scattered nanoparticles [19].
5.4. Microemulsion method
This technique involves mixing chitosan's acetic solution with glutaraldehyde in an organic solvent like hexane and letting the combination settle overnight. When this combination is held at room temperature on a continuous stirring cycle, the nanoparticles will develop overnight when the cross-linking process is finished as seen in Fig. 7. After precipitation with CaCl2 and centrifugation, a surplus surfactant may be removed from this stage of the product. The nanoparticle solution is dissolved in distilled water and recrystallized [19]. Narrow size distributions are produced by this method. It is possible to control the size of the nanoparticles by changing how much glutaraldehyde is used in the production process. Using this method, nanoparticles of petite sizes may be created. Organic solvents are used, the process takes a long time, and the intricacy of the washing step are all negatives [19].
 Fig. 7. Preparation of chitosan nanoparticles via emulsion technique [31].
Fig. 7. Preparation of chitosan nanoparticles via emulsion technique [31].5.5. Emulsification solvent diffusion method
Under mechanical stirring as indicated in Fig. 8, an emulsion is created by adding an organic phase to a chitosan solution containing a stabilizing ingredient, such as poloxamer [32]. Afterward, the emulsion is homogenized under high pressure to generate a long-lasting mixture. A large quantity of water is used to dilute the emulsion to facilitate proper consistency. For nanoparticles to be formed, an organic solvent must diffuse into the water, resulting in polymer precipitation. Particularly problematic aspects include the significant shear pressures required during nanoparticle formation and its reliance on organic solvents.
 Fig. 8. preparation of chitosan nanoparticles by emulsification solvent diffusion technique [33].
Fig. 8. preparation of chitosan nanoparticles by emulsification solvent diffusion technique [33].6. Synthesis of chitosan nanocomposites
Components made of polymer nanocomposites are multiphase components with at least one phase of less than 100 nm in dimension [34]. Research shows that the fabrication of nanocomposites is facilitated by a large interfacial areaand managed stress transmission across the contact. When it comes to the qualities of nanocomposites, the first crucial component is essential. The high surface-to-volume ratio of nanoparticles results in widespread binding between the polymer and the nanofiller due to their crucial specific areas. To transmit the sound characteristics of the nanoparticles into the composite, it is required to achieve a solid interfacial connection between the nanoparticles and the composite [35]. As a result, a stable interfacial connection will enable effective stress transport across the contact. Various methods can improve the importance of the interface's covalent and electrostatic bonding and hydrogen and van der Waals attractions [36]. Nanoparticle aggregation may cause a loss in the contact area and weak mechanical characteristics; therefore, a suitable distribution of the nanofillers is also vital to obtain the desired increased mechanical and electrical properties inside the matrix [37]. In nanocomposites, the interfacial strength substantially impacts the automatic features, such as elongation, toughness, and tensile strength. Of the several nano-fillers developed for use as reinforcement phases in chitosan nanocomposites, the most often employed include layered silicates, metal nanoparticles, carbon nanotubes, and graphene-based materials.
6.1. Layered silicates
Due to their superior material characteristics, nanocomposite polymer–clay has received significant attention. High-aspect-ratio aluminosilicate acid and silicawith strong complexation chemistry build components of clay. This nanofiller, composed of silicon, magnesium, hydroxyl, oxygen, aluminium, and several related cations, has a characteristic layered structure in different forms. Silicon (Si) and aluminium (Al), and magnesium (Mg) are joined together by four and eight oxygen atoms, respectively, in the layers, which are then encircled by another layer of silicon and oxygen atoms. Organic polymers cannot be combined with clays since clays are hydrophilic by nature [38]. Ammonium or Phosphorus ions are typically used to improve interlayer space between layers and allow polymer diffusion. To function correctly, nanocomposites, remarkably layered silicates, rely on a uniform distribution of silicate structures throughout the polymeric matrix.
6.2. Metal nanoparticles
Adding nanoparticles to polymer matrices creates a new group of more functional materials [39]. More specifically, it is undertaken to obtain enhanced mechanical characteristics and, in certain situations, impart bioactivity to a non-biodegradable material by integrating inorganic particles into the chitosan matrix [18]. Chitosan nanocomposites typically have three nanoparticle fillers: bioactive ceramic, bioactive glass, and metal nanoparticles. The most common filler is bioactive glass nanoparticles, a class of surface reactive materials that can form bonds with physiological tissues such as bone. Bioactive glasses are generally created by the melt or sol-gel techniques of fabrication. These Nanoparticles are mainly silicate with varying amounts of sodium, calcium, and phosphorus added for flavour and texture. In addition, they may generate ions and act as an effective substrate for protein adsorption because of their nanometric size. Therefore, using sol-gel methods, it is feasible to create bioactive nanoparticles with precisely regulated compositions.
It is also possible to include ceramic nanoparticles in nanocomposites, which have superior mechanical properties and better interactions with the surrounding tissues because of their integration into the composite. Most notably, hydroxyapatite nanoparticles, which exhibit excellent osteoconductive and osteoinductive biodegradability and high mechanical strength, have shown promise in nanocomposite manufacturing [40]. However, when significant concentrations of ceramic nanoparticles are used in composites, these materials' brittle nature may negatively impact the composite characteristics. Metal nanoparticles, for example, are mixed with polymers to give biosensing and antibacterial properties. All metals, including silver, gold, and zinc oxide nanoparticles, have previously been employed in nanoparticle manufacturing, including silver, gold, and zinc oxide nanoparticles. Chemical composition, particle size, crystallinity, and shape are just a few variables that may be adjusted independently of the nanoparticle kind to optimize the composites' features [41].