1. Introduction
Tomato (Lycopersicon eculentum L.) is grown throughout the world, with an annual production that, in 2014, has exceeded 170 million tons (FAOSTAT, 2014). The majority of tomato fruits produced are consumed in processed form, such as peeled tomato (whole or diced), juices, sauce and ketchup, whose manufacture often requires peel removal (Rock et al., 2012).
Thus, the industrial transformation of tomatoes typically includes a peeling phase of the fruits, consisting in the use of either hot lye solutions or steam blanching (SB), which, however, suffers from various disadvantages such as disposal of caustic, high pH waste solution, and excessive water and energy consumption (Pan et al., 2009, Rock et al., 2012).
Recently, the “FieldFood” (635632-FieldFOOD-H2020) project investigated the possibility of coupling a mild pre-treatment of whole tomatoes by pulsed electric field (PEF) at field strength and energy input below 1 kV/cm and 1 kJ/kg, respectively, with SB, as a less energy-intensive peeling treatment, as compared with a conventional peeling process (TecnAlimentaria–Food Industry, 2017).
However, as suggested by the vast literature on the topic, PEF pre-treatment might be expected to have a beneficial effect also on the permeabilization of the tomato peels, enabling the recovery of valuable intracellular compounds (Barba et al., 2015). The main effect of the application of PEF pre-treatment of plant tissues, at an electric field of moderate intensity (E < 10 kV/cm) and relatively low energy (WT < 10 kJ/kg), is the permeabilization of the cell membranes, which facilitates the selective recovery of intracellular compounds from the inner parts of the cells (Barba et al., 2015, Donsì et al., 2010, Pataro et al., 2017).
Tomato peels, together with seeds and unused pulp, are the main by-products of tomato fruit processing, representing 2–5% in weight of the total processed tomatoes (Knoblich et al., 2005).
The tomato peels currently find low-added value uses as animal feed and fertilizers (Knoblich et al., 2005, Strati and Oreopoulou, 2014), or are directly sent to landfill (Rossini et al., 2013). However, they are still rich in important nutrients, such as proteins, lipids, carbohydrates, and fibers, and constitute a primary source of several carotenoids (Knoblich et al., 2005, Strati and Oreopoulou, 2014).
Carotenoid compounds are natural pigments, with health-beneficial properties, which are accumulated in the chloroplasts and chromoplasts of several fruits during ripening (Pataro et al., 2015, Singh et al., 2015). Lycopene is the most abundant carotenoid in tomato processing by-products. In particular, it accumulates in the peels (Strati and Oreopoulou, 2014), at concentrations about five times higher than in tomato seeds (Knoblich et al., 2005) and pulp (Luengo et al., 2014).
Lycopene, along with β-carotene, is an authorized natural pigment for several types of food products (Strati and Oreopoulou, 2014). Moreover, due to its remarkable antioxidant activity, it is also widely used in skin cosmetic products for its anti-aging properties (Lenucci et al., 2015). Because of the its activity in reducing the risk of cardiovascular diseases, atherosclerosis, prostate cancer and cognitive impairment, it is also used as food supplement or nutraceutical ingredient in the formulation of food products (Lin and Chen, 2003, Queralt et al., 2013, Strati and Oreopoulou, 2014, Zuorro et al., 2011).
For all the above reasons, in the last decade the global market of carotenoids exhibited a tremendous growth, which is expected to reach around US$ 1.53 billion in 2021, with a compound annual growth rate (CAGR) of 3.78% between 2016 and 2021 (MarketsandMarkets, 2016). This increasing trend is also reflected by the growing number of patents deposited worldwide on the extraction processes of carotenoids from natural sources (Riggi, 2010, Strati and Oreopoulou, 2014).
Conventional extraction processes of carotenoids are usually based on the maceration of the by-products using an organic solvent (e.g., acetone, hexane, ethanol, diethyl ether, methanol and petroleum ether) or a solvent mixture with high affinity for lipid-soluble compounds (Lin and Chen, 2003, Strati and Oreopoulou, 2011a, Strati and Oreopoulou, 2011b). However, these methods are time-consuming, and often require large amounts of solvents, relatively high temperature, and may eventually lead to the degradation of the thermosensitive compounds, such as carotenoids, as well as to the co-extraction of undesirable components, thus increasing the downstream processing costs (Luengo et al., 2014, Strati and Oreopoulou, 2014). In addition, before extraction, the by-products often require a pre-treatment, mainly comminution and drying, which is costly and may cause significant losses of valuable compounds (Knoblich et al., 2005, Luengo et al., 2014, Strati and Oreopoulou, 2014).
Therefore, the implementation of an innovative wet disruption method, such as PEF, has been proposed as an intensification pre-treatment in the extraction of valuable intracellular compounds from food residues, which is also able to prevent their degradation, reduce the energy costs, the solvent consumption and shorten the treatment time (Luengo et al., 2014).
Many investigations have proved that PEF can enhance the extraction yield of water-soluble natural pigments and antioxidant compounds such as polyphenols, flavonoids, and anthocyanins from a wide range of food processing by-products (Barba et al., 2015, Bobinaitė et al., 2015, Boussetta et al., 2012, Chemat et al., 2017, Corrales et al., 2008, Luengo et al., 2013, Parniakov et al., 2016, Pataro et al., 2017), while there are limited data about the effect of PEF on the extraction of non-polar compounds (Luengo et al., 2014, Roohinejad et al., 2014, Wiktor et al., 2015, Yin et al., 2008).
In particular, to date, only the study of Luengo et al. (2014) has addressed the PEF-assisted extraction of lipid-soluble compounds, such as carotenoids, from tomato peels, which have been treated by PEF after hand peeling of fresh tomatoes.
In addition, no studies have been published on the extractability of carotenoids from tomato processed by-products (peels), after steam blanching (SB) of whole tomato fruits.
The objective of this work was to investigate the use of PEF, alone and in combination with SB, as pre-treatment of whole tomato fruits, with the aim of improving the extractability of carotenoids from tomato processing by-products (peels).
Specifically, the effects of different electric field strengths and steam blanching temperatures on the cell disintegration index of peel tissue, as well as on the total content and composition of carotenoids and antioxidant activity of the extracts were investigated.
2. Materials & methods
2.1. Chemicals and raw material
Fully ripened tomatoes of the “Pachino” variety were purchased from a local supermarket and stored in dark under refrigerated conditions (4 ± 1 °C) until use, within 5 days from purchase.
Color measurements were performed on the surface of tomatoes with a tristimulus colorimeter CR400 Chroma Meter (Konika Minolta Inc., Japan). Five readings were taken at random positions from each fruit. Data were collected in CIE L*a*b* color space and the values of L*, a* and b* were recorded and used to evaluate the combination parameter hue (H) angle, which indicates the actual color or the redness.
Tomatoes of similar size (about 2.6 cm in diameter) and color (H = 45.8 ± 1.8) were selected prior to PEF, SB or PEF + SB pre-treatments, in order to use fruits that exhibited a homogeneous carotenoid concentration (Luengo et al., 2014, Pataro et al., 2015).
Physical-chemical parameters of selected tomatoes, such as total soluble solids (6.53 ± 0.15°Brix), titratable acidity (0.44 ± 0.05 g citric acid/100 g fresh weight tomatoes) and moisture content (90.2 ± 0.8 gwater/100 g fresh weight tomatoes), were also determined.
HPLC grade methanol and acetonitrile as well as acetone, iron (III) chloride hexahydrate (FeCl3·6H2O), citric acid and 2,4,6-tripyridyl-s-triazine (TPTZ) were purchased from Sigma-Aldrich (Steinheim, Germany). Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was obtained from Acros Organics (Geel, Belgium). Sodium acetate and acetic acid were purchased, respectively, from Panreac (Panreac Quimica, Barcelona, Spain) and Fisher (Fisher Scientific, Rodano, Italy).
2.2. PEF apparatus
PEF-assisted extraction of carotenoids from tomato peels was carried out using a laboratory scale batch system. It consisted of a high voltage pulsed power (20 kV-500 A) generator (Modulator PG, ScandiNova, Uppsala, Sweden) able to generate monopolar square wave pulses (3–25 μs, 1–450 Hz). The generator was connected by a high voltage cable to a batch parallel plate treatment chamber (Donsi et al., 2011) with an electrode area of 75 cm2, while the distance between the electrodes could be adjusted up to 5 cm, depending on the volume of the treated sample. The actual voltage and current signals in the treatment chamber were measured using a high voltage probe (Tektronix, P6015A, Wilsonville, OR, USA) and a Rogowski coil (2–0.1 Stangenes, Inc., USA) connected to a 300 MHz digital oscilloscope (Tektronix, TDS 3034B, Wilsonville, OR, USA). The maximum electric field intensity (E, in kV/cm) and total specific energy input (WT, in kJ/kg) were calculated as reported in Bobinaitė et al. (2015).
2.3. PEF, SB, and PEF + SB-assisted extraction
For each experiments, approximately 150 g of whole tomatoes (10 fruits) were subjected to PEF, SB or PEF + SB pre-treatments. In a first set of experiments, tomato fruits were loaded into the PEF treatment chamber with tap water at a constant solid to liquid ratio (1:1 g/mL) and exposed to different field strengths (E = 0.25, 0.50, and 0.75 kV/cm) at a constant total specific energy input (1 kJ/kg), frequency (10 Hz) and pulse width (20 μs). These PEF parameters were determined on the basis of preliminary experiments to ensure the preservation of the fruit integrity, and improve its peelability (TecnAlimentaria–Food Industry, 2017) while inducing a sufficient degree of cell membrane permeabilization of tomato peels at minimum energy consumption. In all PEF experiments, the initial temperature of the samples was 20 ± 1 °C and no appreciable temperature increase was detected due to the low energy input delivered during the treatment. All the PEF treatments were performed in triplicate.
After the electrical pre-treatment, tomato fruits were hand peeled, and square pieces (1 cm2) were cut out of the removed peels. Approximately 1 g of tomato peels was immediately placed into a 100 mL pyrex flask, where acetone was added at a constant solid to liquid ratio (1:40 g/mL). The flasks were incubated for 4 h in a water bath set at 25 °C, under constant shaking at 160 rpm. These extraction conditions were sufficient to reach significant extraction yields of the target intracellular compounds (data not shown). Moreover, in agreement with previous works, the low extraction temperature contributes not only to limit the operation cost, but also to avoid undesirable degradation reactions of the carotenoids (Singh et al., 2015, Strati and Oreopoulou, 2011a).
Samples of identical size and shape were manually cut from the peels recovered from untreated tomato fruits, to be used as controls.
A second set of experiments investigated the effect of the pre-treatment of tomato fruits, based either on SB alone or on its combination with PEF (PEF + SB), on the extraction yield of carotenoids from the tomato peels. Fresh and PEF treated tomato fruits were subjected to SB in a lab-scale steam oven (Minea, SO25P, France) for 1 min at different blanching temperatures (TSB = 50, 60, and 70 °C). All SB and PEF + SB treatments were performed in triplicates. After treatment, the fruits were hand peeled and subjected to the same extraction protocol described above.
The extracts from untreated and treated (PEF, SB, PEF + SB) samples were then centrifuged at 5700 × g (PK121R model, ALC International, Cologno Monzese, IT) for 10 min at 4 °C to separate the supernatant, which was then filtered through 0.45 μm syringe filters. The final extracts were then stored at −20 °C until further analysis.
2.4. Cell disintegration index
Cell disintegration index (ZP) was used to quantify the degree of cell membrane permeabilization of tomato peel tissues induced by PEF, SB, or PEF + SB pre-treatments of whole tomato fruits before extraction. The determination of ZP via impedance analyses was carried out according to the method described by Bobinaitė et al. (2015). Triplicate measurements of electrical complex impedance in frequency sweep (103–107 Hz) were carried out by loading 5 g of square pieces (1 cm2) cut out of the peels of untreated or treated tomato fruits into the measuring cell connected to an impedance analyzer (Solartron 1260, UK). For each treatment condition investigated, the ZP value, ranging from 0 (for intact tissue) to 1 (for fully permeabilized tissue), was calculated on the basis of the measurement of the absolute value of the complex impedance of untreated (Zuntr) and treated tissue (Ztr) in the low (1 kHz) and high (1 MHz) frequency ranges (Donsì et al., 2010).(1)
2.5. Determination of total carotenoid (TC) content
The total carotenoid (TC) content of tomato peels extracts from untreated and treated samples was determined according to the method described by Lichtenthaler and Wellburn (1983). The absorbance of undiluted extracts was measured at 470 nm (A470), 645 nm (A645), and 662 nm (A662), in a V-650 UV-Vis spectrophotometer (Jasco Inc., Easton, USA). Absolute acetone was used as a blank. The total content of carotenoids, expressed in mg/100 g of fresh weight (FW) peels, was calculated from the following equations for 100% acetone:(2)(3)(4)where Ca is the content of chlorophyll a, Cb is the content of chlorophyll b, and Cx+c is the content of carotenoids. All the assays were performed in triplicate.
2.6. Evaluation of ferric reducing antioxidant power (FRAP) of extracts
FRAP assay of tomato peels extracts was carried out according to the method described by Benzie and Strain (1996) with some modification. Before the measurements, 0.3 M sodium acetate buffer (pH 3.6) was prepared by dissolving 3.1 g of sodium acetate and 16 mL of acetic acid in 1000 mL of distilled water; 10 mM TPTZ solution was prepared by dissolving 0.031 g TPTZ in 10 mL of 40 mM HCl; 20 mM ferric solution was prepared by dissolving 0.054 g of FeCl3·6H2O in 10 mL of distilled water.
The FRAP working solution was prepared by freshly mixing 0.3 M sodium acetate buffer, 10 mM TPTZ solution, and 20 mM ferric solution at a ratio of 10:1:1 (v/v/v). For the analysis, 2.5 mL of freshly prepared FRAP working solution and 0.5 mL of undiluted extract were mixed and incubated for 10 min at ambient temperature. The change in absorbance due to the reduction of ferric-tripyridyltriazine (Fe III-TPTZ) complex by the antioxidants contained in the samples was monitored at 593 nm using a V-650 UV-Vis spectrophotometer (Jasco Inc., Easton, USA). The absorption of blank samples (applying the same analysis conditions) were tested each time before and after analysis. Trolox was used as the standard for calibration curve and the FRAP values were expressed as mmol of Trolox equivalents (mmol TE) per 100 g of FW tomato peels. All the assays were performed in triplicate.
2.7. HPLC analysis of carotenoid compounds
For the identification of individual carotenoids, the tomato peel extracts of untreated and treated samples were further analyzed by high-performance liquid chromatography (HPLC).
Carotenoids were separated using a Waters 1525 series HPLC system, equipped with a Waters 2996 photodiode array detector (DAD) (Waters Corporation, USA). Analytical separation of carotenoids was carried out in a Waters Spherisorb C18 reverse phase column (5 μm ODS2, 4,6 mm × 250 mm, Water Corporation, USA). The temperature of the HPLC column was set at 30 °C. Before the injection, the tomato peel extracts were filtered through 0.20 μm filters. The mobile phase consisted of acetonitrile/methanol (30:70, v/v). The flow rate of the mobile phase through the column and the injection volume were 1.5 mL/min and 100 μL, respectively. The absorbance detection wavelength was 472 nm.
The identification of the major carotenoids in tomato peel extracts was carried out by comparing their retention times and absorption spectra with those described in the literature data (Naviglio et al., 2006).
2.8. Statistical analysis
All experiments and analysis of collected samples were performed in triplicate, and the mean values and standard deviations (SD) of experimental data were calculated. Statistically significant differences (p ≤ 0.05) between the means were evaluated using one-way analysis of variance (ANOVA), and the Tukey's test. The Pearson's product-moment correlation coefficient was used to measure the strength of the linear relationship between two variables. Statistical analyses were carried out using SPSS 20 (SPSS Inc., Chicago, USA) statistical package.
3. Results and discussion
3.1. Effect of PEF treatment intensity on the carotenoid content and antioxidant power of tomato peel extracts
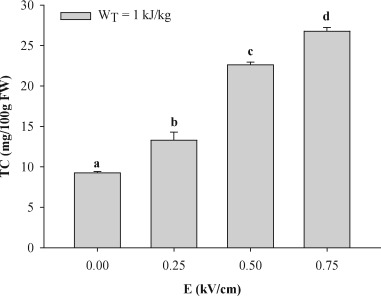
Fig. 1 shows total carotenoid (TC) content in the peel extracts of untreated (0 kV/cm) and PEF-treated tomato fruits.
 Fig. 1. Total carotenoid (TC) content of the extracts obtained from peels of untreated (0 kV/cm) and PEF-treated (WT = 1 kJ/kg) whole tomato fruits at different field strengths. Different letters above the bars indicate significant differences among the mean values (p ≤ 0.05).
Fig. 1. Total carotenoid (TC) content of the extracts obtained from peels of untreated (0 kV/cm) and PEF-treated (WT = 1 kJ/kg) whole tomato fruits at different field strengths. Different letters above the bars indicate significant differences among the mean values (p ≤ 0.05).The amount of TC extracted from the untreated samples was 9.26 mg/100 g FW tomato peels, which is consistent with previous observations showing that a substantial amount of carotenoids (in particular lycopene) are accumulated in the skins of tomato fruits (Knoblich et al., 2005, Luengo et al., 2014, Strati and Oreopoulou, 2014). Moreover, the results also highlight that, despite the compactness of the plant tissue (Zuorro et al., 2011), acetone is a good extraction solvent, because it is able to penetrate the intact plant cells of tomato peels, where carotenoids are enclosed, and to dissolve them (Luengo et al., 2014, Strati and Oreopoulou, 2011a, Strati and Oreopoulou, 2011b). The application of PEF pre-treatment to the tomato fruits before peeling resulted in the intensification of the extractability of carotenoids, with a significantly (p ≤ 0.05) higher TC content in the extracts, compared to the control samples. Moreover, when PEF intensity was increased, the extractability of carotenoid compounds increased by 44%, 144% and 189% at 0.25, 0.50 and 0.75 kV/cm, respectively, compared with the control extraction.
The permeabilization degree of the cell membranes of the tomato peel tissues upon the exposure of the whole fruits to an external electric field was determined in terms of Zp values of the tomato peels, evaluated via impedance measurements. The Zp values exhibited a statistically significant increase (p = 0.05) when the field strength increased from 0.25 (Zp = 0.20) to 0.50 kV/cm (Zp = 0.61), while a slight, not statistically significant increase was observed when the PEF intensity was increased to 0.75 kV/cm (Zp = 0.66). Remarkably, a highly positive correlation was observed between TC content and ZP values (Table 1), which can be explained by the reduced mass transfer resistances, due to the permeabilization the cell membranes of the tomato peel tissues, and consequent increment in the extraction yield of carotenoids (Luengo et al., 2014).
Table 1. Correlation coefficient among cell disintegration index (Zp) of tomato peel, and TC content, antioxidant activity (AA), and lycopene (Lyc) content of extracts from peels of untreated and PEF treated whole tomato fruits at different field strength (0.25-0-75 kV/cm).
| Properties | Zp | TCC | AA | Lyc |
|---|---|---|---|---|
| Zp | 1.000 | 0.978 | 0.961 | 0.994 |
| TCC | 0.978 | 1.000 | 0.997 | 0.998 |
| AA | 0.961 | 0.997 | 1.000 | 0.991 |
| Lyc | 0.994 | 0.998 | 0.991 | 1.000 |
PEF-induced permeabilization of cell membranes is effective in improving pigments extractability from plant tissues, such as anthocyanins from grape pomace, blueberry press cake, purple-fleshed potato, red prickly pear peels and red cabbage (Barba et al., 2015, Bobinaitė et al., 2015, Corrales et al., 2008, Gachovska et al., 2010, Koubaa et al., 2016, Pataro et al., 2017, Puertolas et al., 2013), or betanin from red beets (Chalermchat et al., 2004, Lopéz et al., 2009). Moreover, Luengo et al. (2014), who investigated the extraction of carotenoid compounds from peels of fresh tomato (commercial variety: tomate canario), found that 90-μs PEF treatment at 5 kV/cm increased the extraction yields in acetone by 50%, as compared to a conventional solvent extraction. However, differently from this work, the authors applied PEF pre-treatment directly to the fresh tomato peels rather than to tomato fruits, and found a lower concentration of carotenoids in the extracts (about 3.2 mg/100 g FW tomato peels). This could be attributed to the biological diversity of the tomatoes, which likely led to lower ZP values (about 0.2 at 5 kV/cm and 90 μs), despite an applied field strength higher than this work.
A qualitative analysis of the composition of the peel extracts was carried out via HPLC, with the resulting chromatogram profiles, detected at 470 nm, reported in Fig. 2. The profiles of the extracts from untreated and PEF-treated samples appeared to be similar, suggesting that the electrical pre-treatment neither promoted the selective extraction of specific compounds nor caused isomerization or degradation reactions. This is in agreement with the observations reported by other authors (Luengo et al., 2013, Luengo et al., 2014, Lopéz et al., 2009, Pataro et al., 2017), who found that PEF pre-treatment did not significantly alter the HPLC chromatogram profiles of the extracts, probably due to the relatively mild intensity of the applied treatment (Kahmič-Kalamiza et al., 2014).
 Fig. 2. HPLC-DAD profiles of carotenoids at 470 nm in the extracts from peels obtained after peeling of (a) untreated, and PEF-treated (WT = 1 kJ/kg) whole tomato fruits at (b) 0.25 kV/cm, (c) 0.50 kV/cm, and (d) 0.75 kV/cm.
Fig. 2. HPLC-DAD profiles of carotenoids at 470 nm in the extracts from peels obtained after peeling of (a) untreated, and PEF-treated (WT = 1 kJ/kg) whole tomato fruits at (b) 0.25 kV/cm, (c) 0.50 kV/cm, and (d) 0.75 kV/cm.In particular, in Fig. 2, the main peak can be associated with all-trans lycopene, detected at an elution time of 12.65 min (Naviglio et al., 2006). These results are consistent with those obtained via spectrophotometric analyses, which showed visible spectra with a maximum absorption at the characteristic wavelength (470 nm) of lycopene (data not shown). This is perfectly coherent with the fact that lycopene represents more than 80% of the total carotenoid content in the fully ripened tomatoes (Pataro et al., 2015).
The strong positive correlation, observed also between TC content and lycopene content in peel extracts (Table 1), further confirmed that lycopene was the most predominant carotenoid in the extracts from tomato peels.
Moreover, it is worth noting that, in comparison with the control sample, the application of PEF pre-treatment caused a remarkable increment of the lycopene peak area of 52%, 192%, and 231% at 0.25, 0.50 and 0.75 kV/cm, respectively. Similar results were observed by other authors, when comparing the anthocyanin profile in the extracts from PEF treated blueberries and purple-fleshed potato (Pataro et al., 2017, Puertolas et al., 2013).
Additionally, also the antioxidant power of the carotenoids (particularly lycopene) contained in the peel extracts was assessed using the FRAP assay.
As shown in Fig. 3, the extracts obtained from the peels of PEF-treated tomato fruits possessed a significantly (p = 0.05) higher antioxidant activity than the control extracts (66–372%). In general, the higher the field strength, the greater the antioxidant power, but significant differences (p = 0.05) were detected only between the extracts of PEF treated samples at 0.25 and 0.50 kV/cm. Moreover, as previously observed (Luengo et al., 2014), a highly positive correlation was found between TC (Fig. 1), lycopene content (Fig. 2) and antioxidant activity (Fig. 3) of peel extracts (Table 1), which clearly indicates that the lycopene contained in the tomato peels predominantly contributes to the antioxidant activity of the extracts.
 Fig. 3. Ferric reducing antioxidant power (FRAP) of the extracts obtained from peels of untreated (0 kV/cm) and PEF-treated (WT = 1 kJ/kg) whole tomato fruits at different field strengths. Different letters above the bars indicate significant differences among the mean values (p ≤ 0.05).
Fig. 3. Ferric reducing antioxidant power (FRAP) of the extracts obtained from peels of untreated (0 kV/cm) and PEF-treated (WT = 1 kJ/kg) whole tomato fruits at different field strengths. Different letters above the bars indicate significant differences among the mean values (p ≤ 0.05).The results of this study hence suggest that, within the range of field strength investigated, the cell disintegration level (Zp = 0.61) achieved with the intermediate PEF treatment intensity (0.5 kV/cm) corresponds to the most favorable conditions to intensify the extractability of carotenoid compounds with the highest antioxidant activity. It is likely that higher PEF treatment intensity (>0.75 kV/cm) might further enhance the Zp value of peel tissues and, consequently, improve the extraction yield of valuable intracellular compounds. However, the application of more severe PEF treatment conditions seriously impair the integrity of tomato fruits (data not shown), which is in contrasts with the aim of achieving, in addition to the valorization of tomato by-products, also high quality peeled tomatoes, as envisaged in the FieldFood project.
Further investigations of PEF pre-treatment in combination with SB of tomato fruits were, therefore, carried out at 0.5 kV/cm with a constant energy input of 1 kJ/kg.
3.2. Combined effect of PEF and SB pre-treatments on Zp, carotenoid content and antioxidant power of tomato peels extracts
Steam blanching (SB) is a unit operation typically used to facilitate peel removal from tomato fruits during the manufacturing of several tomato products. Therefore, in view of the exploitation as a cheap and rich source of natural carotenoids of the large amounts of tomato processed by-products (peels) currently produced at the industrial level, the impact of SB pre-treatment on the cell structure of peel tissues and the subsequent recovery of these compounds should be evaluated. Eventually, the application of a mild cell disintegration technique such as PEF in combination with SB of tomato fruits could be used to further intensify the extractability of valuable intracellular compounds.
In this work, extracts obtained from peels of whole tomato fruits pre-treated by SB (1 min) alone or by the sequence of PEF (E = 0.50 kV/cm, WT = 1 kJ/kg) and SB (1 min) at different steam blanching temperature (50, 60 and 70 °C), were analyzed in order to evaluate the impact of either the single thermal treatment or the combined treatment on the extractability of carotenoid compounds with high antioxidant activity.
The results of Fig. 4 show that the extraction yield of carotenoids from peels of mildly SB-treated fruits was significantly improved (60–189%), as compared with the control extraction performed from fresh tomato peels (Fig. 1). However, no significant difference was detected between the TC content of the SB-treated samples at 50 and 60 °C, whereas a significant (p = 0.05) difference was observed when the blanching temperature was increased from 60 to 70 °C.
 Fig. 4. Total carotenoid (TC) content of extracts obtained from peels of whole tomato fruits pretreated by SB (1min) (black bars) or PEF (E = 0.50 kV/cm, WT = 1 kJ/kg)+SB (1 min) (grey bars) as a function of the steam blanching temperature. Different letters above the bars indicate significant differences among the mean values (p ≤ 0.05).
Fig. 4. Total carotenoid (TC) content of extracts obtained from peels of whole tomato fruits pretreated by SB (1min) (black bars) or PEF (E = 0.50 kV/cm, WT = 1 kJ/kg)+SB (1 min) (grey bars) as a function of the steam blanching temperature. Different letters above the bars indicate significant differences among the mean values (p ≤ 0.05).It is likely that in the blanching temperature range examined, the improved extractability of carotenoids when increasing temperature can be related to the thermal damage induced at the cuticular level (Strati and Oreopoulou, 2011a). In fact, as shown in Fig. 5, the ZP values of tomato peels obtained from the SB pre-treatment of tomato fruits at 50, 60, and 70 °C, increased to 0.2, 0.36, and 0,57, respectively, with a significant difference observed only when the temperature was increased from 50 to 70 °C. Moreover, a strong positive correlation was observed between Zp and TC content (Table 2). To the best of our knowledge, no previous work investigated the effect of SB of tomato fruits on the extractability of carotenoids from the peel residues, while several works dealt with the effect of the extraction temperature on the recovery of carotenoids. To this purpose, for example, Strati and Oreopoulou (2011a)observed that an increase of extraction temperature from 25 to 70 °C caused an increase in the carotenoids concentration in acetone extracts from tomato peel powder, which was attributed to the destruction of the cellular structure.
 Fig. 5. Cell disintegration index (Zp) of peels obtained after peeling of whole tomato fruits pretreated by SB (1min) (black bars) or PEF (E = 0.50 kV/cm, WT = 1 kJ/kg)+SB (1 min) (grey bars) as a function of the steam blanching temperature. Different letters above the bars indicate significant differences among the mean values (p ≤ 0.05).
Fig. 5. Cell disintegration index (Zp) of peels obtained after peeling of whole tomato fruits pretreated by SB (1min) (black bars) or PEF (E = 0.50 kV/cm, WT = 1 kJ/kg)+SB (1 min) (grey bars) as a function of the steam blanching temperature. Different letters above the bars indicate significant differences among the mean values (p ≤ 0.05).Table 2. Correlation coefficient among cell disintegration index (Zp) of tomato peel, and TC content, antioxidant activity (AA), and lycopene (Lyc) content of extracts from peels obtained after peeling of whole tomato fruits pre-treated by SB (1min) or PEF (E = 0.50 kV/cm, WT = 1 kJ/kg) + SB (1 min) at different blanching temperature (50, 60, and 70 °C).
| Properties | Zp | TCC (SB) | TCC (PEF-SB) | AA (SB) | AA (PEF-SB) | Lyc (SB) | Lyc (PEF-SB) |
|---|---|---|---|---|---|---|---|
| Zp | 1.000 | 0.876 | 0.830 | 0.912 | −0.128 | 0.906 | 0.705 |
| TCC (SB) | 0.876 | 1.000 | – | 0.997 | – | 0.998 | – |
| TCC (PEF-SB) | 0.830 | – | 1.000 | – | 0.447 | – | 0.981 |
| AA (SB) | 0.912 | 0.997 | – | 1.000 | – | 1.000 | – |
| AA (PEF-SB) | −0.128 | – | 0.447 | – | 1.000 | – | 0.613 |
| Lyc (SB) | 0.906 | 0.998 | – | 1.000 | – | 1.000 | – |
| Lyc (PEF-SB) | 0.705 | – | 0.981 | – | 0.613 | – | 1.000 |
In contrast, when PEF pre-treatment was applied prior to SB, the TC content rose to significantly higher values (p = 0.05) with respect to the thermally treated samples for blanching temperatures of 50 and 60 °C, while a slight but not significant increase was observed when the temperature was increased to 70 °C. No statistical difference was, instead, observed among the PEF + SB treated samples (Fig. 4).
However, it is worth noting that the combined treatment showed an additive effect in the extraction yield of TC at the blanching temperature of 50 °C, whereas a synergistic effect was observed at 60 °C, corresponding to a maximum value of 37.9 mg/100 g FW tomato peels. Further increasing the SB temperature up to 70 °C, instead, caused a slight but not significant decrease in the amount of TC extracted, as compared with the combined treatment performed at lower temperatures. From these results, it might be concluded that the electroporation effect induced by PEF prior to the thermal treatment enables the intensified recovery of valuable compounds at lower blanching temperature, probably because of the reduced thermal stress that could negatively affect the extraction and bioavailability of thermolabile compounds. Similarly, previously published works demonstrated that PEF permeabilization of plant tissue before extraction has the potential of decreasing the extraction temperature without affecting the extraction yield (Loginova et al., 2011, Lopéz et al., 2009, Puertolas et al., 2013).
The results of Fig. 4 positively correlate with the higher values of ZP detected when PEF was applied prior to SB treatment (Fig. 5, Table 2), indicating that the combined treatment has the potential to further enhance the degree of structural damages at the cuticular level, thus facilitating the penetration capacity of the solvent and the recovery of the carotenoid compounds.
Moreover, the results of Fig. 4 are consistent with the HPLC chromatogram profiles of the extracts obtained upon the application of SB (Fig. 6a) alone or of PEF + SB (Fig. 6b). Interestingly, it can be observed that, once again, only the peak of lycopene was detected and that no isomerization or degradation occurred upon the application of either a mild SB treatment or the combination of PEF with SB. In contrast, SB or PEF + SB increased the yield compared to the extraction from untreated fresh peels or peels obtained upon the PEF pre-treatment of tomato fruits (Fig. 2). In particular, the results of Fig. 6 also indicate that the combined PEF + SB treatment markedly increased the area of the lycopene peak, which rose approximately of 200%, 220%, and 20% at blanching temperatures of 50 °C, 60 °C, and 70 °C, compared to the peel extracts of SB-treated tomato fruits at the same temperatures. It is likely that the moderate temperature and PEF treatment intensity used in our experiments were high enough to intensify the extractability of carotenoid compounds but sufficiently mild to induce any degradation of carotenoids. Despite our results show a slight decrease in the TC content at the highest blanching temperature, they appears to be consistent with findings of Strati and Oreopoulou (2011a), who found that the increase of extraction temperature up to 70 °C did not cause any alterations to lycopene and other carotenoids from tomato waste, while it increased the yield, compared to an extraction at 25 °C.