1. Introduction
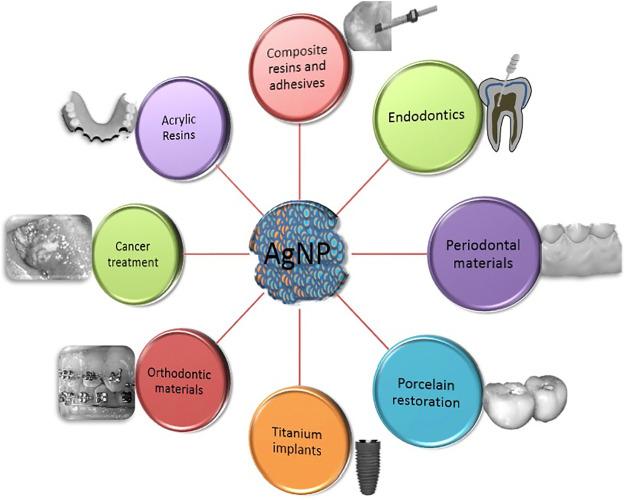
Nanotechnology is an essential technology of 21st century with groups of atom at nanoscale of 1–100 nm [[1], [2], [3], [4]]. Nanoparticles (NPs) can be obtained from natural sources or chemically synthesized or one of the by-products [5,6]. Due to its higher surface to volume ratio and antibacterial properties they have found applications in the field of medicine [7,8]. Among the various existing nanomaterials, AgNPs has gained attention owing to their distinctive physical and bio-chemical properties in contrast with their macro and micro complements. Silver is a safe antimicrobial agent which has a potential to kill 650 different types of organisms causing diseases [9]. AgNPs havebeen synthesized and have shown to possess potential antimicrobial actions [10]. Their smaller particle size with increased surface area provides antimicrobial action [11,12] at decreased filler level preventing negative effect on the mechanical properties of the biomaterial [13]. As biofilm organisms are resistant to antibacterial agents, small particle size of AgNPs makes it possible to penetrate cell membranes causing DNA damage and cell death [14,15]. In this review, we discuss influence of incorporation of AgNPs into different biomaterials used in restorative dentistry (composite resins and adhesives) [16,17], endodontics [18,19], periodontics, implant dentistry (titanium dental implants) [[20], [21], [22]], prosthetic dentistry (porcelain and acrylic resins), orthodontics (cements for brackets), oral cancers [23] (Fig. 1).
 Fig. 1. Applications of silver nanoparticles used for biomaterials.
Fig. 1. Applications of silver nanoparticles used for biomaterials.2. Mechanism of actions of AgNPs
2.1. Antimicrobial action of AgNPs
The AgNPs have exhibited a broad spectrum antibacterial effect on both gram positive and gram-negative organisms and various drug resistant strains. Although various mechanisms have been proposed for antibacterial action, exact mode of action is not completely understood (Fig. 2). According to Jones and Hoek [24], the most common modes of action can be free silver ions uptake causing interruption of ATP molecules and preventing DNA replication or formation of reactive oxygen species by AgNPs or direct damage of cell membrane by Silver ions (Ag+). It is recognised that AgNPs forms pits in the cell wall of gram negative organisms causing increased permeability and cell death. So generally, AgNPs cause denaturation and oxidise the cell wall which leads to rupture of organelles resulting in cell lysis [25,26]. AgNPs also modify the phosphotyrosine profile of peptides which interrupts the organisms signal transduction and prevents multiplication [27].
 Fig. 2. Steps of antimicrobial action of silver nanoparticles. (A) AgNPs diffusion and uptake into the bacterial cell: Accumulation and dissolution of AgNPs at plasma membrane cause cell leakage. (B) Destabilization of Ribosomes: denatures ribosomes inhibiting protein synthesis and plasma membrane degradation. (C) Enzyme interaction: AgNPs bind with the thiol group (-SH) in the respiratory enzymes and deactivate them. (D) Interruption of electron transfer chain: AgNPs interfere with electron transport affecting signaling pathway. (E) Reactive oxygen Species(ROS): Mitochondrial damage induces ROS which oxidizes proteins. (F) DNA Damage. AgNPs binds with DNA preventing its replication and multiplication causing apoptosis. (G) Cell Death: Formation of pits and perforations in cell membrane leads to release of cell organelles and cell death.
Fig. 2. Steps of antimicrobial action of silver nanoparticles. (A) AgNPs diffusion and uptake into the bacterial cell: Accumulation and dissolution of AgNPs at plasma membrane cause cell leakage. (B) Destabilization of Ribosomes: denatures ribosomes inhibiting protein synthesis and plasma membrane degradation. (C) Enzyme interaction: AgNPs bind with the thiol group (-SH) in the respiratory enzymes and deactivate them. (D) Interruption of electron transfer chain: AgNPs interfere with electron transport affecting signaling pathway. (E) Reactive oxygen Species(ROS): Mitochondrial damage induces ROS which oxidizes proteins. (F) DNA Damage. AgNPs binds with DNA preventing its replication and multiplication causing apoptosis. (G) Cell Death: Formation of pits and perforations in cell membrane leads to release of cell organelles and cell death.The antibacterial action of AgNPs is mainly due to the release of Ag + ions. Release of Ag + is higher when fine AgNPs are used (<10 nm particle size) for antibacterial action compared to larger AgNPs [1]. Minimum inhibitory concentration (MIC) of AgNPs is in approximately 0.003 mg/mL for Fusobacteriun nucleatum [2], 0.04 mg/mL for Streptococcus mutans (Holla et al., 2012), and 0.5 mg/mL for Actinomyces oris [3] As per observation of experiment by Sondi etal., 2004, concentration of 50–60 μg cm−3 of AgNPs causes 100% inhibition of bacterial growth Escherichia coli [26]. Also bactericidal properties of AgNPs are size dependent. AgNPs in the range of 1–10 nm with direct interaction with cell membrane surface alters the permeability and causes cell damage [28].
2.2. Antiviral action of AgNPs
It is suggested that AgNPs may bind with the outer proteins of viruses inhibiting their binding and replication. Though antiviral mechanism of AgNPs is yet to be completely known, it remains a scope for future research [29].
2.3. Antifungal action of AgNPs
AgNPs have exhibited antifungal action against 44 strains of various fungal species [30]. AgNPs action against Candida albicans could be destruction of cell membrane integrity inhibiting cell growth [31]. Thus, AgNPs can be one of the agents to prevent fungal infections related to oral structures. Concentration of1 μg/ml of AgNPs incorporated in resins have shown potent antifungal activity without any cytotoxicity [32].
3. Toxicity of AgNPs
The fact of toxic nature towards microbial agents of silver ions have been successfully implemented in research. Nanoparticle formulation of silver would enhance wide spectrum antimicrobial properties through enormous increase in surface area in nanoparticle formulation. Further, these antimicrobial properties are even evidenced against antibiotic resistance microbes and has also been proved synergistic effect with conventional antimicrobials [[33], [34], [35], [36], [37]]. Wide contribution of AgNPs in medical field has extended its role in dentistry, in prevention of bacterial adhesion, proliferation and finally formation of biofilm in several dental procedures [38]. Thus, application of AgNPs in oral preparations directly contacts with the teeth and surrounding cells and tissues within the oral cavity. Therefore, besides undeniable contribution towards oral health of AgNPs against infecting microbes and producing diseases, serious adverse events must be addressed to fulfil its safety requirements.
Adverse events of free Ag+ in the industrial wastes are prominent due to the occurrence of argyria (skin discoloration) and argyosis (discoloration of eyes) of other associated side effects on renal, hepatic, gastrointestinal, respiratory adverse effects [39]. Additionally, several reports on toxic evidences of AgNPs, may be due to co-exposure with fluorides, or due to cytotoxicity to gingival fibroblast by capping agent, confirmed interest towards explorative study on it [34]. Preclinical studies on rats has showed increased accumulation in females particularly in liver, kidney, colon, and jejunum as compared to the males. Further studies confirmed accumulation of silver in the glomerulus of the female rat kidneys, as supported by the pigmentations during histopathological studies. Additionally, affinity of silver towards sulphur, selenium and chlorine interfere in signal transduction [40]. Additional in vivo studies have indicated silver nanocarriers are deposited in the liver to produce its hepatic toxicities. Histopathological diagnosis revealed higher incidence of hyperplasia of bile duct with or without necrosis, pigmentation and fibrosis [41].
In vitro studies on rat liver cells have shown to have oxidative stress and impaired mitochondrial function [42]. Exposure to AgNPs has been associated with tissue damage especially in liver. No Observable Adverse Effect Level (NOAEL) of 30 mg/kg and Lowest Observable Adverse Effect Level (LOAEL) of 125 mg/kg has been observed for Ag NPs in rats [43]. Alternate studies also revealed oxidative damage of AgNPs through generation of reactive oxygen species via mitochondrial respiratory chain, could lead to interference in ATPgeneration. Such interference lead to apoptotic cell damage and DNA destruction and ultimately interfere the cell survival [44]. Contrarily, exposure of nasal AgNPs for 90 days does not produce any genetic toxicity irrespective of sex of the experimental animals [45]. Along with impaired mitochondrial function by AgNPs, these nanocarriers are known to cause leakage of the cell membranes and interfere with its ionic permeability, thus interfere with the action potential [7]. Recent studies have reported its potential to prompt oxidative damage, immune-toxicity, cytotoxicity, and apoptosis via interference in caspase activity. Apoptotic potential has further been explored by mitochondrial involvement through jun-kinase [46]. AgNPs are also known to produce toxicity on male reproductive system, where the nanostructured silver particles easily cross the barrier between blood and testes and reach to the male reproductive organ. Deposition of AgNPs in the testes adversely affect the sperm cell production [47]. Although, studies on AgNPs synthesized with ammonia and polyvinyl pyrrolidone showed to be non-inflammatory at low concentration (≤25 μg/mL) as these nanoparticles did not induce production of inflammatory mediators (interleukin-1β and interleukin-6), however, suggested to further clinical studies [48]. Several studies are supporting the underlying toxicities of AgNPs, although many more evidences are still necessary to support in vitro data through conduction of animal studies with the desired dosage level.
In endodontic treatment for as irrigants, AgNPs in concentration of 50 μg/ml (0.005%)has shown to be antibacterial in action andconcentrations above 80 μg/ml could be considered cytotoxic levels [19]. Additionally, as reported in the observations by Frankova et al., AgNPs with spherical in structure and average size of 10 nm should be biocompatible for fibroblasts and keratinocytes [49].
4. Influence of AgNPs incorporation in dental biomaterials [Table 1]
4.1. Acrylic resins for dentures
Poly (methyl methacrylate) (PMMA) acrylic resins are used for the fabrication of partial and complete dentures for replacement of missing teeth. The rough surface of these resins attract potential harmful organisms [32]. C. albicans is able to colonize these resins and is one of the key opportunistic pathogen [50]. Various mouthwashes and denture cleansing agents have been used for their eradication. However, these agents are not able to achieve complete elimination of these pathogens [51]. As AgNPs have been used with dental biomaterials, this review will highlight on influence of AgNPs on the mechanical and prevention of biofilm formations with acrylic resins.
Table 1. Application of silver nanoparticles for various biomaterials used in dentistry.
| Objective | Type of study | Method of preparation | Characterization parameters | Concentration of AgNP (in % or ugs) influencing mechanical, antimicrobial and antifungalproperties of materials. | Inferences | Ref. |
|---|---|---|---|---|---|---|
| 1] Acrylic resins for dentures | ||||||
| To investigate the effect of silver nanoparticles on mechanical properties of conventional heat polymerized acrylic resin and microwave polymerized acrylic resin | In vitro | Prepared by Turkevich method using thermal and microwave irradiation polymerisation. |
TEM analysis showed that prepared AgNP were Spherical in shape with average diameter of 68 nm. Presence of AgNP in microwave polymerized resins significantly decrease elastic modulus and flexural strength. Similarly, thermal analysis revealed decrease in intra and inter molecular forces within polymeric matrix. |
Mechanical properties Microwave-polymerized resins with 0.3 wt% of AgNPs showed highest flexural strength and elastic modulus Heat-polymerized resin with 0.6 wt% AgNPs showed lowest values. While adding 0.8 nd 1.6 wt% AgNPs in the microwave-polymerized resin significantly decreased and elastic modulus |
Incorporation of 0.8 and 1.6 wt% AgNPs decreased flexural strength and elastic modulus for resin group but no effect on heat polymerized resin group. Addition of AgNPs decreased the glass transition temperature for both groups without any affecting the impact strength. | [52] |
| To explore the effect of AgNPs incorporated in PMMA on mechanical and antimicrobial properties. | In vitro | Light-cure and chemical-cure systems were used to synthesize AgNP-PMMA denture resin. | Durometer hardness and Ultimate Transverse Strength (UTS) study showed that AgNP-PMMA was softer and lower UTS compared to PMMA control. Ag release was studied for 28 days; however, plateau was observed after 7 days. | Antibacterial AgNP-PMMA resins with 1% w/w AgBz (silver benzoate) and extra benzoyl peroxide (B) and dimethyl-p-toluidine (D) [1.5B:0.5D] and [1B:1D] showed long term in vitroantimicrobial activity. | Addition of AgNPs lead to improvement in the mechanical and anti-bacterial properties of PMMA. | [53] |
| To observethe outcome of AgNPs incorporated in PMMA on biocompatibility and anti-fungal properties. | In vitro | AgNP was prepared by reducing AgNO3viaplant infusion and incorporated in during PMMA synthesis by suspension polymerisation technique. | TEM results showed spherical nanoparticle with the size range of 10-20 nm. A flexural strength test showed Mean flexural modulus (GPa) and flexural strength (MPa) of AgNP-PMMA was higher than commercial acrylic resin, Nature Cryl™. |
Antifungal property Concentration of 1 μg/mL of silver nanoparticles in PMMA-silver nanoparticle discs was found to have antifungal activity without any cytotoxicity and genotoxicity. |
Addition of AgNPs lead to decreased adherence of C. albicans and exhibited no cytotoxicity and genotoxicity. | [32] |
| To observe the effects of AgNPs in tissue conditioners on antimicrobial properties, minimum bactericidal concentration and minimum fungicidal concentration of silver nanoparticles. | In vitro | Silver nanoparticles were prepared by gamma irradiation using AgNO3 and polyvinyl pyrrolidon (stabilizer). | TEM analysis showed average size of AgNP was in the range of 100-120 nm. |
Weight percentages Ag+: 2.5 wt%; Zn2+: 14.5 wt%; NH4+: 2.5 wt%, H2O: 16–18 wt% |
Tissue conditioners containing SZ have antimicrobial effects on C. albicans and nosocomial respiratory infection-causing bacteria, S. aureusand P. aeruginosa,and that this effect may be found in vitro after four weeks of immersion in saliva | [23] |
| To investigate whether a modified method can produce AgNP in-situ in resins and release silver ions to produce antibacterial action. | In vitro | AgNP synthesized in-situ inlight cure (LC) and chemical cure (CC) dentalresin via curing method. |
The colours of dental resin become darker due to presence of AgNP. Rockwell hardness of CC was not affected by presence of Ag while hardness of LC was decrease with increase in Ag concentration. Nanoparticles cluster was observed in LC resin while nanoparticles distributed evenly with less cluster in CC. In CC the release of Ag was detected at every concentration while in LC Ag was detected when resin made with 0.1% of AgNPs. |
Mechanical properties Concentration of 0.15% (w/w) Ag benzoate(AgNBz) was the maximum which could be light-cured and for specimens for chemically curable resins, concentration of 0.6% (w/w) AgBz was the highest. Hardness of light cured resins decreased with concentrations AgBz reaching 0.1% (w/w) and above. Chemical cured resins were harder than the light cured resins above 0.2% AgBz. For LC specimens, Ag + ion release with light cured resin was measurable only resins made with 0.1% AgBz. Resin having 0.5%AgBz inhibited 97.5% and those made with 0.2% AgBz inhibited 52.4% of the growth of S. mutans |
The study demonstrated that the modified process generated AgNPs in- situ using the resin's own curing process. Silver ions release was detected but it gradually diminished overtime. The silver nanoparticles loaded resins also demonstrated antibacterial activity against S. mutans. | [55] |
| 2]Composite resins | ||||||
| To analyse the consequence of adding AgNPs to calcium disilicate cements' mechanical properties. | In vitro | AgNP was synthesized by chemical reduction method using NaBH4 and capped with polyvinyl alcohol. These capped nanoparticles were incorporated in dental cements (PC 70%/ZrO2 30% or to WMTA) |
Radiopacity of PC/ZrO2 and WMTA/AgNPs was higher, the lowermost radiopacity was detected for PC/ZrO2/AgNP. Compressive strength of dental cements was increased by incorporation of AgNP. |
AgNP particle size was 4-11 nm. | Incorporation of AgNPs to these cements improved their mechanical and physiochemical properties | [65] |
| To evaluate effect of AgNP incorporated in composites on formation of elutable substances from light cure polymerisation process. | In vitro | Commercial AgNP was dispersed in Tetric Flow and polymerize using an Astralis 10® light source. | Not available |
Polymerisation process of composites: 0.0125, 0.025, 0.05, 0.1 and 0.3% by weight silver nanoparticles were added to composite. 24 h later the elutable compounds were detected with 0.3% AgNP. |
Addition of AgNP to light cure polymerized composites may cause increase in elutable substances. | [17] |
| To analyse bonding materials having quaternary ammonium dimethacrylate (QADM) and compare with bonding material having AgNP for cytotoxicity and antibacterial properties. | In vitro | Quaternary ammonium dimethacrylate (QADM) was synthesized by modified Menschutkin reaction. Silver 2-ethylhexanoate salt was dissolved in 2-(tert-butylamino) ethyl methacrylate and then added to Scotchbond Multi-Purpose bonding system (SBMP) primer. Finally added to adhesive to make composite. | TEM results showed uniform dispersion of Ag in dentin with the particle size of 2.7 nm. Presence of Ag doesn't affect the microtensile bond strengths of composites. |
Antibacterial property: AgNP filler levels of 0.05% in primer and adhesives presented strong antibacterial properties without decreasing microtensile dentin bond strength. |
The AgNP containing adhesive exhibits long distance as well as contact inhibitory effect whereas QADM- adhesive exhibits inhibition of organisms on its surface only. Fibroblast cytotoxicity and microtensile bonding strength remained unaffected by both the adhesives. |
[71] |
| To investigate antibacterial efficacy and bond strength of adhesive with AgNP and amorphous Calcium phosphate | In vitro | Silver 2-ethylhexanoate salt was dissolved in 2-(tert-butylamino) ethyl methacrylate and then added to SBMP primer. Nanoparticles of amorphous calciumphosphate (NACP) were prepared by spray drying method by using dicalcium phosphate anhydrous and calcium carbonate. Finally, the NACP was mixed with adhesive containing AgNP. |
Dentin shear Bond strength of AgNP containing primer and adhesive similar to control while presence of NACP increase the bond strength. SEM images revealed that prepared resins were well filled and nanoparticles well filtered into dental tubules. |
Antibacetrial property Mean particle size of AgNP was 2.7 nm. AgNP were added at 0.1% by mass into adhesive and primer which reduced the metabolic activity of biofilm by 50% without reducing the dentin bond strength |
Biofilm viability was substantially reduced by this novel combination without affecting the bond strength | [62] |
| Evaluation of effects of biocompatibility of AgNPs for use as a restorative material | In vitro |
12-methacryloyloxydodecylpyridinium bromide (MDPB) and AgNP was incorporated into SBMP primer. |
Dentine shear bond strength was not affected by incorporation of MDPB and AgNP. | Can be used as a biocompatible and antimicrobial agent | [73] | |
| To explore the influence of AgNPs on dental plaque and properties of nanocomposite material. | In vitro | Spray drying method was used to fabricate NACP. NACP was incorporated into TEGDMA (triethyleneglycol dimethacrylate and BisGMA(bisphenolglycerolatedimethacrylate) resin. Similarly, QAS and AgNP was also incorporated in resin and finally the resins were photo- polymerized. |
Particle size of formulated NACP and AgNP was 116 nm and 2.7 respectively. TEM analysis showed uniform distribution of nanoparticles in resins. The resin with NACP showed lower flexural Strength. |
Antibacterial and cytotoxicity AgNP were added at 0.05% by mass fraction with the primer had potent antibacterial activity without affecting the bond strength. Also, fibroblasts viability was 100% proving it to be biocompatible. |
AgNPs decreased biofilm formation and improved the mechanical properties of nanocomposites | [60] |
| [75] | ||||||
| 3] Endodontic materials | ||||||
| Comparative analysis between AgNP, chlorhexidinegluconate (CHX) and sodium hypochlorite (NaOCl) for antibacterial action. | In vitro | Ag NP was produced by catalytic chemical vapours deposition procedure and then dispersed into water. | Prepared nanoparticle are spherical with 35 nm average diameter. |
Antibacterial action and cytotoxic levels as intracanal irrigant in endodontics AgNP in concentration of 50 μg/ml (0.005%) can be considered for intracanal irrigantdue to antibacterial action whereas those above 80 μg/mlconcentration could be considered cytotoxic levels. The silver particle size used in this experiment was 35 nm. |
In lower concentrations, AgNPs solution can have the bactericidal effect similar to 5.25% NaOCl, so it can be a used as a new intracanalirrigant.. | [19] |
| To analyse the bactericidal action of AgNPs as an irrigation solution in root canal therapy. | Ex vivo | AgNP was synthesized by reduction method using AgNO3 and gallic acid. | AgNP have spherical structure with 10 nm diameter. The surface plasmon resonance is narrow and located at 420 nm. |
Antibacterial action AgNP particle size around 10 nm with ashowed antibacterial effect on E. faecalis |
The antibacterial potency of AgNP based solution was similar in antibacterial action as an irrigant compared to 2.25% of NaOCl. | [92] |
| To evaluate the effect of adding AgNP (1%) to MTA antibacterial properties. | In vitro | Used commercially AgNPs | Not available |
Antibacterial action Addition of AgNPs by 1% weight with particle size <150um to MTA improved its antimicrobial (E. faecalis, C. albicans, and P. aeruginosa). |
Addition of AgNP to adding MTA enhanced its antimicrobial efficiency. | [18] |
| To investigate bactericidal efficacy of AgNP + Ca (OH)2 medicament vs.AgNP and Ca (OH)2 alone. | In vitro | Used commercially available AgNP gel | Not available |
Antibacterial action Concentration of AgNP in gel used wasof 20 ppm with an average diameter of 2 nm particle size. |
Antibacterial outcome of AgNP was inferior to Ca (OH)2 or combination of both materials. | [140] |
| 4] Periodontology | ||||||
| Examine the effects of AgNPs on human dermal fibroblasts, human epidermal keratinocytes and also on interleukins, growth factors and matrix metalloproteinases. | In vitro | AgNP was prepared by reducing AgNO3 viaNaBH4. | Particle size of AgNP was found to be 10 ± 5 nm with -22 mV zeta potential and 7.1 pH. |
Biocompatibility of AgNPs AgNPs were spherical in structure with average size of 10 nm. The production of IL-12 was by human epidermal keratinocytes (NHEK) after 24 h was increased by AgNPs (2.5 and 0.25 ppm) whereas they decreased (at 25 ppm) expression of COX-2 in normal human dermal fibroblasts(NHDFs). Also the levels of MMP-1 were increased after 24–48 h. |
The study observed AgNPs reduced cytokines like TNF-α, IL-12 and growth factors (vascular endothelial growth factor) after 1-2 days and reduced COX-2 after 1 day at the maximum concentration of AgNPs only. | [49] |
| Analysis of bactericidal effect of AgNPs along with selected antibiotics against multiresistant Enterobacteriaceae. | In vitro | AgNP was prepared by reduction of complex cation [Ag (NH3)2] + by D-maltose in the presence of NaOH also known as Tollens process. | The prepared NP was 28 nm in diameter with narrow size distribution. |
Antibacterial property AT concentration of 0.8 mg/L, AgNPs demonstrated the strongest antibacterial. At lowest concentration of MICAg/16, AgNPs potentiated the effect of antibiotics. The strongest enhancement of antibacterial activity was seen at AgNP concentrations of MICAg/2 and MICAg/4at which the MICs of the antibiotics being as much as 100-fold lower. Biocompatibility AgNPs, antibiotics alone and AgNPs–antibiotics combinations at concentrations of 4 mg/L and 2 mg/L, respectively, did not exhibit any cytotoxic effects. |
Synergistic effect when AgNPs are combined with antibiotics; resulting in a reduction of MICs of the antibiotics. In addition, antibiotics became bactericidal when combined with AgNP. | [100] |
| Investigated anti-bacterial anti- biofilm effect of carboxymethyl cellulose (CMC)and sodium alginate (SA) capped silver nanoparticles (AgNPs) | In vitro | Biopolymers (sodium alginate SA and carboxymethyl cellulose CMC) capped AgNP was prepared by microwave irradiation method. | SEM results showed polygon shape CMC coated NP with the size range of 50-100 nm while the size range of SA coated NP was 100–150 nm without any aggregation.XRD and FTIR data indicated the characteristic peaks of silver nanocrystals. |
Antibacterial activity AgNPs in conc. (15 μg/mL) with CMC@AgNPs and SA@AgNPs were effective in inhibiting growth of Gram-positive and Gram-negative bacteria. |
Carboxymethyl cellulose capped AgNPs exhibited more potency in reducing growth of Gram –ve as compared to Gram+ve bacteria. While results was exactly reverse for sodium alginate AgNPs. | [101] |
| To explore whether size of silver nanoparticles had any effect on the anti-bacterial activity against oral anaerobic pathogenic bacteria. | In vitro | AgNP were produced by using a hydrothermal method or simple reduction method. |
The XRD results showed no diffraction in characteristic peak of AgNP which indicated high purity of sample. Particle size of NP ranges from 3 to 8 nm with spherical shape prepared by reduction method while size of NP synthesized by hydrothermal method was found in the range of 30–80 nm. Stability study revealed that NP are highly stable without any significant change in structure and size. |
Antibacterial activity AgNPs with particle size 5-nmshows the best antibacterial activity. |
Different particle size of AgNPs had different antimicrobial action against anaerobic bacteria; with 5-nm size AgNPs having the best antibacterial activity. | [102] |
| Effect of silver nanoparticles containing periodontal dressing on inflammatory and repair stages of healing of gingival wound. | In vivo (animal model) | AgNP was fabricated by chemical reduction method and incorporated into periodontal dressing. |
Histologic findings The histologic picture with periodontal dressing applications showed more improved healing parameters compared to controls. There was less inflammatory cell infiltration, decreased edema and well aligned collagen fibres after one week of gingivectomy. |
Antibacterial property Periodontal Dressing A had 25% v/v concentration of AGNPs and Periodontal Dressing B had 50% v/v |
Silver nanoparticles containing periodontal dressing showed marked reduction in oedema with moderate inflammation and prominent neovascularization on 4th post-operative day; it also showed complete wound healing on 7th post-operative day. | [105] |
| To synthesize silver nanoparticles from an extract derived from banana peel waste and its antimicrobial effect on human pathogenic organisms. | In vitro | AgNP was synthesized by chemical reduction method using banana leaf extract as capping and reducing substance. | Formulated nanoparticles were spherical in shape with mean diameter of 23.7 nm. EDX analysis and FT-IR showed typical absorption peak of silver nanocrystals which confirms the formation of AgNP. |
Antibacterial property The antibacterial effect was tested against B. subtilis, S. aureus, P. aeruginosaand E. coli, i. Minimum inhibitory concentration (MIC) was observed at 6.8, 5.1, 1.70 and 3.4 mg/ml of silver nanoparticles respectively whereas the minimum bactericidal concentration (MBC) of AgNPs were found to be 10.2, 10.2, 5.1 and 5.1 mg/ml, respectively. |
Silver nanoparticles were more effective against Gram negative than the Gram-positive organisms. Silver nanoparticles also had synergistic effect on the antibacterial action of levofloxacin against Gram-positive and negative bacteria under study. | [103] |
| Study to investigate the colonization and penetration of specific bacteria (Streptococcus mutans, Aggregatibacteractinomycetemcomitans, Fusobacteriumnucleatum and Porphyromonas gingivalis) on silver nanoparticle impregnated guided tissue regeneration membranes. | In vitro | Silver nanoparticles as a colloidal solution (0.1 mg/mL) in water (PlasmaChem GmbH, Berlin, Germany; 100 mL; average particle size: 10 nm) was utilized in this study to prepare GTR-NS membranes. | The concentration of silver nanoparticles used was 100 μg/mL (0.1 mg/mL); |
Mechanical properties Stress-Strain behaviors of membranes were calculated. GTR-NS showed significantly higher tensile strength (10.01 ± 0.92 MPa; p ≤ .001) in comparison with GTR-C (8.02 ± 1.2 MPa) and GTR-DOX (8.01 ± 1.4 MPa) and |
Results showed that mean bacterial adherence scores and colony forming units were lower statistically significant | [104] |
| 5] Porcelain for crowns and bridges | ||||||
| Analyse the effect of silver and platinum (Pt-NS) nanoparticles on fracture resistance of porcelain | In vitro |
10 nm of average diameter of Ag particles and 5 nm of average diameter of platinum (Pt) particles were separately dispersed in water. Noritake Super Porcelain AAA powder was mixed with silver or platinum NP dispersed solution. Then the slurry was dispensed into a metal mold using a vibrational mixer. Green pellet was prepared using hydraulic press then fired in porcelain furnace. |
The addition of metal NPs increased the mechanical strength of porcelain. Inclusion of both the metals increased the fracture toughness and Young's modulus whereas increased of the fracture toughness was more with Ag NPs than Pt NPs. Pt NPs did not show anyeffect on the median crack length and Vickers hardness. |
Mechanical properties Vickers hardness and crack length of sintered specimens was significantly higher with AgNPs (622.3; SD: 14.1) compared to Pt-NS (515.4, SD: 15.0) andcontrol (503.4, SD: 33.8). Also, the fracture toughness, KIC, of AgNPs (1.54 MPa∙m1/2, SD: 0.05), was significantly higher than Pt-NS (1.42 MPa∙m1/2, SD:0.02) and control (1.36 MPa∙m1/2, SD: 0.03) |
Addition of both the nanoparticles enhanced the fracture toughness and Young's modulus. | [108] |
| [110] | ||||||
| Evaluation of influence of silver nanoparticles on Vickers hardness, crack length, Young modulus and toughness of the porcelain. | In vitro | Average diameter of 10 nm Ag NPs were dispersed in purified water. Noritake Super Porcelain AAA (NS porcelain) powder was mixed with Ag NP dispersed solution and the slurry was poured into a 20 nm diameter cylindrical metal mold using a vibrational mixer. Green pellet was prepared using hydraulic press then fired in porcelain furnace. | Inclusion of Ag NPs resulted significant increase of the fracture toughness and Vickers hardness of NS porcelain. UV–Vis and FT-IR analyses revealed that some of the Ag NP countered with the matrix compositions and were converted to silver ions whereas remaining stay as Ag NPs form itself. |
The concentration of silver (Ag) in the solution (water carboxymethyl cellulose) and was adjusted to 100, 200,500, and 1000 ppm. Vikers hardness Hv of Ag1000 (641, SD: 38.5) was significantly greater (P < .01) than that of Ag200 (547.3, SD: 24.2) or Ag100 (526.3, SD: 23.5). The median crack length was higher in Ag200 (113.7 μm, SD: 6.5), Ag100 (117.6 μm, SD: 5.7) compared to Ag500 (104.5 μm, SD: 11.9) and Ag1000 (100.0 μm, SD: 5.5) (P < .01). |
Decreased crack length while other mechanical properties increased due to incorporation of silver nanoparticles. | [141] |
| To analyse the consequence of silver nanoparticles on the subcritical crack growth behavior of dental porcelains by post-indentation method. | In vitro | Average diameter of 10 nm Ag NPs of different concentration of Ag were dispersed in purified water in presence of carboxy methyl cellulose as dispersing agent. NS porcelain powder was mixed with Ag NP dispersed solution. and the slurry was poured into a 20 nm diameter cylindrical die using a vibrator. The slurry was compressed in the die using hydraulic press then fired in porcelain furnace followed by rapid cooling. | Addition of Ag NPs to porcelain increased the fatigue parameter which further improved with increase concentration of Ag. Increased fatigue parameter means higher resistance to its subcritical crack growth behaviour. | Four different concentrations of silver nanoparticle were used: 100 ppm, 200 ppm, 500 ppm, and 1000 ppm | Fatigue parameter increased with increased amounts of AgNPs. The resistance to crack growth was also enhanced by AGNPs. | [109] |
| 6] Titanium dental implants | ||||||
| Evaluation of bacteriostatic effect of AgNPs layer on titanium surface. | In vitro | By an alkaline hydrothermal HCl immersion method, hydrogen titanate nanotube layer was created on a titanium surface, and subsequent absorption of silver nitrate by immersing the dried nanotube layer into silver nitrate solution and lastly, in-situgrowth of AgNPs in the hydrogen titanate nanotube channels was achieved by reducing Ag ions using glucose. |
Uniform distribution of 10–12 nm diameter single crystalline nanotubes on the surface of the titanium foil sample with hydrogen titanate nanotube layer (NT-Ti) was observed in SEM and TEM analysis. The TEM analysis of AgNP-NT-Ti shows that the nanotubes still maintain their morphology, but there are some nanoparticles of Ag with size of 3 to 8 nm inside the nanotubes. Invitro release study demonstrated that the effective silver release from AgNP-NT-Ti can extend to >15 days. |
Ag ion release for antibacterial activity After 15 days, the Ag ion released concentration for AgNPs-NT-Ti, was 50 ppb which was enough to have antimicrobial action compared to AgNO3-NT-Ti which dropped to zero. Compared to AgNO3-NT-Ti, AgNP-NT-Ti possesses a long-term Ag ion release for enhanced antibacterial effect. |
Silver nanoparticles located in hydrogen titanate nanotubes have shown to release enough Ag ions to impart the long-term bactericidal action and excellent biocompatibility | [115] |
| Investigation of antibacterial efficacy and biocompatibility of AgNPs multilayer coating on the phase-transited lysozyme-primed Titanium surfaces. | In vitro |
Phase-transited lysozyme-functionalized titanium substrates were obtained by dipping into a mixture of lysozyme and tris (2-carboxyethyl) phosphine. To develop multilayer coatings on titanium Substrates, hyaluronic acid and chitosan loaded with AgNP coating was performed vialayer-by-layer self-assembly onto the precursor layer of phase-transited lysozyme. |
The results of XPS and SEM represented that the necklace-like phase-transited lysozyme and self-assembled multilayer were successfully immobilized on the titanium substrates. | The average concentration of released Ag from AgNPs nanoparticles-loaded Ti discs was 0.70 ± 0.14 μg/ml. | The PTL priming technique offers a promising technique for producing long-term antimicrobial multilayer coatings to prevent implant related infections. | [118] |
| 7] Orthodontic cements and adhesives | ||||||
| To develop a novel AgNP antimicrobial cement for orthodontic bands for prevention of white lesions by release of AgNPs in situ | In vitro | AgNP-loaded Opal Band Cement (OBC) was developed using in-situ developed Ag NPs. |
AgNP-loaded Opal Band Cement had similar mechanical strength measured in terms of modulus, hardness, and ultimate transverse strength values to those of the control group. Upto 4 months, controlled and sustained Ag + ion release was observed. Further, release of Ag + ion increased with increased Ag loading. |
Biocompatibility 0.5% AgNP-OBC was not cytotoxic or mutagenic. |
The novel AgNP in-situ released cement showed an excellent antibacterial action which will prevent white spot formation with unaltered mechanical properties compared to controls | [124] |
| To investigate antibacterial outcome of orthodontic adhesive with 1%,5%,10%w/w of AgNP/HA | In vitro |
Ag-doped hydroxyapatite NPs were developed by gamma irradiation and Hyaluronic acid (HA) used as a carrier. Transbond XT pastes Ag/HA NPs were developed by precisely mixing appropriate amounts of Ag/HA NPs and composite paste. Composites was filled in 5-mm diameter of circular metal molds. Thereafter composite discs were taken out and sterilized by gamma-rays. |
– | Antibacterial property composite discs having 5 and 10% silver/hydroxyapatite nanoparticles shows better antibacterial properties against biofilms. | 5% Ag/HAdecreased cariogenic bacterial growth, without affecting the non-cariogenic bacteria. | [126] |
| 8] Anticancer treatment | ||||||
| To observe the anticancer efficacy of composite of alginate, chitosan, and biosynthesized silver nanoparticles (AgNPs) | In vitro | Biosynthesized AgNPs using aqueous extract of Eckloniacava were added into the prepared chitosan-alginate polyelectrolyte solution and frozen and lyophilized. Thereafter scaffold was regenerated using 1% CaCl2 solution followed by 1% NaOH solution and water wash. The washed scaffold was frozen and lyophilized. |
Presence of strongchemical interaction between alginate and chitosan was observed in FT-IR spectra whereas, UV–vis spectroscopy and XRD results assured the presence of AgNPs in the composite. SEM analysis revealed that the nanocomposites have a porous structure and AgNPs were dispersed uniformly in the porous membrane. |
The IC50 value of chitosan-alginate-AgNPs composite tokill MDA-MB–231 cells were 4.6 mg | Chitosan-alginate-AgNPs composite depicted an enormous potential for anticancer treatment. | [136] |
| To synthesize AgNPs by novel green route method and tests its anticancer potential | In vitro | Sucrose stabilized Ag NPs was developed by adding AgNO3 to transparent sucrose solution followed by overnight stirring at room temperature. |
UV analysis represents the surface plasmonresonance band for spherical AgNP at 420 nm. XRD and Electron diffraction analysis confirmed that particles were of pure Ag with a face centered cubic (fcc) structure. TEM analysis revealed that particle size was within the range of 10 to 20 nm. |
50% Growth Inhibition Dose (ID50) was observed at 3.6 μM for AgNPs against human cancer cell lines HT144 (malignant skin melanoma) and H157 (squamous cell lung carcinoma). | AgNPs formulated by Green route method showed instant action against cancer cell line without damaging the host cells. | [139] |
Koruglu et al. [52] investigated the effect of AgNPs (three concentrations 0.3 wt%, 0.8 wt% and 1.6 wt%) on the flexural strength, elastic modulus, impact strength and glass transition temperature of conventional heat polymerized acrylic resin and microwave polymerized acrylic resin. Highest flexural strengthand elastic modulus was observed with microwave polymerized resin group with addition of 0.3 wt% AgNPs, while the lowest values were detected for the 1.6 wt% AgNPs added conventional heat polymerized resin group. Incorporation of AgNPs had no effect on flexural strength and elastic modulus in conventional heat-polymerized resin group. However, these values decreased in microwave polymerized with addition of 0.8 and 1.6 wt% AgNPs. Highest impact strength was obtained for conventional resin group without addition of AgNPs and the lowest for microwave-resin group with 0.8 wt% AgNPs. There was no improvement in impact strength of both resin groups with the addition of AgNPs. Glass transition temperatures were highest for heat polymerized resin group but addition of AgNPs decreased the glass transition temperatures for both the groups [52].
Oei et al. [53] investigated the effect of AgNPs incorporated in poly (methyl methacrylate) on mechanical and antimicrobial properties. It was demonstrated that there was improvement in the mechanical and anti-bacterial properties on addition of AgNPs. The release of silver ions reached a plateau at 7 days but the antibacterial activity extended till 28 days [53]. Acosta-Torres et al. investigated the effect of AgNPs incorporated in poly (methyl methacrylate) on biocompatibility and anti-fungal properties.Study demonstrated decreased adherence of C. albicans and exhibited no cytotoxicity, genotoxicity [32]. Matsuura et al. investigated the effect of silver zeolite containing silver ions incorporated in tissue conditioners on antibacterial and anti-fungal properties. The study revealed there was significant antifungal activity against C.albicansand antibacterial activity against S. aurues, Pseudomonas aeruginosa for 4 weeks [54]. Nam [23] investigated the effect of AgNPs incorporated in tissue conditioners on antimicrobial properties and fungicidal concentration of AgNPs. The study concluded that minimum bactericidal concentration against S.aureus, S. mutans at 0.1% Ag+ incorporated and minimum fungicidal concentration against C. albicans at 0.5% Ag+ incorporated after a 24 h and 72 h incubation period [23]. Fanet al. [55] investigated whether modified method can produce AgNPs in-situ in PMMA and bisphenol, a glycidyl methacrylate resin, release silver ions and deliver effective antibacterial activity. The study demonstrated that the modified process generated AgNPs in situ using the resin's own curing process. Silver ions release was detected but it gradually diminished overtime. The AgNPs loaded resins also demonstrated antibacterial activity against S. mutans [55].
The data revealed that addition of AgNPs did not significantly affect mechanical properties of acrylic resin and also prolonged antimicrobial property to the acrylic resin. This is important in dealing with microbial colonization under denture base. Since AgNPs don't affect the mechanical property of acrylic resins, the novel acrylic resin incorporated with AgNPs could be developed as a denture base. They can also be added in tissue conditioners which are routinely used for ill-fitting dentures that are occasionally susceptible to microbial colonization. Having these beneficial effects, AgNPs incorporation in these materials can solve the issues arising from microbial colonization particularly oral Candida infection.
4.2. Composite resins
AgNPs are combined with different materials due to their antimicrobial effect which decreases biofilm formation and maintenance of better oral health [56,57]. Due to its smaller particle size, AgNPs penetrates through cell membranes more readily, resulting in damage and inactivity [58]. In order to avoid biofilm build up over composite and in the restorations margins, few researchers tried to evaluate the effect of AgNPs incorporated restorative materials composite resins [[59], [60], [61]] and adhesive systems [[62], [63], [64]].
Vazquez-Garcia et al. [65] evaluated the effect of addition of AgNPs on mechanical and antibacterial properties of calcium silicate cements like Mineral trioxide aggregate (MTA) and Portland cement (PC) associated with zirconium oxide (ZrO2). Results observed were that the PC/ZrO2 + AgNPs had higher resistance for compression. This could be because of incorporation of AgNPs leading to decrease porosity. Both, MTA + AgNPs and PC/ZrO2 + AgNPs depicted enhanced mechanical properties. Even at the end of 15 h' period both showed higher reduction of test bacteria. Thus, addition of AgNPs showed favourable outcomes with calcium silicate cements [65].Composite resin containing silver ions have antibacterial effects on various oral bacteria e.g. S. mutans [66]. Ag+also influence the mechanical properties ofvarious adhesive systems used with these composites [67]. However, the influence of these Ag+ on polymerisation of these materials is scarce. Durner et al. observed the influence of AgNPs in composite resins on the production of elutable substances from light cure polymerisation hardened specimens. The amounts of these elutable components were roughly proportional to amount of AgNPs. Exception to this was the material with 0.05% of AgNPs. Thus the study concluded that AgNPs does have an influence on formation of these elutable ingredients during the light cure polymerisation method and this could be explained due to different mechanisms like photon reflection and scattering, electron interaction of AgNPs andlight initiators, absorption and emission of photons by AgNPs, different complex of silver ions [17]. In comparison to other restorative materials, composites exhibit more biofilm formation on its surface in-vivo [68]. These biofilms are major reasons for secondary caries formation at the margins of the restoration which leads to failure [69]. To combat these, formulation of adhesive systems have been developed with antibacterial agents to prevent the issue of secondary caries [70]. Similarly, Li F et al. [71] investigated a novel bonding agent with quaternary ammonium dimethacrylate (QADM) to a bonding agent having AgNPs for antibacterial activity. They also were evaluated for contact and long-distance inhibition. AgNPs resins depicted inhibitory action against S.mutans on its surface as well as at a longer distance compared to QADM bonding agent showing only contact inhibitory action. Fibroblast cytotoxicity and microtensile bonding strength were not affected by both the agents. Thus these novel agents demand more research validation and can find valuable applications as agents incorporated in restorative cements and carious preventive agents [71].Similar observations were done by Zhang K et al. (2012) who observed AgNPs and QADM containing adhesive attained strong antibacterial actions against biofilms without any effect on bond strength [72].