1. Introduction
Aquatic environments are being polluted daily by different pollutants because of associated anthropogenic and natural activities as a result of continual industrial development around these watersheds. The most released contaminants in the environment include pharmaceuticals and personal care products, endocrine disrupting compounds, persistent organic pollutants (POPs), heavy metals, volatile organic compounds (VOCs), dichloro-diphenyl-trichloroethane, dioxins, furans, and other contaminants of emerging concerns, just to mention a few. POPs are amongst dangerous pollutants with some toxic chemical substances due to their genotoxicity, carcinogenicity, teratogenicity and mutagenicity properties [1,2]. They are mostly carbon-based toxic compounds that adversely affect the environment and human health around the world due to their persistence for a long time in the aquatic environment. However, they may ubiquitously bioaccumulate and pass from one species to the next through the food web [3]. They are hence divided into many classes such as polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDD) and polycyclic aromatic hydrocarbons (PAHs), among others [4]. PAHs are amongst the predominant organic chemicals which pollute water, soil, waste sludge, air, and sediment due to their hydrophobic and low degradability nature, chemical stability and tendency to bind to particulate matter [[5], [6], [7]]. Several PAHs have been extensively studied and reported in the literature [[8], [9], [10]]. An example of the most studied ones include benzo[b]fluoranthene, benzo[a]pyrene, chrysene, phenanthrene, pyrene, anthracene, indeno[1,2,3-(c,d)]pyrene, naphthalene, acenaphthene, benzo[g,h,i]perylene, fluorene, benzo[a]anthracene, dibenzo[a,h]anthracene, benzo[k]fluoranthene, fluoranthene, and acenaphthylene [[11], [12], [13]]. These 16 PAHs have been recorded by the European Union and U.S. Environmental Protection Agency as high priority contaminants to be monitored and quantified in all aquatic environments due to their chemical properties [8,[14], [15], [16], [17], [18], [19]]. They are divided into the light- (up to four rings) or low molecular weight-PAHs (LMW-PAHs) and heavy- (more than four benzene and/or pentacyclic molecule rings) or high molecular weight-PAHs (HMW-PAHs) depending on their number of fused benzene rings [13,20,21]. However, their chemical and physical properties are highlighted by their conjugated π-electron systems [22]. HMW-PAHs are more toxic and detrimental to human health and environment, and are more stable than the LMW-PAHs [[23], [24], [25]]. Structures of PAHs can be non-alternate or alternate due to their arrangement in aromatic rings or if the non-benzene rings are four, five and six members, respectively [21,26,27] and do not carry substituents or contain any heteroatoms. PAHs in the aquatic environments originate from all kinds of domestic burnings, agricultural sources, natural sources and industrial processes such as steel-making plants, coal mines and dumpsite leachates [22,[28], [29], [30]]. Amongst these sources, their concentration level is mainly increased in air, water, sediment and soil by anthropogenic activities such as tobacco smoke, coal production, various forms of cooking, oil spills and manufacturing, burning of fossil fuels, and wood preservation using creosote [2,8,11,15,31]. They can be transported very far by water and air from their production area due to their tendency to bind to particulate matter and deposit into sediment and soil [32]. However, continuous exposure to them can have numerous health effects on the human systems including lung and skin cancer, diarrhoea, skin irritation, and breathing problem [30].
PAHs can be eliminated from aquatic environments using biological, chemical and physical methods. Studies have revealed that both physical and biological techniques are inefficient to completely remove them from water. To lessen their impact on the environment, it is important to remove them from aquatic environments through advanced treatment and chemical methods such as peroxonation, Fenton's reagent, photocatalytic degradation and ozonationprocesses which are characterized by high performance and low cost [27]. Recently, Fenton oxidation, photocatalysis and photolysis as effective advanced oxidation processes (AOPs) permitted the efficient mineralization and decomposition of PAHs in wastewater and groundwater, unfortunately they included the usage of liquid hydrogen peroxide [27,33]. However, some derivatives from these processes end up in the formation of quinone and other hepatotoxic compounds, which are more harmful to humans than the parent PAHs themselves [27]. Moreover, in recent decades, AOPs combined with different catalysts have been categorised as the most effective methods in photodegrading PAHs in water [33]. Adsorption method and photocatalytic degradation have also been recently used for decontamination of polluted water with organic chemicals [[34], [35], [36]].
Therefore, the adsorption process using polymeric nanocomposite materials (PNMs) under sunlight irradiation emerges as a sustainable alternative for easy implementation, with low cost, less secondary contamination, high removal rate, and high efficiency [36]. Polymers, particularly biopolymers bring another aspect to the removal process as they are good adsorbents and biodegradable compounds. They exhibit some physicochemical properties such as high selectivity depending on the type of surface modification, excellent chelation behaviour, high reactivity, and chemical stability [37]; Mei et al., 2019; Meramo et al., 2019 [35,38]; Lu et al., 2020; [36,39]. This review presents a summary of various polymeric nanocomposite and other photocatalytic materials used for the photocatalytic detoxification and mechanism of PAHs in environmental media like aquatic sediments and associated water bodies.
1.1. Photocatalytic degradation of PAHs
Degradation of organic pollutants such as PAHs must be well performed to avoid the formation of hazardous metabolites. The most known methods and techniques used in this process are Fenton oxidation [40], multiwalled carbon nanotubes (MWCNTs) [41], TiO2 using ultraviolet (UV) light source [42], carbon-based materials (bio-achar) [43], ozonation, and chlorination. It is in that way that advanced technologies have been applied over traditional techniques, which have many limitations such as low performance and viability, low selectivity, and inherent agglomeration associated with Van Der Waals. However, recent studies suggest the degradation of PAHs through the photocatalytic process amalgamated with graphene oxide to reduce the bandgap and improve the absorption of visible light [27,30]. This amalgam of graphene oxide/metal oxide such as zinc oxide presents a low photoluminescence peak and is normally used as a heterojunction in the photodegradation process of PAHs [27]. Nanocomposites are an emerging class of materials in which nanofillers (dimension size <100 nm) are incorporated [44]. They are being used due to their fast dissolution properties, high specific surface area, super-magnetic characteristics, quantum confinement and strong sorption [44,45]. Polymer nanocomposite materials have received great attention among the industrial and scientific communities in recent decades because they can achieve significant improvements in dimensional stability, mechanical properties and gas or solvent barrier properties concerning the polymer matrix at very low concentration of nanofillers as compared with the continuous phase [[46], [47], [48]]. However, nanosized cellulosic materials may be good fillers for the synthesis of PNMs to enhance the overall performance in terms of the mechanical and functional properties of nanocomposites. The main purpose for the addition of nanofillers to polymers focuses on the improvement in the electrical, mechanical, permeation barrier and heat resistance properties of a polymer [49,50]. More information on PNMs, their synthesis, characterization and application are provided in the next sections.
1.1.1. Background on polymeric nanocomposite materials
As the foundation of nanotechnology, PNMs are polymers that are incorporated with nanofillers or nanomaterials homogeneously or uniformly dispersed into the polymer matrix [51,52]. Nanofillers can be inorganic [nano-clay (organically modified clay like hectorite (Na0·3(Mg,Li)3Si4O10(OH)2), kaolinite (Al₂Si₂O₅(OH)₄), saponite (Ca0·25(Mg,Fe)3((Si,Al)4O10) (OH)2·n(H2O)), and montmorillonite ((Na,Ca)0·33(Al,Mg)2(Si4O10) (OH)2·nH2O) and metallic nanoparticles like zinc (Zn), copper (Cu) and titanium oxides (TiO2), silver (Ag), gold (Au), and silica (SiO2), carbon nanofibers such as graphene, carbon nanotubes, carbon fibres, flurrene, and graphite and other particles] or organic (rod-like nanocrystals/nanofibers like cellulose ((C6H10O5)n), chitin((C8H13O5N)n, starch ((C6H10O5)n), and chitosan (C18H35N3O13)) materials [[52], [53], [54]]. These polymer fillers can have different shapes such as three-dimensional or iso-dimensional or zero-dimensional, two-dimensional and one-dimensional nanofillers usually less than 100 nm [55,56]. Nanoparticles such as silver (Ag), titanium oxide (TiO2), zin oxide (ZnO), silica (SiO2) and magnetite (Fe3O4) have intensively been applied in water purification due to their UV resistance, high photocatalytic activity and relative index, non-toxicity, excellent transparency to visible light, hydrophilicity, low cost, and good stability [44]. When they are incorporated into a matrix polymer, they impart different properties that qualify them to be applied in biomedicine, water purification and separation processes [56]. The assessment of the nanomaterial dispersion in the polymer matrix is very important since the thermal and mechanical properties are intensely related to the morphologies obtained. Depending on the level of separation of the nanofillers, three varieties of nanocomposite morphologies are available [51]. Polymer materials differ from other materials in their properties, ease of processing and low weight. However, to improve some of their properties such as mechanical and thermal stability, large numbers of additives are normally added to polymeric matrices to form a polymer matrix composite with a distinguishable interface and different chemical and physical properties [57]. Based on the nature of the matrices, composite materials may be classified into four major groups such as polymer matrix composite, carbon matrix composite, metal matrix composite, and ceramic matrix composite [58].
1.1.2. Preparation methods of polymer nanocomposite materials
Polymer nanocomposite materials can be prepared using various techniques such as co-precipitation and ultrasound-assisted green chemistry method with extract aqueous lemongrass [36], solvothermal or hydrothermal process [[59], [60], [61]] and ionic cross-linking [62]; Patiño-Ruiz et al., 2021), just to mention a few.
1.1.3. Characterization techniques of the polymer nanocomposite materials
The core-shell structure, density and surface morphology of the prepared nanocomposite can be determined using a high-resolution transmission electron microscope and field-emission scanning electron microscope (FE-SEM) coupled to an EDS detector [36,60,63,64]. The crystallinity, thermal stability, physical and chemical characteristics of the polymer nanocomposite material can be explored using X-ray powder diffraction spectroscopy (XRD) and thermogravimetric analyser [63,65]. Their surface area, pore volume and diameter can also be identified using Brunauer-Emmett-Teller (BET) [64] while functional groups are normally characterized by Fourier-transform infrared spectroscopy (FTIR) [36,60,64]. They can also be characterized using atomic force microscope (AFM) and small-angle X-ray scattering [66], rheometric technique, Raman spectroscopy [36], differential scanning calorimeter (DSC), dynamic modulus analysis (DMA) and thermomechanical (TMA) [67].
2. Degradation and mechanism pathways of PAHs
Like other POPs, PAHs may undergo mineralization and degradation processesin the presence of chemical reactions such as AOPs, photolysis, photocatalysis, ultraviolet radiation and hydrogen peroxide (H2O2), among others. Different intermediates such as ring-opening structures and fragments, alcohols, benzoic and phthalic acid derivatives, hydroxylated- and oxygenated-PAHs and acyclic hydrocarbons may be formed in aquatic environments depending on the molecular weight of the parent PAHs [34]. Their detection can also be performed using different mass spectrophotometers.
2.1. Degradation or mineralization of PAHs using photocatalytic materials under sunlight
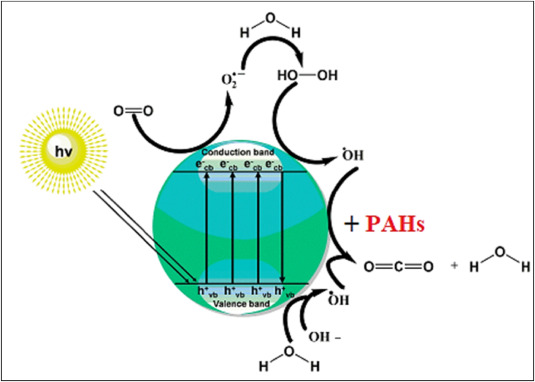
Different research works have been carried out on the degradation or mineralization of both LMW- and HMW-PAHs in aquatic environments using photocatalysts in the presence of solar irradiation [27,68]. Several catalysts are used to absorb the sunlight (λ < 380 nm) which facilitates them to undergo redox reactions: reduction on the conduction band and oxidation on the valence band [42]. These redox reactions support the conversion of oxygen into superoxide (O2−) and water into hydroxyl group (-OH) which will degrade or mineralize organic pollutants such as PAHs as shown in Fig. 1 [27].
 Fig. 1. A summary of photocatalytic decomposition of PAHs under sunlight [27].
Fig. 1. A summary of photocatalytic decomposition of PAHs under sunlight [27].For example [69], effectively confirmed that TiO2 could intensively catalyse the destruction of various PAHs such as fluorene (C13H10), naphthalene (C10H8) and anthracene (C6H4CH)2 using a 500 W high-pressure Mercury lamp [70]. further proved phenanthrene (C14H10) could be easily degraded over TiO2 in an aqueous dispersion under UV light [71]. used TiO2-based zinc hexacyanoferrate (C6FeN6Zn2) framework as a semiconductor catalyst to mineralize three-membered PAHs such as fluorene, phenanthrene and acenaphthene (C12H10) in river sediment, soil and water samples. Slower degradation in sediment and soil has been observed and might be ascribed to the reduced diffusion caused by the interaction between the organic content of sediment/soil with PAHs [71]. Smaller by-products like (E)-3-hydroxyacrylaldehyde (m/z = 71) and (Z)-prop-1-ene-1,2,3-triol (m/z = 91) have been identified, evidently braced e− excitement from encapsulated nano-catalyst followed by hydroxyl radicals (active species) based oxidation of PAHs. In the same year (2019), a faceted TiO2 photocatalytic degradation technique under solar irradiation of anthraquinone in aquatic solution was performed [72]. Later, a novel Ca–Ag3PO4 composite under visible light technique has been used to mineralize phenanthrene in water [73]. Effective and fast photodegradation of phenanthrene and pyrene was also highlighted (Soni et al., 2020). In all photodegradation processes, the main products formed were carbon dioxide (CO2), water and other smaller organic by-products.
2.2. Detection of metabolites resulting from the degradation of PAHs
The determination of derivatives obtained after degradation is normally performed using techniques that are also used to determine the concentrations of parent phenols, PAHs and other alkylated- or hydroxylated-PAHs [16]. Like other POPs, emerging contaminants of concern, PAHs and their derivatives are normally analysed using a gas chromatography-mass spectrometry (GC-MS) [29,64,73]; Rani and Shanker, 2020), high-performance liquid chromatography(HPLC) [74], liquid chromatography-quadrupole time-of-flight mass spectrometry (LC-ToF/MS) [72], HPLC with a fluorescence detector [75], and isotope dilution capillary GC-MS tandem mass spectrometry [76].
3. Mechanism pathways of PAHs
PAHs undergo different mechanism pathways during their degradation or detoxification processes in aquatic environmental samples and each technique or PAH structure has its resultant metabolites or fragments. For example, the photo-Fenton oxidation of PAHs results in inorganic ions, carbon dioxide and water. Serial reactions like ketolysis, ring open reactions and hydroxylationcould be taken place to generate intermediate products and finally be effectively mineralized to carbon dioxide [70,77,78].
4. Polymeric nanocomposite materials for photocatalytic detoxification of PAHs in aquatic environments
As illustrated in the previous section, PAHs are mainly available in aquatic environments such as soil, air, water, sludge, food, and sediment in different concentration levels due to the substrates they are in contact with [29,79]. Thus, their screening and removal processes from these environmental matrices are automatically different. They can be removed from aquatic environments using conventional methods as well as new emerging technologies. The existing treatment techniques include photocatalytic degradation, chemical oxidation, extraction, bioremediation and adsorption (employing adsorbents such as nanocomposites, geo-sorbents, silica, zeolites, polymers, mesoporous biomass derivatives, and graphene-based materials) [77,80,81]. Advanced phytoremediation, enhanced remediation using electrokinetic remediation, integrated approaches, biocatalysts and green nano-remediation hold great potential and are still at the developmental stage [81]. A lot has been done on the photocatalytic degradation of PAHs in environmental matrices [40,42,77,78,[82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95]]. However, most of the work done concerned the degradation of phenanthrene (C14H10), anthracene (C6H4CH)2 and anthraquinone (C6H4(CO)2C6H4) using only photocatalysts under UV light due to their damaging effects on the environment, model and prevalence for scientific investigations [27,73,78,93]. Ag3PO4, ZnO-, TiO2- and MHCFs-based materials and others have received important attention for photodegradation of PAHs [77]. For example, the photodegradation of phenanthrene leads to the formation of 9,10-phenanthrenequinone, which is more harmful to humans than phenanthrene itself [27,96]. However, conventional physical and chemical removal methods simply transferred the PAHs into another form without destroying them into harmless compounds [78]. The amalgamation of bimetallic oxides or a combination of metals as an efficient photocatalyst demonstrated its propitiousness for their degradation from water resources [27]. Most of these techniques may present advantages and disadvantages [23,97,98]. The most applied and concerned are photocatalytic degradation, chemical oxidation and adsorption, among others. Their advantages and disadvantages are presented in Table 1.
| Methods | Advantages | Disadvantages |
|---|---|---|
| Photocatalytic oxidation |
|
|
| Chemical oxidation |
|
|
| Adsorption process |
|
|
Recently, there has been an increasing interest in developing sorbents by incorporating inorganic (metal and metal oxides) into organic polymers aiming at fusing their properties into one material. In the hybrid nanocomposites, the polymer matrix can be natural or synthetic and the nanofiller or photocatalyst is usually a metal or metal oxide. The nanofillers facilitate the polymer strengthening or the condition of a supplementary interaction mechanism with the metal ions. The addition of low contents of these photocatalysts into the polymers can lead to improvements in their barrier, thermal, flammability and mechanical properties without affecting their processability. They received important attention due to the ease of production, flexibility and lightweight [51]. The most applied PNMs for degradation of PAHs in environmental matrices are chitosan beads modified with TiO2 and iron oxide (FeO) nanoparticles (Chitosan-FeO/TiO2) [64] and magnetic metal oxides-chitosan nanocomposites (ZnFe2O4-Chitosan) under natural sunlight [99]. Thiourea-magnetite-TiO2modified-chitosan beads (Chitosan-T-FeO-TiO2) [36], graphene oxide silverphosphate (GO/Ag3PO4) and magnetic/graphene/chitosan nanocomposite (MG-Chitosan) [60] as shown in Table 2. Other PNMs found in the literature are poly-aminated Fe3O4@chitosan core-shell magnetic nanoparticles (Fu et al., 2020), cross-linked magnetic ethylenediaminetetraacetic acid (EDTA)/TiO2/chitosan [63], organic montmorillonite sodium alginate (Dai et al., 2020). These studies indicated that the immobilization of FeO nanoparticles within the chitosan structure enhanced the stability features of these nanoparticles against oxidative and acidic conditions. Chitosan-FeO/TiO2 showed the highest pore size and BET surface area that are attributed to the presence of nanoparticles, which increased the surface area and promoted the formation of porosity [64].
Table 2. Available PNMs, their obtained parameters and performances [36,60,64,89,99].
| Parameters and references | Polymer nanocomposite materials | ||||
|---|---|---|---|---|---|
| Chitosan-FeO/TiO2 | Chitosan-T- FeO/TiO2 | ZnFe2O4-Chitosan | MGO-Chitosan | GO/Ag3PO4 | |
| Pore volume (cm3/g) | 4.60 × 10−2 | 0.000147 | – | – | – |
| Pore size (nm) | 6.80 | 10.20 | – | – | – |
| BET surface area (m2/g) | 27.10 | 1.58 | 80.11 | – | – |
| Optimum pH | 8.02 | 6.50 | Neutral pH | 2.00 | – |
| Adsorption type | Langmuir and Freundlich models | Freundlich model | Langmuir model | Freundlich model | – |
| Adsorption capacity (mg/g) | 33.10 | 133.69 | 1.50 | 169.49 | – |
| Degradation efficiency (%) | 90.00 | – | 92–95 | – | 49.7–100 |
| Reusability performance of the catalyst (%) | – | – | 1st cycle: 95; 10th cycle: 91 | – | 5 times: 44.6–95.2 |
| Order model and kinetics | Pseudo-second order | Pseudo-second order | First-order | Pseudo-second order | Pseudo-first-order |
| Material | Mesoporous | Mesoporous | Mesoporous | Nano porous | – |
| Pollutant | Anthracene and phenanthrene | Naphthalene | Naphthalene | 2-Naphthol | Phenanthrene, pyrene and naphthalene |
| Aquatic environment | Seawater | Industrial effluents | Synthetic wastewater | Aqueous solution | Simulated wastewater |
| References | [64] | [36] | [99] | [60] | [89] |
This available literature reveals that a small number of PNMs have been used to photodegrade PAHs in industrial effluents, seawater, aqueous solution and synthetic wastewater samples [36,60,64,89,99]. From Table 2, anthracene, phenanthrene, naphthalene and its derivative 2-naphthol as LMW-PAHs were photodegraded using these PNMs under natural light. Only [100] evaluated the photodegradation of pyrene as an HMW-PAH, which is the most toxic in the environment due to its low solubility in water. However [101,102], highly recommended the application of photocatalytic degradation on HMW-PAHs as a suitable method because of its high efficiency, friendly and economical to the environment. The oxidation products of PAHs produced by a photooxidationprocess are more soluble in water than the parent PAHs [101]. Table 2 also shows that only chitosan- and graphene oxide-based PNMs have been employed to photodegrade these kinds of PAHs in water matrices. This was because they only need sunlight for action, and they are stable, recyclable, reusable and inexpensive materials. Chitosan is normally produced by a deacetylation of chitin and is one of the most abundant biopolymers in nature, with its unique properties such as crystallinity, biocompatibility, biodegradability, non-toxicity, hydrophilicity, adsorbing nature, solubility in a wide range of organic solvents, film-forming ability, and chelation metal ions by hydroxyl and amino functional groups [63,[103], [104], [105]]. Most of the PNMs found in the literature (Table 2) were based on the iron oxide or magnetiteincorporation, which generates reactive oxygen species via AOPs [106,107]; Solano et al., 2020). Another finding was no study was conducted on the photodegradation of PAHs in soil and sediments which are their main sink in the environment.
4.1. Photocatalytic degradation of PAHs in water
PAHs are among the organic chemicals that have increased attention for their potential carcinogenic effects [108]. They are organic hydrocarbons that are ubiquitous in the environment [6,109]; Sun et al., 2017). Their solubility in water depends upon pH, water matrix components, temperature and ionic strength [75,110]. They are commonly applied for the production of pigments, agrochemical production, plastics, dyes, pharmaceutical industries and even in wood preservatives [6]. PAHs can be produced primarily by incomplete combustion of various organic substances [19,111]. They are considered to be mutagenic, endocrine-disrupting and carcinogenic agents [112]. This class of pollutants can be often detoxified in the aqueous phase by photocatalytic materials such as ZnO-, TiO2-, MHCFs-, Ag3PO4-based and other types due to their potential photocatalytic activity. Photocatalysis is widely used to describe the process based on a series of light-induced redox reactions occurring when a semiconductor (e.g.: ZnO, TiO2, Al2O3, WO3, and SnO2), interacts with light to produce reactive species [22]. However, TiO2-based photocatalysts were found as the most popular candidates for the degradation of PAHs in the aqueous phase while others need reinforcement of graphene oxide or being incorporated into different polymers [77]. TiO2 is one of the most useful semiconductors due to its efficiency, high activity, photochemical inertness and low cost [22].
4.2. Photocatalytic degradation of PAHs in sediment and soil
PAHs are known as a subclass of POPs that are highly associated with anthropogenic activities such as industrial development, transport and settlement [79,112]. They are very persistent in soil, sludge and sediment than in water due to their lipophilic and persistent nature which increases with their molecular weights [113]. Once they reach the aquatic systems, they are easily absorbed in particulate matter and ultimately deposited in the sediments. PAHs in the sediments can be released into the water body and may continue to harm human health and the entire ecosystem. After different biochemical reactions, soils and sediments are the final deposition sinks of PAHs [79]. They can enter the food chain by deposition and transfer from soil [114]. However, their degradation rate varies depending on their solubility and molecular weight. PAH compounds can be assembled into different degradation and sequestration fractions in both sediments and soil depending on their bioavailability [115]. divided them based on decreasing availability into the following phases: water-extractable phase (or easily degraded), slow molecular diffusion of PAHs into microsites (transition) and sequestered residual phase which requires extraction with organic solvents. The important goal of the degradation processis the complete mineralization of PAHs to CO2, microbial carbon, water and other inorganic substances [116]. Regrettably, degradation of PAHs may result in the accumulation of metabolites (mainly quinones (C6H4O2), ketones (C₃H₆O), coumarins (C9H6O2) and dicarboxylic acid anhydrides (HO2C−R–CO2H)) that can be more soluble and/or more toxic than the parent compounds (Lundstedt, 2003). For example, fluoranthene degradation has been found to generate potentially leachable and more soluble metabolites [117]. [118] showed an enhanced but incomplete degradation of PAH compounds and noted a brief spike in leachate toxicity due to the accumulation of more soluble metabolites. However, after remediation was complete, final toxicity was negligible because the metabolites tended to be less stable and more soluble than the parent compounds, making them more available to degraders [118]. Intermediates of PAH degradation are not always bioavailable and can also be incorporated into the humic fraction of soil, making them less available and less toxic [119]. However, a suitable photocatalytic degradation method under sunlight irradiation using polymer-based nanocomposite materials has to be applied to completely degrade and mineralize these PAHs in soil and sediments. These polymer-based nanocomposite materials can work in almost the entire pH range and contain biodegradable polymers which are biocompatible and soluble in a wide range of organic solvents. They also contain different nanofillers which have been reported with the potential results for the photodegradation of PAHs due to their hydrophilic nature, good stability, UV resistance, high photocatalytic activity, non-toxic, low cost, good dispersion, high relative index, and excellent transparency to visible light [56,77].
5. Conclusion and perspectives
In conclusion, photocatalysis under sunlight irradiation is very promising for the degradation of toxic organic pollutants such as PAHs in aquatic environments. In this review, an overview of the various polymeric nanocomposite materials for the photocatalytic degradation and mechanism of PAHs in environmental media was presented. The paper provides a brief account of the chemistry, origin and photocatalytic transformation of PAHs, highlighting their bioaccumulation in aquatic environments. The use of polymer nanocomposite materials under sunlight as an adsorbent for PAH removal was discussed in this paper, together with the mechanism of the adsorption process. The paper finally recommends the application of polymeric nanocomposite materials for the treatment of PAHs-contaminated soils, sediments and waters especially the HMW-PAH ones as they were revealed to have high adsorption capacity, good degradation efficiency and are reusable several times. They can also work in almost the entire pH range and contain biodegradable polymers which are biocompatible and soluble in a wide range of organic solvents.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful for the financial support received from the South African National Research Foundation (NRF) [grant No. SRUG190420431876, 2021] for the postdoctoral fellowship to the first author.