1. Introduction
Metals are fundamental to the economy and to human well-being. Global metal usage in 2005 was 19 times that at the beginning of the 20th century (Krausmann et al., 2009), and, on a basis of the historical trends, it is expected to continue to increase (Rogich and Matos 2008). While demand for metals grows, the risk of metal depletion also grows, since all the metals on earth have a mineable limit (e.g. (Stephens et al., 2010),). For this reason, technological improvements to improve the efficiency of pure metal production from natural ores and to recycle waste products, or urban ores, with higher productivity are much needed.
A variety of approaches is used to obtain pure metals at both the industry and laboratory scale. The most ubiquitous of the conventional approaches is the use of furnaces. For example, the blast furnace and the converter furnace are widely used in the ironmaking industry (Smith 2015). Blast-smelting iron and steel production has increasingly become important in the manufacturing ironmaking field because of its efficiency (Zhang et al., 2016). In the furnace-based conventional approach, because heat energy is not quickly transferred from the sample surface to the inner part, melting takes more time (Mandal et al., 2015). Another major approach is the use of leaching methods. This approach has been applied to oxide solutions (e.g., zinc oxide solution) with acid substances (Yoshida 2003). In this process, the acid or alkaline chemical agents fuse samples which contain the target metals. Then the pH of the liquids is adjusted to facilitate the concentration of the target metals in order to obtain pure metals (e.g. (Sokic et al., 2015),). The leaching process has increasingly fallen out of favor since it results in environmental pollution (Raghavan et al. 1998) and presents potential health risks to the general population (Min et al., 2013).
Microwave technology has been recently used as a new, efficient heating source with a low environmental impact. Rapid heating is achieved because the microwave directly transfers electromagnetic energy to the samples at the molecular scale (Bansala et al., 2014). Moreover, because the microwave assists the reactivity of leaching, the chemical reaction becomes faster. These characteristics of the microwave heating have led to significant improvements in the metallurgical field in recent years.
There is an increasing number of review articles focused on products treated with the use of the microwave (e.g. (Matli et al., 2016),). In other studies, the microwave-based metallurgical processes have been reviewed, each with a specific focus: on the physical and theoretical aspects of microwave-based heating (Mishra and Sharma 2016), on the milestones of microwave-based heating applications (Khaled et al., 2018), on the practical applicability of the microwave for industrial use (Singh et al., 2015), on the theoretical phenomena of microwave irradiation in material processing (Mishra and Sharma 2016), on the absorption mechanism of metallic powders when irradiated by microwaves (Zhang et al., 2018), and on the relationship between the heating behavior and physical characteristics (i.e., magnetic permeability and permittivity) (Amini et al. 2021). However, to the best of the authors’ knowledge, there are no existing reviews focused on obtaining pure metals through smelting and recycling with the microwave-based approach.
To further explore the potential for microwave-based heating technology to be employed in the metallurgical field, a review on the use of microwave metallurgical processes to obtain virgin metals from metal oxides and recycled metals from wastes is of importance. The purpose of this paper is to summarize the earlier studies on efforts to obtain pure metals under microwave irradiation and to suggest the future possibilities of microwave-based heating.
This paper is structured as follows. The definition and principle of the microwave heating method is presented in Section 2. The microwave-based production of virgin metals is reviewed in Section 3, and the microwave-based production of recycled metals is presented in Section 4. The research gaps and future prospects of microwave-based approaches to obtain pure metals are discussed in Section 5. Finally, Section 6 concludes this paper.
2. Methodology of microwave heating
2.1. The band designation of electromagnetic waves and the microwave definition
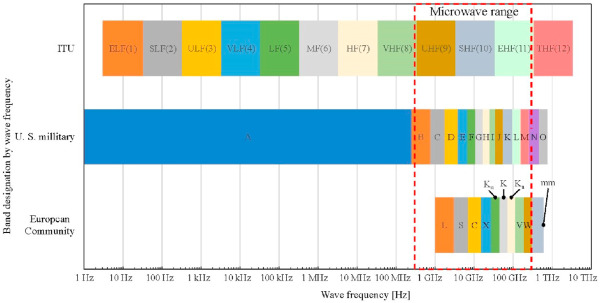
Visible light, infrared light, ultraviolet light, and radio waves constitute the electromagnetic waves, which are divided into two factors: the electric field (E-field) and the magnetic field (H-field) (Cao et al. 2010). The band standard designations represent the type of electromagnetic wave based on its wavelength and frequency. One of the most globally recognized band standard designations is formulated by the International Telecommunication Union (ITU). In addition, the United States military tri-service and the European Community (the forerunner to the European Union) created the band standard designations for use in their regions.
Even though the microwave is categorized as an infrared radiation wave, there is no distinct consensus for defining the microwave among the aforementioned band standard designations. In general, the band range of microwaves is recognized to be from 300 MHz to 300 GHz (Williams et al., 2013). A comparison of the specific band standard designations of microwaves for three organizations in terms of wave frequency and band range is summarized in Fig. 1.
 Fig. 1
Fig. 12.2. The principle of microwave heating
Microwaves propagate in a straight line, with electric and magnetic propagation as well, as demonstrated in Fig. 2 (Omran 2015). Electromagnetic waves have a particular reaction with the heated target material (Bradshaw et al. 1998), unlike that of conventional furnace-based heating (Huang and Lu 2009). One of the notable distinctions in microwave heating is the electric polarization resulting from the electric field. There are the four electric polarization processes in microwave heating: the limited space charges in materials, the spinning of bipolar molecules, ionic polarization and electronic polarization (Newnham et al., 1991).
 Fig. 2
Fig. 2The temperature rise of the sample in microwave heating is estimated by using the following heat conduction equation (Zhang et al. 1994);where is the density of the heated sample, is the specific heat capacity, is the temperature, and is the time of the microwave heating process. The four variabilities of represent the various heat flow rates. These are determined as follows.
denotes the microwave heat transfer into the sample. It is obtained by the following equation;where is the power dissipation of the microwave, is the surface resistance of the sample and is the magnetic field intensity on the surface.
represents the heat radiation consequence of sample. It is estimated by the Stefan-Boltzmann law in the following equation;where is the thermal emissivity on the surface, and is the Stefan-Boltzmann constant. The Stefan-Boltzmann constant is equivalent to by experimental and theoretical study.
corresponds to the thermal conduction in the sample based on the Fourier law. It is attained by the following equation;where is the thermal conductivity of sample, is thickness, and is the polar coordinates.
Finally, indicates the convectional heat energy transportation in sample. It is estimated by the following equation;where is the value of convective heat transport efficiency, and is the temperature of the surrounded atmosphere around the sample.
3. Producing virgin metals
The microwave-based reduction approaches used to obtain virgin metals are summarized in this section.
The focus of earlier papers tends to be on the microwave-based pyrometallurgical process alone. Very little attention has been given to the hydrometallurgical process. As such, only the pyrometallurgical processes used to obtain virgin metals by microwave technologies are summarized in this section. The iron making approaches have been widely assessed (Section 3.1), while other metals have also been considered (Section 3.2).
3.1. Iron and steel
As previously noted, the blast furnace and the converter furnace are the most common methods in the industrial pig iron and steel making process (Zhang et al., 2016). This process allows continuous steelmaking with high efficiency (Smith 2015). However, in order to improve the recovery rate and reduce the processing time, the microwave-based furnace has been used as an alternative to the conventional approach to obtain pure iron. The relative studies are summarized in Table 1.
Table 1. Microwave-based reduction approaches to obtain pure iron from iron oxides.
| Reference | Element | Maximum Temperature [°C] | Processing time [min] | Maximum recovery [%] | Maximum Power [kW] |
|---|---|---|---|---|---|
| Aguilar and Gomez (1997) | Iron ore | 1200* | 5–40 | Fe: 40 | 0.8 |
| (Mourao et al.2001) | Iron ore | 1150 | 14–30 | -** | 1.1 |
| Ishizaki et al. (2006) | Magnetite ore | 1400 | 9–30 | Fe: 99 | 5.0 |
| Ishizaki and Nagata (2007) | Magnetite | 1200 | 7–8 | Fe: 82 | 2.8 |
| Ishizaki et al. (2007) | Magnetite ore | 1250 | 7–16 | Fe: 87 | 5.0 |
| Ishizaki and Nagata (2008) | Magnetite | 1200 | 3–8 | – | 2.8 |
| Stir et al. (2009) | Magnetite | 1150 | 10 | Fe: 58 | 0.5 |
| Kashimura et al. (2010) | Iron ore | 1350 | 7–30 | – | 3.0 |
| Hara et al. (2011) | Iron ore | 1400 | 155–258 | Fe: 98 | 12.5 |
| de Castro et al. (2012) | Iron ore | 820 | 0–30 | Fe: 68 | 3.0 |
| Yin et al. (2012) | Hematite ore | 970 | 20–40 | Fe: 98 | 1.0 |
| Hara et al. (2012) | Magnetite ore | 1400 | 14–60 | Fe: 100 | 20.5 |
| Kashimura et al. (2012) | Magnetite | 1370 | 17–21 | – | 0.9 |
| Huang et al. (2012) | Hematite ore*** | 1050 | 15–90 | – | 1.5 |
| Hayashi et al. (2013) | Hematite, Magnetite | 1100 | 7 | Fe: 95 | 12.0 |
| Tang et al. (2014) | Iron ore | 1550 | 60–120 | Fe: 83 | 1.0 |
| Chun et al. (2017) | Iron ore | 1050 | 29–65 | Fe: 93 | 3.0 |
| Lei et al. (2017) | Hematite ore | 1250 | 5–60 | Fe: 85 | 1.5 |
| Amini et al. (2018) | Magnetite | 600 | 15–60 | Fe: 70 | 1.1 |
| Nagata et al. (2019) | Iron ore | 1400 | 20 | Fe: 99 | 16 |
| Zhang et al. (2020) | Iron ore | 1300 | 0–50 | Fe: 96 | 0.5 |
| Roy et al. (2020) | Hematite ore | 860 | 2–26 | Fe: 64 | 10 |
| Agrawal et al. (2021) | Iron ore | 1000 | 10 | Fe: 95 | 0.8 |
*: estimated minimum temperature rate by analyzing samples with the use of SEM after microwave heating.
**: only reported the reaction rate in the pellets.
***: considered by the compositions result.
The studies on the microwave-based reduction approaches can be divided roughly into three main streams: the feasibility of reduction under microwave irradiation, clarification of the microwave-based reduction mechanism, and practical applications of microwave-based heating technology.
3.1.1. Feasibility of microwave-based reduction approaches
In this section, the fundamental research on the feasibility of the microwave-based reduction approach to obtain pure iron is summarized.
The detailed behavior of the reduction of pure iron oxide powders, particularly pure magnetite and hematite, has been reported in the following studies:
Ishizaki et al. analyzed the reactivity of pure magnetite and carbon black mixed powder with SiC at a maximum power of 2.8 kW and at 2.45 GHz (Ishizaki and Nagata 2007). Although both samples rapidly heated immediately after the start of heating, the temperature of samples without SiC rose more rapidly due to the higher reactivity of microwave. The reduction rate of iron was shown to gradually improve by increasing the peak temperature. In this study, a high 81.5% reduction rate was obtained with a maximum temperature of 1200 .
Hayashi et al. evaluated the applicability of microwave-based reduction from pure hematite powder to iron compared to the reduction from magnetite at a maximum power of 12.0 kW and at 2.45 GHz (Hayashi et al., 2013). In the microwave approach, the reduction of hematite required a longer heating time than the reduction of magnetite, as is also the case in the conventional furnace-based reduction approach. In this study, it was shown that despite being heated at a higher temperature than magnetite, hematite requires longer heating time to obtain a similar reduction rate to that of magnetite.
In another study, natural iron ore powders were used as samples in addition to iron oxide powder samples. Hara et al. assessed the reduction of natural magnetite ore powder to pig iron under the high density of microwave beams at a maximum power of 20.5 kW and at 2.45 GHz (greatest-scale equipment among earlier studies) (Hara et al., 2012). A 100% recovery rate was obtained for pig iron when irradiated for 30 min at 1400 . Moreover, the high intensity of electromagnetic energy at this scale resulted in less phosphorus and silicon contamination, for example, in the pig iron than in the conventional furnace-based reduction approach.
The microwave-based reduction approach has been investigated using of pellets made from natural iron ore containing reductants with the aim of achieving efficient reduction in the following studies:
Ishizaki et al. investigated the reduction behavior of the pellets composed of natural magnetite ore and coal at a maximum power of 5.0 kW at 2.45 GHz (Ishizaki et al. 2007). The pellets were heated under a step power supply. The three steps of reduction behavior were as follows: when pellets are heated at nearby 800 , no reduction reaction occurred; after reaching 800 , the reduction of magnetite to wustite gradually progressed until the temperature reached 1000 ; and finally, wustite was reduced to iron at the temperature range between 1000 and 1250 .
Zhang et al. studied the applicability of one of the major biomass contents, lignocellulose, as a reducing agent at a maximum power of 0.5 kW and at 2.45 GHz (Zhang et al., 2020). By comparing the recovery rate of iron ore with the use of lignocellulosic biomass and also with powdery coal, the potential of lignocellulose for use as a reducing agent under microwave-based heating conditions was shown.
3.1.2. Clarification of the microwave-based reduction mechanism
In this section, the existing research works based on the clarification of the microwave-based reduction mechanism are summarized with a particular focus on the different reduction behavior of the electric and magnetic field and the measurement of the apparent activation energy.
To separate the electromagnetic field and analyze the natural behavior of electric field and magnetic field independently, the resonance cavities apparatus was applied (Klein et al., 1993).
Stir et al. studied the pure magnetite reduction of iron with the use of the cavity at a maximum power of 0.50 kW and at 2.45 GHz (Stir et al., 2009). The magnetite and carbon black mixed powder was irradiated in the microwave cavity at the maximum electric field position. The detailed reduction behavior from magnetite to iron in the electric field was determined: the magnetite was reduced to stoichiometric wustite via non-stoichiometric wustite, and then the stoichiometric wustite was finally reduced to iron after the 200 s irradiation. A 58.8% recovery rate was achieved for iron after 300 s of heating.
Kashimura et al. investigated the pure magnetite reduction to iron with the use of the cavity at a maximum power of 0.90 kW and at 915 MHz (Kashimura et al., 2012). A comparison of the rates of the rise in temperature among in the E-field, the H-field and the electromagnetic field indicated that the temperature of the magnetite sample containing carbon potentially exceeds 1370 in the electromagnetic field. A comparison of XRD data of the samples indicated that more magnetite was reduced to -Fe in the H-field than in the electromagnetic field. Although samples were also heated in the E-field, the maximum temperature was below 1200 , which was the lowest peak temperature among the three fields. Based on these results, it was found that the H-field heating has a higher reactivity in the microwave-based magnetite reduction approach than E-field heating.
In another study, the apparent activation energy through the reduction of iron oxides to iron was compared under different heating sources to determine the advantage of microwave irradiation. Huang et al. evaluated the apparent activation energy of natural hematite ore in the non-thermal effect of the microwave with a maximum power of 1.5 kW and at 2.45 GHz (Huang et al., 2012). In the temperature range between 554 and 800 and between 820 and 1050 , the apparent activation energy in the reduction of hematite ore to iron under the microwave irradiation was 75.3 kJ/mol and 53.2 kJ/mol, respectively. The activation energy in these cases was lower than that of the conventional furnace-based approach (158–261 kJ/mol), indicating the microwave-based furnace achieves higher energy efficiency in the reduction of iron ore to pure iron.
3.1.3. Practical application of the microwave-based heating
To further explore the utilization of the microwave as a new heating source in the iron reduction approach, the results of the earlier studies led to suggestions that it can be used in practical applications. In particular, its benefits were considered significant in the continuous reduction approach, in the mitigation of the environmental burden and for the removal of impurities.
The continuous system in the microwave-based reduction approach has been developed in the following studies. Kashimura et al. developed the framework of microwave-based continuous smelting furnace and empirically assessed its framework at a maximum power of 3.0 kW and at 2.45 GHz (Kashimura et al. 2010). The advantages of the microwave-based iron-smelting furnace compared to the conventional furnace approach were identified as follows; nearly half the amount of carbon emissions can be reduced since the samples do not need to be burned; the processing time is shorter; the controllability of furnace is higher; and the iron purity is improved and other processes for pollution removal are not required. The fastest processing time was shown to be approximately 7 min at 3.0 kW power: this is only 2% of the processing time in the conventional blast furnace approach.
Hara et al. developed a system for the continuous production of pig iron at a maximum power of 12.5 kW maximum power (Hara et al., 2011) and at 2.45 GHz. This system was later investigated by Nagata et al. at a maximum power of 16 kW and at 2.45 GHz (Nagata et al. 2019). It was shown that the use of the microwave-based furnace allows the rapid production of pig iron. According to Hara et al. (Hara et al., 2011), reduction time is approximately 6 h in the conventional blast furnace approach, while it is approximately 3 h in the microwave-based approach. A maximum recovery rate of 90% of the pig iron was achieved for the total amount of iron ore.
The use of a non-carbonaceous reductant has gained significant attention even under microwave heating conditions recently. Since carbon dioxide (CO2) emissions are associated with the use of carbon-based materials such as graphite powder, which is one of the main reductants in the reduction processes, the following studies were dedicated to a system with lower CO2 emissions.
Amini et al. used H2 in the gas state as a reductant in the magnetite reduction approach based on microwave heating with the use of 1.1 kW maximum power at 2.45 GHz (Amini et al., 2018). The use of alternative reductants not made from carbon-based materials resulted in much lower CO2 emissions than in the conventional carbon-based reduction approaches. The recovery rate of iron increases as the heating time increases, and the absorption depth of microwave from the surface area to the inner area of samples was shown to depend on the size of the grains in the powder.
Since phosphorus contamination is known to have a negative impact on the quality of iron and steel (Suzuki et al. 1981), the microwave processing was applied in the extraction of impurities from the reduced iron and steel in the following studies.
Yin et al. investigated the potential of removing the phosphorous in oolitic hematite ores at a maximum power of 1.0 kW and at 2.45 GHz (Yin et al., 2012). Natural oolitic hematite ore highly contaminated by phosphorous was heated with coal as the reductant in a commercial microwave oven for 20–40 min. A high iron recovery rate of over 98% was achieved in the 40 min heating, and it was shown that the recovery rate was not affected by the amount of reductant. It was also shown that microwave irradiation for a short processing time leads to a higher dephosphorization rate.
3.2. Non-ferrous metals
The focus of earlier studies of the microwave-based pyrometallurgical approaches tended to be on the reduction of iron oxides. However, some studies also addressed the reduction of some non-ferrous materials, including copper, zinc, magnesium, scandium and vanadium. Those elements in the microwave-based reduction approaches are summarized in Table 2.
Table 2. Microwave-based reduction approaches to obtain non-ferrous metals.
| Reference | Element | Maximum Temperature [°C] | Processing time [min] | Maximum recovery [%] | Maximum Power [kW] |
|---|---|---|---|---|---|
| Samouhos et al. (2011) | Copper(II) oxide | 900 | 0–8 | Cu: 97 | 0.8 |
| Fukushima and Takizawa (2016) | Copper(II) oxide | 1000 | 2 | Cu: 60* | -** |
| Fujii et al. (2017) | Scandium(III) fluoride | 880 | 30 | -*** | 0.2 |
| Wada et al. (2017) | Calcined dolomite ore | 1000 | 80–240 | Mg: 71 | 6.0 |
| Omran, Fabritius and Heikkinen et al. (2018) | Zinc oxide, Zinc ferrite | – | 1–12 | Zn: 99**** | 0.7 |
| Inazu et al. (2020) | Vanadium oxide | – | 60 | V: 56 | 0.8 |
*: estimated from the results chart.
**: only reported the microwave frequency at 2.45 GHz.
***: only reported the observation of pure scandium.
****: obtained in the reduction of zinc oxide.
3.2.1. Copper
Copper is one of the most-highly-consumed non-ferrous metals in the world, with consumption expected to increase remarkably in the years leading to 2050 (Halada et al. 2008). Therefore, the development of an efficient reduction approach to obtain pure copper is important. In some studies, the microwave-based reduction approach has been investigated for CuO to pure copper.
Fukushima et al. compared the conventional furnace-based approach with the microwave-based approach with the use of a cavity at 2.45 GHz (Fukushima and Takizawa 2016). The CuO powder was mixed with boron nitride. This mixed powder was then heated to 1000 in a magnetic field in a microwave-based furnace, and to 1200 in a conventional furnace. When the sample was heated in the magnetic field, a Cu2O layer was observed on the surface area, while a CuO layer was observed on the surface area after heating in a conventional furnace. The difference between the peak temperatures in the heating processes resulted in different compositions in the copper oxide layers. Excluding the weight of the copper oxide layer, the recovery rates of pure copper in the case of heating in the magnetic field and heating in the conventional furnace were nearly 60% and 30%, respectively.
By changing microwave frequencies between 912 MHz and 2.45 GHz, Samouhos et al. investigated CuO reduction using a number of different reductants, including graphite, coke, and lignite, in a microwave-based approach using a cavity and a maximum power of 0.80 kW (Samouhos et al. 2011). The results indicated that CuO powder mixed with twice the stoichiometric amount of lignite achieved the highest recovery rate of 97% when subjected to 4 min of heating.
3.2.2. Zinc
The zinc is one of the most highly consumed non-ferrous metals in the world, and as is the case with copper, consumption is expected to increase considerably in the years leading to 2050 (Halada et al. 2008). The microwave-based reduction approach has been investigated with the aim of obtaining pure zinc.
Omran et al. investigated the microwave-based reduction approach from zinc oxides and ZnFe2O4, or zinc ferrite, with the aim of obtaining pure zinc with a maximum power of 0.7 kW and at 2.45 GHz (Omran, Fabritius and Heikkinen et al., 2018). The zinc oxide and zinc ferrite were mixed with the graphite powder and heated for 12 min. The recovery rates of zinc from the zinc oxide mixed with graphite powder were over 95%. The highest recovery rate was over 99% from the zinc oxide mixed with twice the stoichiometric amount of graphite powder.
3.2.3. Magnesium
Magnesium is used extensively in industrial products, such as cars (Kulekci 2008). It is mainly reduced from CaMg(CO3)2, or calcined dolomite, which is produced by heating natural dolomite ore. In the conventional reduction approach to obtain pure magnesium, the Pidgeon process is one of the most commonly adopted approaches (Halmann et al. 2008). It has been revealed that this process for producing magnesium emits a high amount of CO2 (Gao et al. 2009).
In one study, Wada et al. attempted to replace the conventional Pidgeon process with the microwave-based reduction approach using a maximum power of 6.0 kW at 2.45 GHz (Wada et al., 2017). While carbonaceous materials are used as the reductant in the conventional Pidgeon process, ferrosilicon was used as a reductant in this study in an effort to reduce CO2 emissions. The calcined dolomite was mixed with the ferrosilicon to produce sample briquettes. When the sample briquettes were heated in the microwave-based apparatus under 0.80 kW power irradiation, a 70% recovery rate of magnesium was achieved. The energy consumption in this approach was 58.6 MJ/kg, which represented just one-third the energy consumption of the conventional furnace-based reduction approach. A clear mitigation in CO2 emissions and energy consumption was achieved by using the microwave-based reduction approach instead of the conventional Pidgeon process.
3.2.4. Scandium
Scandium, a rare earth element, is used in light-weight aluminum-based alloys. In the conventional smelting approach, natural scandia (Sc2O3) is reduced to pure scandium via scandium(III) fluoride (ScF3) (Harata et al., 2008).
Fujii et al. used the microwave-based reduction approach to obtain pure scandium with the use of a cavity with a maximum power of 0.18 kW (Fujii et al., 2017). The ScF3 powder was mixed with carbon powder as a reductant, and then the sample was heated under 0.10 kW power irradiation in the magnetic field. After heating for 30 min, pure scandium was adhered to the glass tube in the microwave-based apparatus. The maximum temperature of this approach was 880 , which was 45% lower than that of the conventional furnace-based reduction approach. The lower maximum temperature lends itself to the potential simplification of apparatus since the use of heat insulators can be minimized.
3.2.5. Vanadium
In the modern refining process, vanadium metal is produced under a thermite reaction between aluminum and vanadium oxide (V2O5). This typically takes place at a high temperature of over 2000 . The use of other reductants, such as magnesium oxide (MgO) (Miyauchi and Okabe 2010) and calcium chloride (CaCl2) (Oka and Suzuki 2009), has been shown to reduce the maximum temperature range.
Inazu et al. reported an alternative reduction process using magnesium oxide as a reductant and microwave irradiation at a maximum power of 0.82 kW and at 2.45 GHz (Inazu et al., 2020). Vanadium oxide was mixed with the reductant and was heated for 1 h. The 1000 high temperature under microwave-based heating was almost half that associated with the conventional-based heating method, and a 56% recovery rate of vanadium was achieved.
4. Producing recycled metals
The microwave-based pyrometallurgical and hydrometallurgical recycling approaches are summarized in this section.
4.1. Pyrometallurgical processes
Pyrometallurgical recycling methods are approaches to extract metals from wastes through the physical and chemical transformations by providing thermal energies (Abdelbasir et al., 2018). Microwave-based pyrometallurgical approaches have been investigated for recycling a number of waste materials, such as blast furnace sludge (BFS), electric arc furnace dust (EAFD), and chromium converter waste (CRC). These studies are summarized in Table 3.
Table 3. Microwave-based pyrometallurgical recycling approaches to obtain pure metals.
| Reference | Element | Maximum Temperature [°C] | Processing time [min] | Maximum recovery [%] | Maximum Power [kW] |
|---|---|---|---|---|---|
| Sun et al. (2008) | EAFD | 1200 | 9–15 | Fe: 70, Zn: 100 | 1.1 |
| Kang et al. (2012) | BFS | – | 15 | Fe: 87, P: 93 | 1.7 |
| Kim et al. (2012) | EAFD | 2000 | 4–7 | Fe: 90 | 1.7 |
| An et al. (2014) | EAFD | 1500 | 15–35 | Fe: 87 | 1.7 |
| Omran and Fabritius (2017) | BFS | 1200 | 3–21 | -* | 0.9 |
| Omran and Fabritius (2018) | BFS | 850 | 0–20 | Zn: 95 | 1.1 |
| Cong et al. (2018) | Red mud | – | 25–45 | Fe: 88 | 3.0 |
| Omran and Fabritius (2019) | CRC, EAFD | 1200 | 5–20 | Zn: 97** | 1.1 |
| Ye et al. (2019) | EAFD | 1050 | 15 | Fe: 95, Zn: 100, Pb: 93 | 1.5 |
| Omran et al. (2021) | CRC, EAFD | 1200 | 10 | Zn: 98 | 1.1 |
| Mizuno et al. (2021) | EAFD | 550 | 5–30 | Zn: 80 | 7.5 |
*: only reported the potential of microwave as a heating source.
**: the CRC mixed with graphite powder reached the maximum recovery rate.
4.1.1. Blast furnace sludge (BFS)
The blast furnace sludge (BFS) is generated through the iron and steel making processes from the blast furnace. Metallic ingredients such as iron, CaO, Al2O3, MgO, zinc and K2O are contained in the BFS. In addition, the BFS also contains the carbonaceous material which could potentially act as a reductant in the microwave-based recycling approach.
Omran et al. examined the effect of a microwave-based pyrometallurgical recycling approach from the BFS with the use of a cavity with a maximum power of 0.90 kW at 2.45 GHz (Omran and Fabritius 2017). After heating for 15 min, the reduction to pure iron was observed. The same research group conducted a further investigation of BFS recycling by the microwave-based approach with the use of cavity with 1.1 kW maximum power at 2.45 GHz (Omran and Fabritius 2018). The BFS was crushed into less than 63 grain powder size to improve the metallic recovery rate. After heating for 20 min at a maximum temperature of 800 , the recovery rate of zinc was nearly 95%. After heating at the maximum temperature over 850 for 20 min, the recovery rate of zinc slightly improved. In addition, the over reduction of the BFS was observed: that is, iron oxides were reduced to pure iron. It would appear that this system enables the simultaneous recycling of the BFS to obtain zinc and iron.
4.1.2. Electric arc furnace dust (EAFD)
Electric arc furnace dust (EAFD) is mainly generated through the recycling process of zinc-galvanized steel plates in the production of iron with the use of electric arc furnace (Mantovani et al. 2004). The EAFD contains some vital metals, such as zinc and iron (Khattab et al. 2017). One of the main conventional approaches in the EAFD recycling is the Waelz Kiln process (Lin et al., 2017). Despite permitting a continuous recycling system of EAFD, this approach requires a longer processing time and the recovery rates of iron and zinc are relatively lower. Therefore, the microwave-based approaches have been to EAFD recycling as a new method to reduce the processing time and to improve the recovery rates of iron and zinc.
Sun et al. developed the microwave-based pyrometallurgical recycling approach of EAFD with the use of 1.1 kW maximum power at 2.45 GHz (Sun et al. 2008). The EAFD was mixed with carbonaceous materials like petroleum coke, graphite, and coke performed as a reductant. During heating, the vaporous zinc was reduced from the EAFD due to the low boiling point of pure zinc (Belardi et al., 2012). After heating, 70% and 99% recovery rates for iron and zinc were achieved, respectively. With increasing the heating time and the amount of added carbonaceous materials, the recovery rates of iron zinc improved. It took only 15 min to obtain the reduced iron and vaporous zinc, which achieved one-sixteenth shorter processing time than the conventional Waelz Kiln process.
Mizuno et al. reported a low-carbon recycling process for EAFD using a maximum power of 7.5 kW at 2.45 GHz (Mizuno et al. 2021). In this study, silicon powder was utilized as a non-carbonaceous reductant under the microwave-based recycling of zinc. In their microwave-based recycling approach, the highest removal rate of zinc was nearly 80%, which was more than twice that of the conventional electric furnace. The apparent activation energy of zinc removal under microwave irradiation (64.52 kJ/mol) was lower than that of the conventional electric furnace (179.91 kJ/mol), which implies the enhancement of the chemical reaction speed of zinc removal under microwave irradiation.
4.1.3. Other wastes
Omran et al. studied the microwave-based pyrometallurgical recycling approach of zinc from dusts generated through the iron and steelmaking processes using a cavity with a maximum power of 1.1 kW at 2.45 GHz (Omran and Fabritius 2019). This study considered the following three kinds of dust: the chromium converter (CRC) generated in the treatment of liquid ferrochrome in the ferrochrome production process, the electric arc furnace stainless steel dust, and the electric arc furnace carbon steel dust attained in the recycling of metal scraps by the electric arc furnace. Each dust was mixed with both the BFS and graphite powder performed as a reductant to obtain the pure zinc. After heating for 20 min, the zinc recovery rate in all the samples was over 72%, and the highest zinc recovery rate was almost 97% in the case of the CRC mixed with the graphite powder. These results indicated that the BFS has great potential as a reductant in the microwave-based recycling approach. It should also be noted that the same research group assessed the efficiency of microwave-based furnace with a maximum power of 1.1 kW at 2.45 GHz and compared it with the conventional furnace (Omran et al., 2021).
4.2. Hydrometallurgical processes
Hydrometallurgical recycling methods are approaches for extracting metals from wastes through the chemical reaction between the samples and solutions or solvents (Abdelbasir et al., 2018). The acid or alkaline liquids (e.g., sulfuric acid and calcium hydroxide) are used as the leaching reactors. The microwave-assisted leaching approaches have been investigated for the blast furnace sludges (BFS), the electric arc furnace dust (EAFD), the fluorescent lamps shredder wastes (FLSW), the lead smelting residues (LSR) and copper smelting dust (CSD). The results are summarized in Table 4.
Table 4. Microwave-assisted leaching approaches to obtain pure metals.
| Reference | Element | Maximum Temperature [°C] | Processing time [min] | Maximum Extraction [%] | Maximum Power [kW] |
|---|---|---|---|---|---|
| Xia and Pickles (2000) | EAFD | 120 | 1–60 | Zn: >95 | 0.9 |
| Dutra et al. (2006) | EAFD | 700* | 5–240 | Zn: 60 | 1.0 |
| Veres et al. (2012) | BFS | – | 0–30 | Zn: 90, Fe: 12 | 0.9 |
| Zhang et al. (2013) | Indium-bearing zinc ferrite | – | 5–90 | In: 60 | -** |
| Hobohm et al. (2016) | FLSW | – | 20 | Y: 97 | -** |
| Kim et al. (2017) | LSR | 160 | 50 | Ni: 79, Cu: 94, Zn: 74 | -** |
| Turan et al. (2017) | CSD | – | 5–100 | Cu: 100, Zn: 35, Fe: 5 | 0.9 |
| Sabzezari et al. (2019) | CSD | 90 | 30 | Cu: 89 | 1.0 |
| Laubertova et al. (2020) | EAFD | 104 | 1–60 | Zn: 49, Pb: 93 | 0.9 |
*: observed in the pretreatment process of sample.
**: only reported the microwave frequency at 2.45 GHz.
4.2.1. Blast furnace sludge (BFS)
The extraction rate of iron and zinc was investigated by Veres et al. by employing three different acids for leaching in a microwave-assisted leaching approach using a cavity with a maximum power of 0.90 kW at 2.45 GHz (Veres et al., 2012). Sulfuric acid, hydrochloric acid and nitric acid were separately mixed with BFS before the samples were heated in the microwave-based apparatus. After 30 min of heating under 0.16 kW power irradiation, the highest extract rates of iron and zinc from the BFS was found for sulfuric acid, at nearly 13% and 90%, respectively. According to their study, the obtained extract rates were higher than those achieved by conventional leaching methods.
4.2.2. Lead smelting residues (LSR)
Lead-acid battery scraps are recycled in a smelting furnace to obtain molten lead. The secondary lead smelting residues (LSR) generated in this process, a matte and slag, contain various metals. Some of the vital metals in the LSR, including nickel, copper, and zinc, are recycled using such conventional leaching approaches as high-pressure acid leaching (Li et al. 2009) and sulphation roasting (Dimitrijevic et al., 2016).
To improve the extraction rates of nickel, copper and zinc in the LSR, Kim et al. assessed the microwave-assisted leaching approach of LSR using a microwave at 2.45 GHz (Kim et al., 2017). The LSR was heated with 0.5 mol/L nitric acid as a leaching reactor. After heating at 160 for 15 min, the extraction rates of 79%, 94% and 74% were obtained for nickel, copper, and zinc, respectively. The extraction rates of nickel and copper were higher than those of the conventional sulphation roasting approach, while that of zinc was slightly lower.
4.2.3. Fluorescent lamps shredder wastes (FLSW)
Fluorescent lamps mainly consist of cylindrical glass tubes, electrodes and phosphors (Leslie and Conway 2007). Those lamps are filled with noble gases such as argon, neon, or krypton to generate visible light. Since the phosphors in the fluorescent lamps contain rare earth elements, such as yttrium, lanthanum, cerium, europium, gadolinium, and terbium (Wu et al., 2014), the recycling of fluorescent lamps shredder wastes (FLSW) allows these rare earth elements to be obtained.
In the conventional recycling approaches used with FLSW, several hours are required to extract rare earth elements with high efficiency (Tunsu et al. 2014). Therefore, Hobohm et al. developed a microwave-assisted leaching approach for FLSW to reduce the processing time (Hobohm et al., 2016). The FLSW was mixed with SiO2 sand, and then several different types of acids were added to this FLSW mix as leaching reactors to allow extraction rates to be compared. Among the rare earth elements contained in FLSW, the optimal condition for yttrium extraction was a mixture of nitric/hydrofluoric acid (i.e. aqua regia), with an extraction rate of nearly 97%. This result, which was almost the same as that of conventional leaching approaches, was achieved with an almost 90% reduction in the processing time.
4.2.4. Electric arc furnace dust (EAFD)
The use of microwave-assisted leaching approaches to the recycling of EAFD has been investigated. Xia et al. investigated a microwave-assisted leaching approach for zinc recovery from EAFD using a maximum power of 0.90 kW at 2.45 GHz (Xia and Pickles 2000). Calcium hydroxide was selected as the leaching reactor in this study, and the optimal condition for extracting zinc with high efficiency was determined. With increases in the microwave irradiation power, the extraction rates of zinc gradually improved, reaching over 90% under 0.90 kW power irradiation. In addition, with the increase in the concentration of calcium hydroxide, the extract rate of zinc improved, reaching nearly 95% for a concentration rate of 8 mol/L. These results show that the microwave irradiation power and the concentration rate of calcium hydroxide had a significant influence on the extraction rate of zinc. In addition, the extract rate of zinc in the microwave-assisted leaching approach was 5–10% higher than that of the conventional-based leaching approach.
Laubertova et al. compared the efficiency of microwave-based leaching with that of conventional furnace-based leaching with the use of 0.9 kW maximum power at 2.45 GHz (Laubertova et al., 2020). Sodium hydroxide was used as the leaching agent, and the extract rates of Zn and Pb were measured at the same condition in both leaching methods. In the results, the recovery rates of both metals by the microwave-based leaching were higher than those by the conventional furnace-based leaching due to the uniform heating of the samples made possible by the heating mechanism of microwave irradiation. The highest extract rates obtained for Zn and Pb were 48.72% and 92.84%.
4.2.5. Copper smelting dust (CSD)
In the conventional pyrometallurgical smelting of copper from natural ore, a huge amount of CSD is generated as a by-product (Montenegro et al. 2008). Recycling CSD is important to obtain valuable metals, such as copper, zinc and iron.
A microwave-based leaching approach was applied to CSD in an investigation by Turan et al. using hydrogen peroxide and acetic acid as solvents and a maximum power of 0.9 kW at 2.45 GHz (Turan et al. 2017). The extraction rates of copper, zinc and iron were determined along with the kinetics mechanism of CSD in hydrogen peroxide with acetic acid. After heating the sample for 30 min, a 100% extraction rate was obtained for copper, while that of iron was less than 5% because of the selective leaching of copper contained in the CSD. In addition, the apparent activation energy of CSD under microwave irradiation was assessed at 16.64 kJ/mol by applying the shrinking unreacted core model (Dickinson and Heal 1999) to a kinetic analysis in a multiple solid-liquid chemical reaction.
5. Discussion
Section 3 and Section 4 include reviews of the studies on the microwave-based pyrometallurgical and hydrometallurgical approaches to obtain pure metals. In this section, we identify the research gaps based on those studies and consider and the future prospects.
5.1. Microwave-based approaches to obtain virgin metal
In the conventional iron and steelmaking processes using the blast furnace, pure hematite and hematite ores, not magnetite ores, are utilized as raw material to obtain pig iron (Fu et al., 2016). In the earlier studies on the use of microwave as a new heating source, the attention was on pure magnetite and magnetite ores. This trend in preferable technologies is likely due to the differences in physical properties between hematite and magnetite in terms of permittivity and magnetic permeability. Basically, any metal-oxide or reductants with high reactivity to the microwave over a wide range of temperatures would be suitable for use in microwave-based reduction approaches. The reactivity of metal-oxides and reductants with microwave mainly depends on the two physical property values: permittivity and magnetic permeability. The permittivity of magnetite is nearly the same as that of hematite, while the magnetic permeability of magnetite is higher than that of hematite (He et al., 2015). Given that a higher magnetic permeability is associated with the greater microwave energy absorption, magnetite rather than hematite is reduced more efficiently using microwave-based heating.
The introduction of microwave technology diversifies the options of the reduction process, which assists in determining the technological selection based on the physical properties of iron ores. As seen in the case of iron oxides, there are metal ores that are not suitable for use in conventional furnace-based heating but are suited to microwave-based heating. Further investigations of metal-oxides and reductants highly matched with the microwave-based heating, determined by referring to their reported permittivity and magnetic permeability, are required to deeply explore the microwave-based reduction approaches in a practical manner.
However, while these physical properties for various metal-oxides and reductants have already been measured at room temperature, few studies have been conducted to determine their permittivity and magnetic permeability at high temperature. The studies that have been done have been for just a few materials: magnetite (Hotta et al. 2011), goethite (Pickles et al. 2005), nickel zinc ferrites (Peelamedu et al., 2006), chromium oxide (Dube et al., 2007), alumina and zirconia oxide (Arai et al. 1995)). It is essential that accurate measurements of permittivity and magnetic permeability at high temperature are reported for metal-oxides and reductants to determine the range of materials suitable for use in microwave-based reduction approaches.
5.2. Microwave-based approaches to obtain recycled metal
In addition to depletion issues, the dependency on other countries for raw materials is a matter of concern due to the insecurity associated with external dependency and the requirement for continuous supply (Kosai et al., 2018). To address those concerns, the development of efficient recycling approaches to extract various metals such as microwave technology is of great importance. As yet, recycled metal production using both pyrometallurgical and hydrometallurgical approaches has yet to be fully evaluated or compared to virgin metal production using the microwave-based method. Therefore, further investigation is required on the recovery of various metals under microwave irradiation.
To improve energy efficiency, the scale of the conventional furnace under the pyrometallurgical recycling approaches has become larger (Lee and Jou 2012). Clearly, the energy efficiency at the microwave-based furnace scale is different from that of conventional furnace-based heating. Bermúdez et al. assessed the rates of energy efficiency in the microwave-based furnace with the use of 0.70 kW maximum power at 2.45 GHz and found that increasing the weight of samples from 5.0 to 200 g leads to a decrease in the energy efficiency of microwave absorption, and that for samples over 200 g the energy efficiency remains the same (Bermúdez et al., 2015). Therefore, microwave-based recycling approaches are suitable for small-scale recycling systems. A small-scale microwave-based recycling system is also suitable for use as a laboratory scale facility with more than 286 g/kW, which is equal to a power density of 200 g/0.7 kW.
One of the benefits of small-scale microwave-based pyrometallurgical recycling is that it can be practically delivered as a distributed recycling system. The distributed recycling process has been already studied for the polymer recycling system. In the recycling of organic compounds such as polylactic acid (PLA) and acrylonitrile butadiene styrene (ABS), which are used in fused filament fabrication-type 3D printers, a distributed recycling system has been recommended to reduce the transportation energy (Lee et al., 2018). Typically, waste materials are collected and delivered to a central recycling facility since large-scale facilities have high recycling efficiency. However, since distributed recycling systems do not require long-distance delivery, their use would amount to a massive reduction of energy use during the transportation stage. Also, the distributed recycling system is suitable for use with small-scale recycling facilities due to the simplicity of installation in domestic locations, including offices and homes. Considering the advantages of small-scale recycling with the use of microwave, there is great potential for microwave-based pyrometallurgical recycling approaches to be the base for the establishment of a distributed recycling system. This small-scale microwave-based pyrometallurgical recycling approach has the potential to dramatically reduce the excessive energy consumed in the transportation from the storage area of waste materials to the conventional large-scale recycling facilities.
The recycling approaches which use acid or alkaline chemical agents to obtain pure metals have been widely reported. There has been some attention given to the recovery of rare earth elements from waste materials using hydrometallurgical approaches: from nickel metal hybrid batteries (Yao et al. 2018), from LED flat panel displays (Ruiz-Mercado et al., 2017), and from neodymium magnets (Vander-Hoogerstraete et al., 2014)). As yet, microwave-assisted leaching approaches to the treatment of those waste materials have not been studied. Given the advantages of the use of microwave as a heating source, such as the improvement of reactivity of chemical agents and the reduction in the processing time compared to the conventional-based leaching approaches, the investigation of reactivity between the microwave-assisted leaching approaches and those waste materials containing rare earth elements is expected to contribute to the development of a new recycling system.
6. Conclusion
Microwave-based extractive metallurgical approaches to obtaining pure metals were reviewed in this paper. The principles of the microwave-based furnace to heat the samples were first summarized. Then, key studies of microwave-based approaches to produce virgin metals were presented along with those on microwave-based approaches to recovering metal in recycling. Finally, through the review, the limitations of current progress and the future prospects of microwave-based metallurgical technology were identified and possible directions for the future were discussed.
The findings can be summarized as follows. The reported microwave-based reduction approaches to obtaining virgin metals have mainly been focused on the reduction of iron oxides due to the reactivity in the microwave between hematite and magnetite. Since so few studies have been done on the suitability of other metal-oxides to microwave-based reduction approaches, there is a large amount of research to be done exploring various non-ferrous metal-oxides, and especially those associated with the greatest depletion risks. Microwave-based recycling approaches to obtain recycled metals can be divided into two streams; pyrometallurgical approaches and hydrometallurgical approaches. The microwave-based recycling approaches are mainly focused on the recycling of industrial waste materials such as EAFD and BFS. Furthermore, because of the great potential of using waste materials as a reductant to obtain recycled metals, microwave-based recycling approaches can be expected to contribute to improve industrial symbiosis.
Through the review, the following significant points emerged regarding heating by microwave:
-
1.
It is possible that materials difficult to reduce using conventional technology can be well-treated under microwave irradiation. In the modern conventional steelmaking process, magnetite is not used as a raw material because it is difficult to reduce. However, magnetite is reactive under microwave irradiation, and a high recovery rate of more than 90% of Fe at was observed. That is, microwave technology has the potential to allow the magnetite ore to be used as a new raw material in the steel industry.
-
2.
The apparent activation energy is lower than in conventional technology. It has been pointed out that the apparent activation energy in the chemical reaction can be reduced by using microwave-based heating to obtain pure metals through both smelting and recycling (a 52.4% reduction in the production of virgin iron metal (Huang et al., 2012) and 64.2% reduction in the production of recycled zinc metal from EAFD (Mizuno et al. 2021)). Considering the more than 50% of reduction in the apparent activation energy under microwave irradiation, microwave technology has the potential to facilitate a more rapid chemical reaction with fewer impurities.
Considering the advantages of the microwave-based furnaces, the following investigations are recommended to help determine the real potential of applications of microwave-based extractive metallurgy:
-
1.
Measurements of the permittivity and the magnetic permeability of metal-oxides and reductants at high temperature;
-
2.
Analysis of the difference in kinetic mechanics between microwave-based heating and conventional furnace-based heating;
-
3.
Evaluation of the distributed recycling system by employing small-scale microwave-based recycling technology, compared with the conventional centralized large-scale recycling approach from the lifecycle perspective.
Declaration of competing interest
All authors have participated in (a) conception and design, or analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content; and (c) approval of the final version.
This manuscript has not been submitted to, nor is under review at, another journal or other publishing venue.
The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript.
The following authors have affiliations with organizations with direct or indirect financial interest in the subject matter discussed in the manuscript: