1. Introduction
Tissue engineering devices, principally those used as implantable scaffolds, are generally made from biomaterials that offer different structures and properties. To this end, many biomaterials – both synthetic and naturally occurring – have been used for tissue engineering (TE) applications, where additional modifications to the scaffold material such as anchoring of biologically active entities are usually required. Recently, naturally occurring materials such as cellulose, chitosan, hyaluronic acid, and collagen have attracted significant amount of interests as potential materials for applications in TE [1]. Among these materials, cellulose is the most widespread naturally occurring material on earth, with a biomass production estimated to be about 1.5 × 1012 tons annually [2]. It is an important structural component found in cell walls of green plants and some species of bacteria also produce cellulose (i.e. bacterial cellulose, BC) in the form of biofilm [2]. While it is necessary to employ different chemical treatments to obtain pure cellulose from plants [3], BC is readily produced in pure form and does not contain any other compound present in the plant pulp or from animal origin [4]. Bacterial cellulose was originally discovered in the 1880s by A. J. Brown [5], [6]. The first documented BC applications in biomedical were reported by a Brazilian company in 1986, 1989, and 1990 in a series of patents (see patents WO 08602095, WO 08908148 and US 4912049) that discussed the applications of Biofill® in different TE applications, such as skin substitutes for burns and ulcers [7]. In parallel Johnson & Johnson explored the use of BC as a liquid loaded pad for wound care in 1986 and 1987 (see patents US 4588400 and US 4655758). Subsequent studies investigated biocompatibilities of BC using L929 cells (mouse fibroblasts cells) [8] and in rats [9]. Recently more extensive studies on BC have been conducted, and its full potentials in TE applications have started to be gradually realized [10], [11], [12].
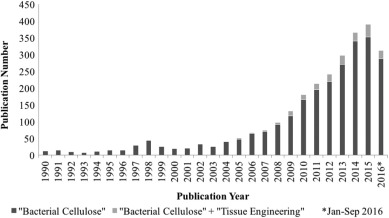
Unmodified BC has unique physical and mechanical properties not displayed by other biomaterials, such as high purity, ultrafine fibrous network structure with a variable pore geometry [13], [14], high water holding capacity, absorbing over 100 times of its own weight in water [15], [16], [17], high crystallinity (i.e. 84–89%) [18], broad chemical and physical modifying ability [19], [20], and the ability to mold into different structures [21]. Moreover, BC sheets and fibers have Young's modulus of 15–18 GPa and 78 GPa, respectively [22], [23]. However, its potential as a biomaterial in TE applications has not yet been fully explored. As shown in Fig. 1, there is a significant difference between the number of annual publications when “bacterial cellulose” was used as a key word and those when “bacterial cellulose” + “tissue engineering” were used (data obtained from Web of Science in September 2016). A closer look at the publications of BC with TE applications reveals that the majority of these papers mainly deal with investigations to modify BC scaffold porosities, introducing functional groups and/or antimicrobial molecules, increasing BC degradation rates, and enhancing BC biocompatibilities. Therefore, this review will first discuss general properties of BC and will subsequently focus on recent progress in in situ and ex situ modifications of BC in TE applications.
 Fig. 1. Annual publications on BC and BC with TE applications since 1990 to September 2016. Search engine used Web of Science™, search term “bacterial cellulose” and “bacterial cellulose” with refined term “tissue engineering”.
Fig. 1. Annual publications on BC and BC with TE applications since 1990 to September 2016. Search engine used Web of Science™, search term “bacterial cellulose” and “bacterial cellulose” with refined term “tissue engineering”.2. Bacterial cellulose
Cellulose (C6H10O5)n is a naturally occurring homopolysaccharide formed by a linear chain of monosaccharide β-d-glucose linked by β(1 → 4) bond. The repeating unit is cellobiose formed by the union of two glucose molecules (Fig. 2(A)). Bacterial cellulose is produced by a few strains of bacteria, such as Agrobacterium, Alcaligenes, Pseudomonas, Rhizobium, Sarcina, and Gluconacetobacter [4], [24], and secreted outside of the cells (Fig. 2(B)). These cellulose chains subsequently form elementary sub-fibers, which are then linked together by hydrogen bonding to form microfibrils. Ultimately microfibrils are organized together to form ribbons [25]. Bacterial cellulose biosynthesis has been extensively documented by Ross et al., who presented an account on BC synthesis “machinery”, genetics and its regulatory mechanism in a model organism Acetobacter xylinum, a non-pathogenic bacteria [26], [27]. Recently, many other publications provided overviews of genetic modifications of cellulose producing bacteria, focusing mainly on BC production yields [25], [28], [29], [30]; interested readers are referred to consult other excellent studies for examples of genetic engineering approaches to produce BC [31], [32], [33], [34], [35], [36].
 Fig. 2. BC structures. (A) Chemical structure of cellulose; (B) SEM micrographs of BC membrane showing cellulose nanofibers and bacteria excreting them reproduced from [23] with permission of Springer; (C) photograph of BC membrane after purification.
Fig. 2. BC structures. (A) Chemical structure of cellulose; (B) SEM micrographs of BC membrane showing cellulose nanofibers and bacteria excreting them reproduced from [23] with permission of Springer; (C) photograph of BC membrane after purification.After BC synthesis, the membrane is further processed to remove bacterial cells, organic acids, salts as well as residual sugars and other components of the culture medium, which could be integrated within the cellulose network. This purification process can be accomplished by various methods, including washing, centrifugation, filtration, and chemical extraction. Among these methods, washing BC with hot and diluted sodium hydroxide solution, rinsing with distilled water, and finally sterilizing the BC by autoclaving is commonly used [9], [37], [38], [39]. Fig. 2(C) shows a BC membrane obtained by washing with 0.1 M NaOH at 50 °C followed by extensive washing with distilled water. It should be noted that there are no reports in the literature concerning the presence of pathogen-associated molecular patterns in the purified BC; in fact, it will become clear later in this review that BC has been shown to be biocompatible. It is believed that bacteria produce cellulose biofilm in order to protect the organisms from ultraviolet radiation and other chemical or mechanical insults to the bacteria [26], as well as to improve nutrient transport [23]. Bacterial cellulose membrane is formed in a structure with asymmetrical layers at the air/liquid interface, resulting in a denser surface where it is in contact with air and a more gelatinous network on the other side where it is in contact with the liquid [9], [40], [41], [42], [43].
Bacterial cellulose can be produced in different shapes and molded into 3D structures during in vitro cultures, depending on the production method chosen (i.e. static culture, agitated culture or airlift reactor) [21], [41], [44], [45]. In addition, the resulting BC properties, such as mechanical properties as well as micro- and macro-structures are also influenced by bacterial culture conditions [25], [46], [47], [48], [49]. An ideal biomaterial for TE applications must be capable to promote correct tissue regeneration; it must also provide a variety of shapes and sizes, promote cell–biomaterial interactions, and have microstructures suitable for new tissue formation [50]. Due to its structural similarities to extracellular matrix (ECM) components, BC has been reported to show good biocompatibilities. For example, using MTT assay and confocal imaging, Recouvreux et al. showed that human vein endothelial cells proliferated and migrated vertically into a BC hydrogel [21]. In addition, Zang et al. studied differentiation of human adipose-derived mesenchymal stem cells (HASCs) cultured on BC, and showed that the HASCs were successfully differentiated into osteoblasts and formed a consistent layer of osteoblasts on the BC [51]. Moreover, BC scaffolds seeded with the HASCs were implanted into ulna defects of rabbits, and significant mineralization was observed in the defects after 8 weeks when compared with the control group; the researchers also noted that there were no signs of any inflammation responses [51].
Furthermore, BC has been used in many other in vivo studies. In a meticulously designed study, Helenius et al. subcutaneously implanted BC membranes on the back of Wistar rats, and subsequently evaluated host responses to the implanted BC material in terms of foreign body reactions, chronic inflammation, angiogenesis, and cell growth for a period of 12 weeks. The study showed that BC was fully integrated with the host tissue and did not induce any inflammation or rejection during the course of the study [52]. In another study, BC tubes were shown to exhibit good biocompatibility when they were cultured with primary Schwann cells and were subsequently implanted in a sciatic nerve injury model in Sprague Dawley rats for 6 weeks [53]. Moreover, to evaluate their potentials as substitutes for small-diameter blood vessels, the BC tubes were used to replace carotid arteries in Texel sheep for a period of 12 weeks. It was observed that a confluent endothelial cell layer without any signs of inflammation was formed along the BC tubes and that vascular smooth muscle cells migrated into the BC tube matrix [54]. Similar results were also observed in a study by Kowalska-Ludwicka et al. who used BC tubes for peripheral nerve regenerations for a period of 6 months [55]. Table 1 summarizes findings described above. Indeed, many studies have demonstrated that BC can be well integrated with host tissues for many different TE applications, as can be seen in Table 2.
Table 1. Summary of unmodified BC applications in TE application.
| Application | Experiment | Results | References |
|---|---|---|---|
| Scaffold material |
|
|
[21] |
|
|
[52] | |
| Bone tissue engineering |
|
|
[51] |
| Nerve regeneration |
|
|
[53] |
|
|
[55] | |
| Blood vessel replacement |
|
|
[54] |
Table 2. List of additional examples of BC applications in TE.

3. Modifications of bacterial cellulose for applications in tissue engineering
Despite recent advances, there are still many challenges to overcome before the full potentials of BC can be completely realized as a choice of material in TE applications. These challenges include optimizing culture conditions to control porosities of BC scaffold, introducing functional groups to BC matrix, and increasing BC degradation rate for specific applications [76], [77], [78]. To achieve these goals, BC has been modified using different methods, such as chemical modifications: modification of the chemical structures and functionalities, and physical modifications: modification to change porosities, crystallinities, and fiber densities. In general, there are two main strategies to implement these modifications, i.e. in situ and ex situ methods (Fig. 3). In situmodification is to modify BC during bacterial cell culture by varying the culture conditions, adding additional materials such as additives or reinforcement materials to the culture, or changing the carbon source which may or may not cause chemical changes in the resulting BC (see Fig. 3(A)). In contrast, ex situmodification of BC is carried out after the BC has been formed, and it is done by either chemical or physical methods (see Fig. 3(B)).
 Fig. 3. Schematic representation of BC modification methods. (A) In situmodification of BC, in which the culture medium composition is changed, usually with the addition of other materials, such as additive/reinforcement material. (B) Ex situ modification, in which BC is modified by chemical treatment or absorption of others materials after the BC membrane has been formed in culture.
Fig. 3. Schematic representation of BC modification methods. (A) In situmodification of BC, in which the culture medium composition is changed, usually with the addition of other materials, such as additive/reinforcement material. (B) Ex situ modification, in which BC is modified by chemical treatment or absorption of others materials after the BC membrane has been formed in culture.There are many studies on in situ [17], [79], [80], [81], [82], [83] and ex situ [17], [18], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96]modifications of BC; however there are few researches focusing on TE applications of these new composites. In the following sections, we summarize recent literatures on modifications of BC for applications in TE.
3.1. In Situ modification of bacterial cellulose
In this technique, additive materials are added to BC culture medium at the beginning of the BC production. In situ modification represents a common and important modification method of BC. The additive materials are incorporated into the growing BC fibrils networks, resulting in BC composite materials that possess combinations of both desired properties. In addition, this modification method can also change physicochemical, mechanical, and morphological properties of the resulting BC composite biomaterials [97], [98], [99], [100], [101], [102]. A schematic representation of the modification of BC composites through the in situ strategy is shown in Fig. 3(A).
Bacterial cellulose has been increasingly used in bone tissue engineering as a scaffold material [65], [67], [100], [103]. To control the porosity of the BC scaffolds, in situ modifications are generally employed. For example, paraffin microspheres were added in BC culture medium to produce BC scaffolds with enhanced porosities [56], [59], [65], [103]. Specifically, Zaborowska et al. incorporated paraffin wax microspheres into Gluconacetobacter hansenii culture medium to prepare micro-porous BC scaffold for bone regeneration (Fig. 4) [65]. After the BC formation, all paraffin wax microspheres were removed by extensive washing in surfactant Berol EZ-1, and osteoprogenitor cells were cultured with the resulting micro-porous BC scaffolds. The study showed that the new micro-porous BC scaffold significantly enhanced cell infiltration into the scaffold (Fig. 4(B) and (C)), suggesting a potential TE application in bone regeneration [65], [103]. In addition, the resulting scaffold had a Young's modulus of 1.58 ± 0.78 MPa. While this value was four to five orders of magnitude lower than that of human bones, it was similar to hydrogels that have been shown to successfully regenerate bone tissues [104]. As a result, the authors argued that the micro-porous BC scaffold might be suitable for certain bone regeneration applications, such as plate bones of the face and skull. Likewise, another study reported the preparation of microporous BC scaffolds using a similar technique by adding various sizes of paraffin particles to a tubular fermentation vessel containing Acetobacter xylinum in order to form BC ducts for use in urinary diversion [56].
 Fig. 4. Micro-porous BC scaffold prepared by incorporation of paraffin wax microspheres into bacteria culture medium and its use in vitro. (A) Schematic drawing of a bioreactor used for in situ modification of BC. Confocal microscopyimages of osteoprogenitor cells seeded on (B) modified scaffold and (C) control. Cell nucleus is stained blue and actin cytoskeleton is stained red adapted from [65] with permission from Elsevier.
Fig. 4. Micro-porous BC scaffold prepared by incorporation of paraffin wax microspheres into bacteria culture medium and its use in vitro. (A) Schematic drawing of a bioreactor used for in situ modification of BC. Confocal microscopyimages of osteoprogenitor cells seeded on (B) modified scaffold and (C) control. Cell nucleus is stained blue and actin cytoskeleton is stained red adapted from [65] with permission from Elsevier.In addition, hydroxyapatite nanoparticles (HA - Ca5(PO4)3(OH)) were used as an additive in the BC culture medium to evaluate the biological properties of the new BC scaffold for bone regeneration [67]. To test the effectiveness of the composite scaffolds in promoting bone regeneration, the BC scaffolds were implanted in defects of rat tibiae in vivo. The results showed that no inflammatory reactions were found around the implant, and the bone defects were completely filled with new bone tissue 4 weeks post implantation [67]. The synthesis of a porous BC composite was reported by Barreiro et al. [102]who used sand dollar skeleton (Clypeaster subdepressus), mainly composed of CaMg(CO3)2, to prepare an interconnected porous BC scaffold structure in Gluconacetobacter hansenii culture. The study showed that the sand dollar skeleton served as bio-inert support for the BC composite, and apatite particles were deposited over the BC surface. The scaffold showed a compressive strength of 3.6 ± 0.8 MPa, which the authors suggested was adequate for bone regeneration, specifically for tibial cancellous bone.
An ideal wound dressing should preserve a humid environment at the wound site, have a good oxygen permeability, provide mechanical protection, and absorb excess exudates. In addition, it should also be made from a readily available biomaterial that requires minimal processing, possesses antimicrobial properties, promotes wound healing, and allows removal without pain or trauma [69]. To this end, a wide variety of BC composites has recently been explored as potentially promising wound dressing materials. For example, BC/glucose and BC/dextrin composites prepared by in situ fermentation method were reported, and the composites were shown to have higher porosity and better biocompatibility as demonstrated by a MTS assay ((3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium), in comparison with the BC controls [105]. Furthermore, this study also showed that the modified BC composite materials had enhanced water holding capacity and rehydration rate, both of which were important as wound dressing materials [105]. Similarly, BC/potato starch [106], BC/cotton gauze [107], and BC/aloe vera [82] composites were also synthesized through the in situ method for wound dressing and temporary artificial skin applications. It should be noted that in situ modifications only alter BC membrane morphologies and its physical properties; however the chemical compositions of the BC membrane are not changed in the in situ modifications.
In practice, one important attribute of any wound dressing material is its antimicrobial properties against infections. In an attempt to incorporate antibacterial properties into BC scaffolds, many efforts have been made. For instance, Butchosa et al. modified BC with deacetylated chitin nanocrystals (D-ChNCs), materials that are known to have antimicrobial effects, by both in situand ex situ (see Section 3.2) modification approaches [108]. More specifically, for the in situ modification, D-ChNCs were added in the cell culture during BC production. The in situ modification resulted in a homogeneous incorporation of chitin nanocrystals into the BC network, and exhibited a tensile strength in the range of 377–449 MPa, depending on the composition of the membrane. In addition, the modified BC membrane showed a significantly increased antibacterial activity in comparison with the unmodified controls [108]. In a recent study, Orelma et al. reported the preparation of BC tubes modified initially by an in situ approach with carboxymethyl cellulose (CMC) (i.e. BC/CMC tubes) and subsequently with an ex situ approach by an antibody [109]. Specifically, for the in situ modification, CMC was first added in the BC culture to produce BC/CMC tubes. The as-prepared BC/CMC tubes were then modified ex situ by covalently conjugating with anti-human serum albumin (HSA) antibody via EDC/NHS chemistry, as shown in Fig. 5 [109]. While this study was designed for detection and separation of specific target proteins and not specifically for incorporation of antimicrobial properties into BC membranes, the approaches it took to introduce antibodies to native BC could be useful to introducing antimicrobial moieties to BC.
 Fig. 5. BC tubes modified by in situ approach with carboxymethyl cellulose (CMC) and subsequently by ex situ approach by an antibody. (A) Illustration of in situ BC tubes modification with carboxymethyl cellulose (BC/CMC tubes) and further functionalization with antibodies. Micrographs of the CMC/BC tubes (B) before and (C) after purification adapted from [109] with permission of The Royal Society of Chemistry.
Fig. 5. BC tubes modified by in situ approach with carboxymethyl cellulose (CMC) and subsequently by ex situ approach by an antibody. (A) Illustration of in situ BC tubes modification with carboxymethyl cellulose (BC/CMC tubes) and further functionalization with antibodies. Micrographs of the CMC/BC tubes (B) before and (C) after purification adapted from [109] with permission of The Royal Society of Chemistry.BC has also been used as an artificial blood vessel. Wang et al. used in situmethod to modify BC with chitosan (CS) to produce BC/CS composite that was subsequently ex situ modified by chemically coupling heparin (Hep) to the BC/CS to obtain a BC/CS/Hep composite. The composite showed excellent antimicrobial and anticoagulant properties and a high mechanical strength. Moreover, MTT assays indicated that the BC/CS/Hep composite had excellent biocompatibility, suggesting its potential application in vascular grafts [110].
Although in situ modification of BC has been widely used in many TE applications (see Table 3), the restrictive microbial fermentation conditions limit the introduction of wider varieties of additives. Furthermore, other issues with the in situ modification method, such as interactions between the externally introduced additives and BC fibril growth as well as structure controls of BC nanofibers, still need to be addressed.
Table 3. Summary of in situ modifications of BC for TE applications.
| Application | Bacteria⁎ | Additive material | Modified properties | Reference |
|---|---|---|---|---|
| Scaffold for TE | A. xylinum | Collagen | Color, thickness, roughness, stiffness, porosity, and crystallinity | [99] |
| A. xylinum | Polystyrene and optical fibers | Porosity, fiber network, crystallinity, mechanical property, and swelling behavior | [101] | |
| Bone regeneration | G. hansenii | Sand dollar skeleton | Porosity, density, thickness, and mechanical property | [102] |
| G. xylinus | Hydroxyapatite | Thermal property, fiber diameter and orientation, porosity, crystallinity, mechanical property, and biocompatibility | [67], [97], [98] | |
| G. hansenii/G. xylinus | Paraffin | Porosity, mechanical property, and biocompatibility | [59], [65], [103] | |
| Wound dressing/artificial skin | G. hansenii | Glucose or dextrin | Surface area, water holding capacity, rehydration rate, porosity and biocompatibility | [105] |
| G. xylinus | Potato starch | Thickness, density, porosity, mechanical property, rheological, crystallinity, and biocompatibility | [106] | |
| A.xylinium | Cotton gauze | Water absorbency, wicking ability, drying time, and porosity | [107] | |
| A. xylinum | Aloe vera | Porosity, surface area, mechanical property, water absorption capacity, water vapor permeability, and crystallinity | [82] | |
| A. aceti | Deacetylated chitin nanocrystals | Mechanical property and antimicrobial activity | [108] | |
| Vascular grafts | A. xylinum | Chitosan and heparin | Porosity, roughness, surface area, and biocompatibility | [110] |
| Biofiltration | G. medellinensis | Carboxymethyl cellulose | Thickness, porosity, water retention, charge, and composition | [109] |
| Urinary reconstruction | A. xylinum | Paraffin | Porosity, mechanical property, and biocompatibility | [56] |
- ⁎
-
G. = Gluconacetobacter; A. = Acetobacter. Ex situ modification of bacterial cellulose.
Bacterial cellulose is a biopolymer that can be subjected to almost all techniques used for polymers [47]. In ex situ modifications, BC polymer matrixis impregnated with different materials to modify BC membranes (Fig. 3(B)), and this usually happens after the BC purification process. There are two types of ex situ modifications: chemical and physical. In the case of chemical ex situmodification, BC is reacted with reagents to modify its chemical composition. Since the chemical composition of BC is cellulose, it can be first phosphorylated and then modified by graft copolymerization or crosslinking reactions to achieve BC modification [111]. In contrast, physical ex situ modification is commonly done through physical absorption – porous BC matrix can be filled with solutions or particle suspensions – the presence of hydroxyl groups of cellulose chains often results in strong hydrogen bonding between the BC molecules and absorbed molecules to achieve modification [25]. The ex situmodification through absorption is much simpler and more versatile than the in situ modification method [20].
3.2. Physical ex situ modification
In order to enhance its cell permissiveness, BC nanocomposite and silk fibroin (SF) were prepared using a physical ex situ modification method by Barud et al. [112]. More specifically, BC membranes were soaked into different SF solutions of 25%, 50%, and 75% for 24 h. Results by SEM revealed a well inter-connected porous network structure. The best result achieved was with 50% fibroin content (BC/SF50), where the equal ratio offered a synergistic effect while preserving individual properties of BC and SF. It was further demonstrated that BC/SF50 scaffolds improved cell permissiveness in comparison with pure BC. Furthermore, cytotoxicity and genotoxicity tests showed that the BC/SF50 composite was non-cytotoxic and non-genotoxic, suggesting its potential applications in biomedical applications [112]. Similarly, Gao et al. investigated poly(ethylene glycol) 400 (PEG400) absorbed into BC sponge [113]. The resulting sponge exhibited high surface area, high porosity, and excellent biocompatibility as shown by MTT assays with mesenchymal stem cells [113].
To incorporate antibacterial properties into BC, sodium alginate (SA) and silver sulfadiazine (AgSD) were used to prepare a BC film [114]. Bacterial cellulose was synthesized in a static Acetobacter xylinum culture. Subsequently, the BC membranes were sliced and compressed to form BC slurry. The BC/SA was prepared by mixing BC slurry with SA solution (2.0%, w/v), to which AgSD was added; finally, the mixture was crosslinked by CaCl2. The resulting BC/SA–AgSD composites showed excellent antibacterial performances against Escherichia coli, Staphylococcus aureus and Candida albicans while MTT assays suggested good biocompatibility with human embryonic kidney cells. These results demonstrated that BC/SA–AgSD composites could potentially be used as a wound dressing material [114]. Similarly, benzalkonium chloride [115] and polyvinyl alcohol (PVA) with potassium sorbate [116] were absorbed into BC film to introduce antimicrobial activities to BC [115]. In another study, PVA was used to produce BC/PVA composite, which was used to further produce an antimicrobial composite comprising potassium sorbate. The antimicrobial properties of the composite was tested against Escherichia coli and showed antimicrobial activities that positively correlated to the concentration of potassium sorbate used in the BC composite [116].
Nanostructures of BC membranes can act as a template in the synthesis of a variety of nanomaterials, such as silver nanoparticles (AgNPs), which have been known to introduce antimicrobial properties to BC through ex situmodifications. These nanostructures of BC membranes play a crucial role in the formation of AgNPs by acting as both nano-reactors for nanoparticle nucleation/growth and particle-size restrictor to limit out-of-control growth of the particles, producing small size nanoparticles with a well-controlled narrow particle size distribution. For example, Yang et al. prepared a BC membrane impregnatsed with AgNPs (BC/AgNPs) by initially immersing the BC membrane in silver nitrate (AgNO3) solution after which the impregnated silver ions (Ag+ 1) were reduced to Ag0 particles [117]. Antimicrobial properties of the BC/AgNPs was tested against Escherichia coli and Staphylococcus aureus. The results showed that the BC/AgNPs composite exhibited clear inhibition zones against both model bacteria tested (i.e. 2 mm for E. coli. and 9 mm for S. aureus), while no inhibition zone was observed for native BC (Fig. 6) [117]. Similar methods were described in others studies which showed comparable antimicrobial results [118], [119], [120].