Hemodialysis membrane fouling and human serum protein adsorption
Hemodialysis is the most prescribed renal replacement therapy (RRT) for patients who lost kidney function due to chronic kidney disease (CKD). Hemodialysis is a membrane-based treatment that allows the removal of toxins and excess fluids. In the classic modality of hemodialysis, toxins are removed via diffusion across the membrane where the counter-current flow allows a maximized concentration gradient. Other treatment modalities (hemofiltration, hemodiafiltration) allow the removal of toxins and fluids via diffusion as well as convection, ultrafiltration, and adsorption [1]. Currently, there is a wide variety of membrane materials and structures applied to hemodialysis. However, they all face common challenges associated with plasma protein adsorption. As the hemodialysis treatment is initiated and the patient's blood comes in contact with the dialyzer membrane, plasma proteins almost instantly adsorb to the membrane surface due to the electrostatic interactions.
The adsorption of proteins adversely affects hemodialysis performance in two ways: by creating a secondary filtration layer (protein cake layer) which reduces the selectivity of the membrane and the overall efficacy of the treatment, and by triggering the activation of biochemical cascades that lead to the release of inflammatory cytokines. Even though extensive investigations have been reported regarding the adsorption of blood proteins to biomaterials such as HD membranes, authors still differ as to which protein initially triggers complement activation and how the composition of the adsorbed layer changes over time. In our previous review article [2], we discussed the main factors affecting protein adsorption during hemodialysis including blood flow rate, dialysate flow rate, and treatment time. In the present critical review, we focus on the specific characteristics of three main protein molecules to better understand their adsorptive behavior during hemodialysis and their role in the patients' health. We will also discuss the competitive adsorption and occurrence of the Vroman effect and its potential impacts on hemodialysis.
Because of its vital significance, protein adsorption was extensively studied using a variety of experimental techniques [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. However, despite this long-lasting effort, the understanding of these phenomena still remains incomplete. Conflicting views persist concerning the most fundamental issues such as the nature of interactions driving the protein adsorption, reversibility, monolayer structure and stability, conformational changes, maximum coverages, isotherm interpretation etc.
Membranes comparison
HD membranes can be classified according to their chemical composition as biological (cellulose), modified biological (modified cellulose), or synthetic. Cellulose was the primary material used in HD membranes due to its desirable transport properties until its severe bioincompatibility issues were associated with poor clinical outcomes. Nowadays, commonly used HD membrane materials include polyethersulfone (PES), polyacrylonitrile (PAN), cellulose triacetate (CTA), and polyvinylpyrrolidone: polyarylethersulfone (PVP: PAES) [22], [23], [24], [25].
Despite the fact different membranes have different chemical composition the surface chemistry is a principal area that should be considered when designing potentially biocompatible materials. There are various ways to functionalize the surface with –OH,−CH3, −OSO3H, −COOH and –NH2 groups [26] thus nihilating the difference between membrane bulk chemistry. Some of these functional groups can be created by plasma treatment [27], [28], [29], [30]. There are number of researches devoted to surface modification to improve membrane antifouling properties and to enhance biocompatibility [31], [32], [33], [34], [35].
Surface charge is another key factor that plays a major role in protein adsorption. It should be pointed out that all cell membranes have negative charges [36], [37], [38]. Also, -SO3H and -COOH groups that result in negative charge are present in natural anticoagulant heparin and are believed to be to determine its anticoagulant activity [39], resulting to study of heparin mimicking polymers [34, 35].
However, the latest trend in hemocompatible membranes development includes approach of creating near zero surface charge by applying zwitterionic (ZW) polymer coverings that are reported to enhance membrane biocompatibility [22, 40, 41].
Other factors like hydrophilic-hydrophobic character, wettability and surface free energy [42], [43], [44], [45], [46] and polymer crystallinity [47], [48], [49], [50], [51] were also studied.
Protein structure, function, and adsorptive behavior in hemodialysis
Human blood is mainly composed of water and proteins which are fundamental pieces in a variety of physiological functions. Proteins spontaneously fold secondary structures into tertiary structures where amino acids are rearranged forming active sites responsible for performing different biological functions including adsorption. Some proteins can assume a quaternary structure if they are composed of multiple polypeptide chains (Fig. 1). This folding is governed by hydrophobic effects so in an aqueous solution the protein structure forms a hydrophobic core (like a pocket), and the polar residues stay on the surface in contact with the solvent and are free to interact and adsorb. In this section, we will review the structure, function, and adsorptive behavior relevant to the context of hemodialysis of human serum albumin (HSA), fibrinogen (FB), and transferrin (TR). HSA, FB, and TR are acute-phase proteins and their synthesis by the liver is affected by the presence of inflammatory cytokines commonly observed in HD patients. These proteins have specific transport and adhesion properties in the human organism, however, there are specific roles these proteins play in HD patients. HSA influences the removal of protein-bound uremic toxins (PBUTs) and its excessive leakage across the membrane can lead to severe outcomes to HD patients related to hypoalbuminemia. FB is an adhesive protein and triggers coagulation and complement activation upon contact with HD membranes. Therefore, understanding FB structure, function, and adsorption are key to assess the biocompatibility of HD membranes. TR levels are significantly influenced by the presence of inflammatory cytokines and HSA. Additionally, TR is a small but relatively abundant protein in human plasma, therefore it plays a key role in the competitive adsorption phenomena described as the Vroman effect. For these reasons, the present review focuses on these three highly abundant proteins and their influence on HD patients.
 Fig. 1
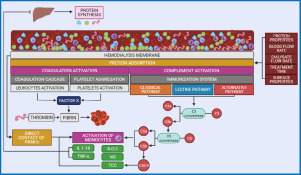
Fig. 1The formation and growth of a protein cake layer on the dialyzer membrane surface increase the overall resistance to flow across the membrane. The mechanisms involved in blood protein adsorption to HD membrane is a complex phenomenon due to the highly heterogeneous composition of blood. In overly simplified terms, first, the proteins approach the membrane via the diffusion mechanism. Then, protein molecules adhere to the membrane as a result of static interactions and finally, the protein molecule undergoes conformational changes at the membrane surface [52,53]. Protein adsorption on the surface is the first step of many undesired bio-reactions and bio-responses, followed by platelet adhesion and activation of coagulation pathways, leading to thrombus formation [54].
Albumin
Human serum albumin (HSA) is the most abundant protein in human blood circulating at 35–45 g/L. HSA participates in multiple functions including regulation of osmotic pressure, protection against oxidative stress, and transportation of several molecules [55]. The HSA molecule is synthesized in the liver and it is a single peptide chain protein formed of 585 amino acids and it's presented in three homologous domains (I, II, and III) which are further divided into subdomains (A and B) of similar structural motifs, but which have different ligand-binding functions [56]. The HSA molecule is a 67 kDa globular protein and in the physiological environment, 68% is found in α-helix configuration. As a transport protein, HSA can easily bind to a wide range of molecules via ligands, including protein-bound uremic toxins (PBUTs). Additionally, the HSA molecule interacts with polymeric surfaces and undergoes conformational changes upon adsorption [57].
In CKD patients, uremic toxins accumulate due to the decline in renal function characterizing the uremic syndrome. Most of these toxins can be successfully removed during hemodialysis via diffusion or convection, however, some of these toxins referred to as PBUTs possess a high affinity to plasma proteins, in particular to HSA. Upon binding to the HSA, these toxins are unlikely to be eliminated using the traditional hemodialysis modality of treatment [58,59]. With that, one of the options to remove these harmful toxins is to utilize medium and high cut-off membranes which remove HSA molecules from the bloodstream by convection or targeted adsorption [60]. Several strategies such as protein-leaking membranes and absorptive nanomaterials have been extensively investigated and were discussed in detail by Daneshamouz et al. (2021). Some argue that the increased mortality in patients with hypoalbuminemia is due to malnutrition and inflammation rather than the low HSA level. With that, controlled leakages and adsorption of HSA during different modalities of hemodialysis have been deemed acceptable to maximize the removal of a wide range of toxins [60]. However, it is of extreme importance to monitor the HSA levels to prevent or control hypoalbuminemia.
Even when HSA loss is minimal due to filtration or adsorption, hemodialysis patients suffer from hypoalbuminemia as a result of malnutrition inflammation syndrome [61]. Clinically significant hypoalbuminemia is diagnosed by HSA level lower than 25 g/L [55]. Renal failure has been discarded as the root cause for suppression of albumin synthesis but hypoalbuminemia still a risk factor and a mortality/morbidity predictor regardless of the underlying condition. Patients with hypoalbuminemia have increase mortality/morbidity, longer hospitalization periods, and higher readmission rates. Hypoalbuminemia has been linked to malnutrition and inflammatory responses, with the latter having a more predominant effect. Patients with malnutrition have reduced dietary intake of protein and it's not an uncommon scenario for dialysis patients. Evidence points out that underdialyzed patients can suffer a loss of appetite leading to suboptimal intake of dietary proteins which results in reduced levels of blood urea nitrogen (BUN). Consequently, this low level of BUN provides clinical evidence for further reducing the dialysis dose keeping the patients underdialyzed.
Hemodialysis patients present high levels of chronic inflammation caused by the release of cytokines as a consequence of the activation of biochemical cascades upon contact between blood and dialyzer membrane [62]. HSA is a negative acute-phase protein (APP), therefore its synthesis is suppressed by the inflammatory response [63]. This means that the presence of cytokines such as interleukin (IL)−1, IL-6, and TNF-α suppresses the synthesis of albumin in the liver while increasing the fractional catabolic rate contributing to hypoalbuminemia [55]. This inflammatory state promotes vascular permeability leading to an increase in the exchange of HSA from the intravascular to the extravascular space.
The properties of the dialysis membrane play a critical role in the inflammatory response and consequently in hypoalbuminemia. In our recent study, we compared the inflammatory profile of hemodialysis patients from St. Paul's Hospital in Saskatoon (Canada) treated with Polyaryl Ether Sulfone-Polyvinylpyrrolidone (PAES:PVP) and cellulose triacetate (CTA) membranes [62]. Although both membranes promoted inflammatory response, those treated with the polymeric membrane presented higher levels of IL-1β, IL-6, and TNF-α. The differences in inflammatory response were attributed to the membrane morphological and chemical properties. The PAES:PVP membranes have a significantly rougher and more hydrophilic surface, which favored red blood cells dehydration and promoted more severe protein adsorption. This membrane material also presented a trace amount of sulfur and nitrogen which have been linked to decreased biocompatibility. We also performed docking studies to evaluate the binding affinity between the membranes and different protein molecules, including HSA. We observed that PAES:PVP membrane has a higher affinity to HSA as well as FB and TR when compared to the biopolymer (cellulose) membrane. We were able to determine the HSA amino acids involved in the hydrophobic and hydrophilic interactions (Fig. 2). Upon interaction with the polymeric membrane the following amino acid were involved in hydrophobic interaction: Phe211, His242, Ala215, Trp214, Leu219, Arg218, Ala291, Ile290, Leu260, Ala261, Tyr150, Ser287, and Leu238. Additionally, hydrophilic interactions with Lys199, Arg222, and Arg257 were identified. The biopolymer (cellulosic) membrane, on the other hand, interacted with Asp451, Arg218, Arg222, Lys199, Lys195, and Glu292. Since we can identify which structures of the membrane material are involved in the interactions with specific amino acids, these studies play a critical role in the development of novel more biocompatible membrane materials.
 Fig. 2
Fig. 2The antifouling properties of the HD membrane can be enhanced by surface modification with graphene oxide (GO-Ag) nanosheets [64], heparin [65], ozone treatment [66], UV [67], coating with hydroxyl containing polymers [68] and pluronic based on polyethylene oxide (PEO) [69] and other methods.
Albumin adsorption on various surfaces was thoroughly studied [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91] including water surface at different pHs [70], colloidal particles [71], nanoparticles [77, 92, 93] and carbon nanotubes [94]. Albumin adsorption kinetics at various pHs and ionic strength was demonstrated to be governed by the bulk transport [72]. Adsorption takes place despite an electrical repulsion between the protein and the membrane surface when pH exceeds isoelectric point (pI = 4.7), indicating that coulombic attractions do not dominate the protein interaction at all conditions [95], though negligible adsorption of HSA was observed at pH 7.4 and 9.7 at negatively charged polystyrene microparticles [90]. Kelly and Zydney [96] also reported that albumin aggregation increased with increasing pH, despite the increase in electrostatic repulsion between individual albumin molecules at higher pH. At the same time, less adsorption was observed on PES membranes at pH 6.80 compared with the adsorption at pH 3.78 due to decrease in hydrophobicity with increase of absolute value of zeta potential with pH raise [97]. When the surface contains NH2 groups, increased albumin adsorption at lower pH (4.0–4.9) is associated with electrostatic interaction between positively charged surface (due to appearance of NH3+) and negatively charged protein [82]. Surface roughness of the size comparable with protein molecule dimensions exerts a decisive influence on albumin adsorption kinetic derived from quartz microbalance (QCM) measurements [73, [98], [99], [100]].
Fibrinogen
Human fibrinogen (FB) is a glycoprotein synthesized in the liver and circulates in plasma at 1.5–3.5 g/L in the human body. The molecular structure consists of 2 identical monomers which are constituted by 3 non-identical peptide chains (α, β, and γ). The structure stability is attained by the interaction of the chains through disulfate bridges. The FB molecule is organized in a trinodal structure with one central node and two distal nodes connected through 3 α-helices chains of the 3 FB chains. The molecular weight of FB is 340 kDa and crystallographic studies indicate that the length is approximately 45 ± 2.5 nm [101,102]. The synthesis of FB is related to that of HSA which we have discussed to be strongly influenced by the inflammatory response [103]. As a positive APP, the synthesis of FB is up-regulated by pro-inflammatory cytokines, especially IL-6. These components trigger intercellular signaling pathways in hepatocytes and modulate gene expression via various transcription factors [104].
The role of FB in protein-mediated blood activations has been extensively investigated. The adsorption of FB plays a central role in hemodialysis due to its participation in the activation of the coagulation and complement system. The literature reports that the binding strength between FB and membrane surface is higher than that for other highly abundant proteins such as HSA and immunoglobulin. While HSA does not influence platelet adhesion, FB plays a key role in biomaterial-induced thrombosis. Once adsorbed to the membrane surface, FB binds to platelets via cell membrane receptors leading to platelet adhesion, aggregation, and activation which mediated thrombus formation [105]. The extent of these reactions depends not only on the membrane properties but also on the hydrodynamic conditions set by blood and dialysate flow rates. Our recent studies demonstrated that the adsorption of FB although faster is milder when high blood flow rates are applied [106]. Tanaka et al. (2000) [107] demonstrated that not only the amount of FB adsorbed but also the protein conformational change affect platelet activation.
The coagulation and complement systems are interconnected and even though the mechanisms are not yet fully elucidated. Since FB isoforms are associated with activation of the coagulation cascade, they directly or indirectly influence the activation of the complement system. The complement system is able to differentiate between components of the body from foreign species. Its role is to attack and destroy foreign structures via direct lysis or by activating inflammatory and immune responses [108]. The complement system can be activated via different pathways (classical, lectin, or alternative) depending on the properties of the biomaterial. The contact between blood and foreign surfaces like hemodialysis membranes has been reported to activate the complement system via the alternative pathway (AP) [108]. Regardless of the pathway, complement component 3 (C3) is cleaved into C3a and C3b. As the C3b level increases, C5-a convertase generation is promoted which leads to cleavage of C5 into C5a and C5b. C5a is a powerful anaphylatoxin and promotes a procoagulant state in hemodialysis patients, whereas C5b binds to the surface and interacts with C6-C9 to form the membrane complex attack (MAC/C5b-9) [109].
During hemodialysis, the activation of the complement system is been observed to be triggered by the adsorption of ficolin-2 to the membrane surface, which activates the lectin pathway (LP) [110]. The ficolin-2 molecule possesses a collagen-like chain and a fibrinogen-like head and is able to bind with many partners. Polysulfone membranes have been observed to adsorb ficolin-2 and mannose-binding lectin (MBL) which trigger the LP, as well as properdin, which activates the AP. Additionally, complement inhibitors can be absorbed by the membrane, leading to further dysregulation of the pathways. Complement activation can lead to serious clinical outcomes related to inflammation, coagulation, and reduced host defense. Koga et al. (2018) reported that reduced FB adsorption onto HD membranes reduced inflammatory response and oxidative stress which minimizes cell activation [111]. Based on the literature reports and our recent studies, enhancing the biocompatibility of hemodialysis membranes is without a doubt a top priority.
Many researches are devoted to fibrinogen adsorption process [24, 65, 67, 68, 81, 86, 89, 93, [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127]]. Fibrinogen was adsorbed to a broad range of surface chemistries from a wide range of solution concentrations, with the amount and conformation of adsorbed fibrinogen determined by absorbance and circular dichroism (CD) spectropolarimetry, respectively [114]. Platelet adhesion response was determined by lactate dehydrogenase (LDH) assay and scanning electron microscopy (SEM). Conformation of adsorbed fibrinogen was shown to be the critical determinant of platelet adhesion, not the amount of fibrinogen adsorbed. Adsorbed fibrinogen conformations favorable for platelet adhesion were identified on both hydrophobic and hydrophilic surfaces [119]. However, in the hydrophobic surfaces these conformations seem to be present only when the molecules are involved in fiber formation. Conformational changes of adsorbed fibrinogen on hydroxyapatite surface were also observed [118]. Brash et al. compared 125l-fibrinogen adsorption in the presence of normal plasma to plasma deficient in specific proteins [116]. Fibrinogen was found to be displaced from the surface. The extent of displacement was greatly reduced, however, but not eliminated in high-mol-wt kininogen (HK)-deficient plasma. Factor Xll-deficient plasma also showed reduced fibrinogen displacement. These data indicate that HK can actually displace fibrinogen; however, factor XII or a factor XII-mediated reaction also appears to be necessary for this displacement to occur. Study of fibrinogen adsorption on hydroxyl‑reach glass surface revealed that hydrophilicity increases the tendency of fibrinogen molecules to interact with the surface rather than with other molecules, thus inhibiting fibrinogen self-assembly [117].
The development of novel membrane materials of improved biocompatibility for hemodialysis patients has been a great challenge for many decades. The transition from biopolymer (cellulosic) membranes to synthetic polymers represented a great advance, but it came with new biocompatibility concerns. Before synthesizing novel membrane materials, we must understand how the material can potentially interact with blood components, especially with proteins. To that end, we have invested in docking and molecular dynamic simulation studies. These computational techniques provide insight into the preferred binding sites, orientation, and binding energy between two molecules that allow us to understand the binding energy as well as the forces involved in the interactions. We have observed that FB possesses an affinity to the polymeric materials than the biopolymer (cellulosic) ones we have investigated [62]. Saadati et al. (2020) observed that PAES interacted with chains A and B from the FB molecule while the ligand model did not fit into the active pocket of chain H. The interaction between chains A and B and the PAES model involved the amino acids Ala218 B, Ser123 B, Asn215 B, Asp224 A, and Phe160 A. Mollahosseini et al. (2020) reported that FB interacts more intensely with PAES:PVP when compared to HSA and TR. The reported final stable interaction energy for FB was two orders of magnitude higher. The interaction was mostly associated with strong hydrogen bonding. The size of the FB molecule and the higher number of active sites are believed to important factors in these results.
Transferrin
Another highly abundant plasma protein in humans is transferrin (TR). TR is a monomeric glycoprotein of 80 kDa. The molecule is structured in two homologous lobes (N- and C-lobes) connected by a short peptide. C-lobe contains a carbohydrate moiety attached to it. Each lobe is divided into subdomains that connect two antiparallel β-sheets that act as flexible joints. The shape of the TR molecule allows iron binding and transportation which takes part in the immune response [128]. The normal concentration of TR in adults varies between 2 and 5 mg/L [129]. However, the physiological levels of TR depend on the iron intake, so patients with low protein dietary intake or malnutrition may present higher TR levels (hypertransferrinemia). On the other end of the spectrum, hypotransferrinemia is associated with hypoalbuminemia which as previously discussed can be a result of malnutrition, inflammation, and transmembrane loss of HSA for dialysis patients (Fig. 3). A high prevalence of hypotransferrinemia has been observed in chronically dialyzed patients and it's has been observed to be a complex multifactorial phenomenon. Hypotransferrinemia in combination with non-inflammatory functional iron deficiency has been identified as major mechanisms of iron disorders in CKD anemia patients. Similar to HSA, TR is a negative APP so its concentration in the serum concentration decreases in response to the presence of inflammatory cytokines as previously discussed.
 Fig. 3
Fig. 3Even though these associations have been found, very little information can be found on the mechanism of transferrin adsorption and the competitive effect with other proteins during hemodialysis. In our previous work, we used molecular docking to estimate the binding affinity of TR to one polymeric membrane material as well as one biopolymer (cellulosic) material, both widely used in hemodialysis treatment in Canadian hospitals. We observed that TR possesses a higher binding affinity to the polymeric material (PAES:PVP) than to the biopolymer (cellulose triacetate - CTA) one. When compared to other highly abundant blood proteins, TR presented a higher affinity to PAES:PVP than FB but lower than HSA. Regarding the biopolymer (cellulosic) material, the affinity values were only slightly different, and TR presented binding affinity sightly higher than the two other proteins. This can be an indication that TR molecules have to compete with HSA and FR for adsorption sites during HD in a phenomenon described as the Vroman effect (Fig. 4).
 Fig. 4
Fig. 4Adsorption kinetics
According to the literature, protein adsorption can be quantified using adsorption isotherm, the kinetics of adsorption/desorption, the conformation of adsorbed proteins, the number and character of protein segments in contact with the surface, in addition to several parameters related to the adsorbed protein layer (i.e. refractive index and the layer thickness) [130], [131], [132]. The protein adsorption isotherm is defined in the literature as “a function that relates the measured adsorbed amount of protein (per unit area) to the solution concentration of a protein” [130]. According to Hlady et al. (2009), there are two models of adsorption isotherms of protein: lattice binding function (Eq.1) and bulk ligand-binding function (Eq.2) [133].
Where θs represents the fraction of saturation of lattice sites, Γsres is the lattice sites surface concentration, Ks is adsorption constant of the lattice site, and ns is the adsorption coefficient of the lattice site [133]. Furthermore, the θB is the fractional saturation of binding units on the surface at a constant lattice site concentration with the bulk protein as an independent variable, while cp is the bulk protein concentration at equilibrium, KB is the bulk-ligand adsorption constant, and nB is the bulk-ligand adsorption coefficient [133]. Adsorption isotherms can be used not only to describe the process but also to understand the affinity of the protein and the surface [130]. The main disadvantage of lattice models is related to the limitation in defining the size of a ‘site’ on the membrane surface. This difficulty is associated with the fact that protein molecules can occupy more than one site and the lack of information on protein structure upon adsorption [132].
The expected shape of protein adsorption kinetics is a monotonically increasing curve that after a sufficiently long time period reaches saturation. A rather unexpected phenomenon of an overshoot during the adsorption kinetics was observed, which refers to a situation where the adsorption kinetics passes a local or global maximum before the saturation is reached [134], [135], [136], [137], [138], [139].
In a number of colloid and polymer adsorption studies overshoots and even oscillations of adsorption kinetics are reported and mechanistically explained by the so-called time delay model [140], [141], [142]. According to this model an overshoot during the adsorption occurs when the surface is temporarily oversaturated and equilibration is reached through a net desorption of polymers despite a further supply of polymer solution. This means, adsorption begins when desorption from the surface is not allowed. After a certain time delay, however, desorption starts due to conformational rearrangements which may cause the overshoot provided the surface is fairly covered and consequently oversaturated. However, the properties and shapes of these overshoots differ broadly which has led to a variety of concepts seeking to explain this peculiar behavior. The most prominent work on this issue was conducted by Vroman et al. [143], [144], [145] who investigated the adsorption of proteins from blood plasma to a solid interface. It turned out that the protein Fibrinogen rapidly adsorbs to the surface but after a short time passes through a coverage maximum and finally covers the surface in smaller amounts at the equilibrium state than in the intermediate state. Experiments with differing protein compositions revealed that this behavior is actually a displacement effect due to which Fibrinogen is replaced by other proteins of higher surface affinity, predominantly by the protein high molecular weight kininogen (HMWK). Numerous subsequent studies confirmed this mechanism which was hence concluded to be of general validity [146], [147], [148]. In recognition to his initial studies this effect is now called ‘Vroman effect’ [149, 150]. Elofsson et al. observed an overshoot during the adsorption of β-Lactoglobulin which was attributed to an initial adsorption of metastable octamers that were subsequently replaced by the more stable monomers and dimers [137].
Based on comprehensive experimental investigations Rabe et al. have suggested a consistent explanation of the overshooting effect that combines the idea of orientational or conformational rearrangements with some aspects of the time delay model [136]. The authors studied the adsorption kinetics of the model protein β-Lactoglobulin (β-Lg) on a hydrophilic glass surface using fluorescence detection. It turned out that in the beginning all proteins bind in an irreversible manner to the surface as no desorption can be observed upon buffer rinse. However, once a certain coverage level has been exceeded in the course of adsorption a sudden alteration of the binding behavior from irreversible to reversible takes place. In contrast to some previous explanations that suggested the formation of a first irreversible protein layer on which a second reversible layer is built [151], [152], [153], here it was proven that all proteins, including the new ones and those which were already adsorbed before on the surface, are affected by this affinity alteration.
Competitive adsorption, Vroman effect, and its influence on hemodialysis patients
Human blood is a highly heterogeneous protein solution and each protein interacts differently with a foreign surface such as a hemodialysis membrane. As proteins adsorb to the surface, the composition of the protein layer is gradually changing, and this change affects the blood filtration and the blood activation reactions. The multiprotein adsorption process has been observed to take place as a combination of transport, adsorption, and repulsion processes [154], [155], [156]. It has been proposed that smaller proteins adsorb first due to higher diffusivity and hence they are the predominant species in the primary adsorption stage. These small proteins are rigid and experience a lower degree of spreading when compared to larger proteins. Larger proteins diffuse at a slower rate towards the membrane surface, but they tend o bind more strongly to the surface due to their larger surface area. This proposed mechanism is based on factual knowledge, but it seems to be oversimplified for the present context. According to Horbett (2013) [154], [155], [156], the multiprotein adsorption process is affected by three main driving forces, namely the intrinsic affinity between proteins and the solid surface, the chemical nature of the solid surface, and the relative bulk concentration of each protein. The adsorption of proteins to the membrane surface takes place within milliseconds, but the spreading of the proteins is a slower process than can take several hours [154], [155], [156]. The interaction between protein and surface induces a gain of free energy so the protein molecule undergoes conformational changes and spread on the solid surface to maximize its footprint. Depending on the degree of spreading, proteins can be divided into two groups: hard and soft proteins. Depending on the degree of interaction, a large protein can dislocate a small one in a phenomenon called the Vroman effect. This dynamic interaction between multiple proteins and the membrane surface leads to progressive changes in the composition of the protein cake layer, affecting the filtration performance and biocompatibility profile of the surface. The experimental observation of the Vroman effect is very challenging due to the microscopic scale and dynamic nature of the process. However, obtaining an accurate description of this effect can help the assessment of the biocompatibility of hemodialysis membranes and lead to the development of membrane materials that promote attenuated blood activation reactions.
We have observed that the binding affinities between protein and biomaterials are dependent on the chemical composition of the biomaterial. For instance, the ligand of PAES:PVP presented two important interactions that differentiate the polymeric ligand interaction from the cellulosic ligand with FB were identified. One was the polar contact from the SO2 substituent inserts into the hydrophilic pocket of proteins and receptor active sites and the second was the hydrophobic interaction from protein residues interacts with phenyl groups in the PAES:PVP model. Consequently, when the same proteins are exposed to a different material the adsorptive behavior will be different and will activate different biochemical cascades.
Several biocompatibility enhancement strategies have been reported [154], [155], [156], [157]. We have synthesized a zwitterionic (ZW)-coated polyether sulfone (PES) clinical membrane and we utilized ATR-FTIR analysis to assess the fouling tendency due to FB adsorption. A significantly lower fouling tendency on the ZW-coated membrane was reflected on reduced IR intensity corresponding to amide 1 and a decrease in the α-helix content of the adsorbed FB. We also utilized synchrotron-based micro-computed tomography (SR-μCT) for in-situ visualization of the fouling phenomena and assessment of protein adsorption in different microlayers of the membrane. We were able to confirm the improvement provided by ZW-coating, observing a smaller fraction occupied by protein molecules on the coated membrane. Synchrotron-based imaging is a powerful tool for protein adsorption studies for the high spatial and temporal resolution which allows the 3D visualization of the protein deposition without interfering in the process. Many studies have demonstrated that we can synthesize biomaterials that preferably adsorb one protein over another to improve its biocompatibility. But, without knowing precisely how each protein interacts with the material in the presence of other blood components, how can we determine which protein should adsorb first? The adsorption of each protein leads to the activation of different mechanisms and consequently different patient outcomes.
Fibrinogen adsorption on Ti6Al4V particles research revealed the difference between fibrinogen and albumin adsorption. The fibrinogen saturation degree is limited at early absorption stages while this limitation affects the whole range studied for albumin [67]. Fibrinogen was found as well as albumin to be adsorbed preferably by negatively charged surfaces rather than neutral ones [86]. Brimeel et al. showed that functionalization of PEG with sulfonate groups results in increased adsorption of albumin from its 0.15 M salt solutions compared with fibrinogen [89]. Under lower ionic strength conditions albumin adsorption was again minimized as a result of the increased electrical double-layer interaction observed with the PEG-SO3Н modified surface. This unique and unexpected adsorption behavior of albumin provides an alternative explanation to the “negative cilia” model used by others to rationalize observed thromboresistance on PEG-sulfonate coatings. The difference in albumin and fibrinogen adsorption was also demonstrated using C2C12 cell line [113]. Results showed that the amount of albumin absorbed was greater than the amount of fibrinogen (comprised in the range of 70%–85% and 10%–22% respectively). Park et al. studied fibrinogen and albumin adsorption on glass surfaces from single protein solutions or from a mixture of the two proteins at varying bulk concentrations [122]. It was found that fibrinogen molecules always formed multimeric aggregates even at surface concentrations much lower than a monolayer coverage. On the other hand, albumin did not form multimers when the surface concentration was lower than that of the monolayer. The results indicate that patchwise adsorption is the unique feature of fibrinogen adsorption.
Outlook
During HD protein adsorption occurs spontaneously due to the electrostatic interactions between protein molecules and membrane surface. However, each protein has unique features and interact with the membrane surface differently. In the context of HD treatment, HSA plays a critical role in the removal of PBUTs and its synthesis can be suppressed due to inflammatory response. FB participates in the activation of clotting/coagulation events and can give rise to serious outcomes in the short- and long term. TR is fundamental for the transport of iron in the body and it's negatively affected by the presence of inflammatory cytokines. The literature reports that transmembrane protein loss and inflammatory response lead to hypoalbuminemia and hypotransferrinemia which can have a severe impact on patients’ health. Based on the current scientific knowledge, these low protein abnormalities induced by HD treatment can be mitigated by enhancing membrane biocompatibility and optimizing HD operating conditions. The development of more biocompatible materials has been a challenge for material scientists. Utilizing computational methods such as molecular docking and molecular dynamic simulations are time-efficient, cost-effective methods for identifying potential interactions between membrane ligands and different protein molecules. With this knowledge, new membrane materials can be developed targeting to promote or hinder specific interactions improving the biocompatibility of the materials. Understanding the mechanisms of competitive protein adsorption during HD is also an important piece of the biocompatibility puzzle. Advanced imaging techniques such as SR-μCT allow the assessment of the occurrence of membrane fouling with negligible interference in the protein adsorptive properties. An in-depth understanding of the effects associated with HD operating conditions is also critical to avoid protein-adsorption-induced side effects. Statistical and mechanistic mathematical models can aid the prescription of more favorable blood flow rate, dialysate flow rate, treatment time based and membrane material base don patients’ profile and treatment goals. With an increasing incidence of CKD worldwide, more patients have been prescribed some modality of HD every year. A combined effort should be continuously made to improve not only membrane biocompatibility but also to optimize HD operating conditions to avoid serious outcomes and improve HD patients’ quality of life.
Declaration of Competing Interest
No conflict of interest to declare.
Acknowledgments
The authors would like to acknowledge and express their gratitude to Saskatchewan Health Research Foundation (SHRF) for the fund provided. The authors are also grateful for the Department of Chemical and Biological Engineering and the Division of Biomedical Engineering at the University of Saskatchewan.