1. Introduction
1.1. Metallic biomaterials as implants
The use of metallic biomaterials for biomedical implants has been traced back from the 19th century [1], [2]. In recent years, manufacturing and fabrication feature as a primary concern in biomaterial engineering [3], [4], [5], [6], [7]. The selection and design of these implants crucially rely on the proposed application [8], [9], [10], [11]. The main tenacity in the development of metal-based implant is due to the demands in internal fracture fixation and bone repair. Before the 1860s, metals such as iron, silver, and gold was the primary candidate for bio-metallic devices and used as spinal wires or bone pin [12], [13]. The usage of these metals has further dominated other materials in orthopedic surgery since the introduction of Lister's aseptic surgical technique in the 1860s. In orthopedic, depending on the metal implant devices, they are either used as temporary implants (e.g. bone plates, screws, and pins) and permanent implants (e.g. total joints replacement). Recently, there is also increasing trend of using these metal-based implants in the dental and orthodontic applications [3], [5], [12].
Despite the enormous number of available metals and alloys in the materials industry, only a few metals and alloys can fit the requirements for development as bioimplants. The commonly used metallic biomaterials are 316L stainless steel (316L SS) [14], [15], [16], cobalt-based (Co-Cr) alloys [17], [18], [19], [20]and titanium and its alloys [2], [12], [21], [22], [23], [24]. Apart of these metals, shape memory alloys such as NiTi [25], [26], [27], magnesium (Mg) [28], [29]and tantalum (Ta) [12], [30], [31] which categorized as “miscellaneous material” implants [27], [32] are also progressing as a potential candidate. To date, nickel titanium (NiTi) has been used as a vascular stent for non-conventional reconstructive surgery of hard tissues or organs. Where else, magnesium and its alloys showed a bright prospect for application in orthopedic and craniofacial repair [25], [29], [33]. These are because, Mg exhibits an unique ability to degrade in vivo and have similar physical properties as natural bones [28], [34], [35]. As a result, Mg and its alloys typically used to develop orthopedic fixation plates and screw device. Table 1 shows the primary metallic materials approved for use as a medical implant by the United States Food and Drugs Administration (FDA) and their typical application area in the human body.
Table 1. Four classes of metallic biomaterials and their primary applications as implants.
| Materials | Applications | Primary utilizations | Applications status | References |
|---|---|---|---|---|
| Stainless steels |
Orthopedic Orthodontic Cardiovascular |
Temporary devices (screws, plates and hip nails), total hip replacements | Routinely applied | [12], [14], [15], [16] |
| Co-based alloys |
Orthopedic Orthodontic Cardiovascular |
Total joints replacements, dental implants, removable partial dentures, orthodontic wire leads, femoral stems, bone implant applications, load-bearing implants, bearing surface implant, | Routinely applied | [12], [17], [18], [19], [20] |
| Ti-based alloys |
Orthopedic Orthodontic Cardiovascular |
Dental implants, orthodontic wire leads, cardiovascular, vascular stents, heart valve parts, stem, total hip replacements | Routinely applied | [2], [12], [21], [22], [23], [24] |
| Miscellaneous | ||||
| NiTi |
Orthodontic Cardiovascular |
Vascular stents, Vena cava filter, Intracranial aneurysm clips, catheter guide wires, orthopedic staples, orthodontic dental arch wires | FDA approved | [25], [26], [27] |
| Mg |
Orthopedic Craniofacial |
Biodegradable orthopedic implants | Animal test | [28], [29], [30], [31], [32] |
| Ta | A radiographic marker, wire structures for neurosurgery and plastic surgery | FDA approved | [2], [12], [33], [34] |
1.2. Material selection criteria for implant
The selection of specific metal to be an implant greatly depends on its specific medical application. To serve safely and retainable for a longer period without rejection, these metals should have a few essential properties such as excellent biocompatibility, high corrosion and wear resistance, suitable mechanical properties, osseointegration, ductility and high hardness [2], [25], [36], [37], [38].
Intuitively, the primary essential properties for metallic implant biomaterials to be classified as outstanding candidates for biodegradable medical devices are their innate biocompatibility towards living cells [39], [40], [41]. Biocompatibility is defined as the ability of material to be used in close connection with living tissues without causing adverse effects to them [19], [42], [43]. The body parts or tissue of a patient that comes into contact with the implants should prevent from any physical irritation, inflammation, toxicity, mutagenic, or carcinogenetic action [44], [45], [46]. The success of the bioimplants highly depends on the level of compatibility and acceptance of the implant by the human body [47], [48], [49]. However, the biocompatibility of implants extremely depends on their corrosion behavior [25], [50], [51]. Hence, the higher the corrosion of implants, the more of its toxic ions rates are released into the body routinely, and greater risk of adverse effects can be expected [29], [52], [53].
Human body are made up of a significant number of natural elements with water (H2O), comprising of about 65 to 75 wt% of the total composition. Accordingly, most of a human body's mass contain oxygen and carbon [12], [54]. Table 2 shows a list of elements found in the human body. Where, about 96% of available elements are off oxygen, hydrogen, carbon and nitrogen which are the building blocks of both water and proteins. Additional ~ 4% of the body mass comes in the form of bone minerals and blood comprising of Ca, P, Mg and extracellular fluids comprising of Na, Cl and K. As such, any implants developed based on these elements would compatible with the human body. However, there are few trace elements which toxic at high levels. Hence, the proper composition required for the metallic implant be free from being toxic. Henceforth the implant will not release toxic metal ions, which causes inflammatory or allergic reactions in the human body.
Furthermore, the bioimplants should possess appropriate mechanical strengthto withstand all the related forces and loads. Principally, the selected material for a specific application should have the load withstanding capacity, so they will not be likely to suffer from the fracture [55], [56]. Additionally, the implanted biomaterial should be high wear, tear, and corrosion resistance, since they are normally exposed to critical humidity level and high percentage environment that promote localized corrosion surroundings [2], [57]. All the considered main criteria discussed above would result in the development of suitable and reliable implant to the human system. However, the current study reveals that most of the bioimplants start to physically fall apart within the period of about 12–15 years. The causes of the failure are due to the chemical, mechanical, surgical, tribological, manufacturing and biocompatibility-related problems [2], [57], [58].
Among of the critical issues and challenging clinical problem faced today is the failure of an implant due to the corrosion. Despite being naturally occurring, this corrosion resistant biocompatible metal, need to undergo modifications, to enhance their useful properties, especially when used as body implants. The classification of biomaterial for implants are reliant on the main leading features, which are (i) biocompatibility of the implant (ii) the mechanical, chemical and tribological properties of the biomaterial and (iii) the health condition of the patients [45], [48], [59]. Thus, following sections discusses in details the causes that lead to the significance of bioimplants corrosion, some of the common types of corrosions that very frequently observed, the effect of the corrosion and its preventions through few appropriate techniques such as deposition of bioactive coatings, the formation of a surface oxide layer and several surface modifications and surface texturing methods.
2. Corrosion of biocompatible metals
Corrosion defined as degeneration of material into its constituent atom due to chemical reactions occurred between the materials and its surroundings. In another word, corrosion is the process of unstable metal changes in the material's thermodynamic state and electrochemical oxidation of metals in reaction with oxygen [60], [61]. Most of the metallic implants claimed to have high corrosion resistance, which prolongs the implant's life in the human body. However, study shows corrosion occurs slowly due to electrochemical reactiononce a metal implanted in the human bodies [58], [61]. The internal surrounding of the body is tissue fluid that producing very reactive interstitial fluid solution surrounds the tissue cells and ionically responses to biomaterials due to the presence of water, chloride and sodium ions, plasma, proteins and amino acid alongside much in the case of saliva [62]. The metal ions released from bioimplants caused not only serious type of infection and other health issues in the patients but also to the statutory authority in the biomedical industry [63], [64].
Human body system subjected to unusual changes in temperature and pH due to changes in local, environmental, systemic, social and economic conditions of individuals. Coupled with the presence corrosive elements such as hydrogen ion (H +), dissolved oxygen, free radicals (O2, O−), sulfide compounds (S2−), and chloride ion (Cl−) in the body further affects the implants and consequently adverse tissue reactions [54], [65]. For example, the concentration of chloride ion in the interstitial fluid are 11 mEq l− 1 whereas in the plasma are 113 mEq l− 1, which might also cause the exposed materials to corrode [58]. Besides, the strand of amino acids that makes up proteins in body tissues and fluids tend to speed up the chemical reactions by increasing the acid-base amount in the body fluids that increase the corrosion rate further [66], [67]. In addition, alterations in pH value did not significantly promote in lowering the corrosion level since the body fluids react as a buffer solution. Generally, the pH value of the healthy human body is right around 7.35–7.45 [58]. After initial implantation process, the pH changes from 5.2 to 5.6 in dense tissues due to infections and other factors but recovers to a pH value of 7.4 within two weeks [58], [66], [67], [68]. Okazaki and Gotoh [69] proved that metal ions concentrations in the patient blood could segregate between well-functioning metal-on-metal resurfacing and increase the wear rates of prostheses. The study showed that the quantity of Fe and Ni released from 316L SS decreased gradually with increasing pH. Correspondingly, the amounts of Mo and Cr released from 316L SS and Co–Cr–Mo casting alloy were also smaller at a pH of 4 or higher. The observations are in accordance with other studies reported by Okazaki and Gotoh [70] in which they stressed that the strongest effect of pH could decrease in the quantities of Fe and Ni released from the SS that will increase the pH of the bodies. Thus, corrosion due to an abrupt change in pH of the body fluid appears to be negligible. Apart from that, the wear and initial cracks that formed on the implant surface would initiate and further accelerate the corrosion rate [71], [72]. Moreover, metal ions that constantly diffused in the body fluids following a latency period of exposure causing an increase in the amount of a cationic form of a toxic ions metals combine with biomolecules like enzymes and proteins in the body [58], [73] shown to cause significant deleterious effects in health issues.
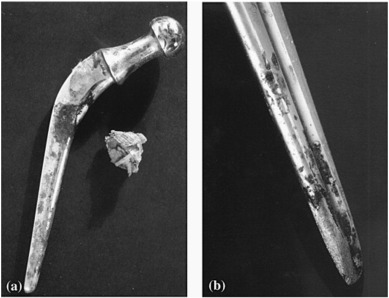
Sir John Charnley designed a Total Hip Arthroplasty (THA) implant in the 1970's [74], [75]. The design consisted of three parts: stainless steel femoral stem and head, a polyethylene acetabular component and a PMMA bone cement [75], [76], [77]. He observed an initial stem fracture from the fatigue failure analysis. After 9–20 years implantation service, failure analysis of the prosthesis revealed that the corrosion occurred on the surface of stem or head of the implant as shown in Fig. 1. The corrosion on the implant surface correlated to periprosthetic metallosis and rapid failure [2], [12], [77]. These were the first defect reported in stainless steel stem material. However, in the stainless steel hip implant, an initial success was reported when implanted to a young population with a longer life expectancy. Nonetheless, allegations of failure of the hip implants emerged in 1990 due to aseptic loosening. Based on the clinical application of THA from 1970 to 1990 demonstrated that 316L stainless steels are not suitable to be used for a longer period as hip joint implant materials due to their insufficient corrosion resistant properties. Table 3 lists the commercially available and applied metal implants for hip replacement prostheses.
 Fig. 1. (a) Corrosion scale on a Charnley stainless steel stem, and (b) pitting and corrosion of a Muller stainless steel stem after implant removal [12].
Fig. 1. (a) Corrosion scale on a Charnley stainless steel stem, and (b) pitting and corrosion of a Muller stainless steel stem after implant removal [12].Table 3. Biomaterial used total joint replacements [12], [78], [79], [80], [81]
| Products | Stem | Head | Line | Cup | Year(s) |
|---|---|---|---|---|---|
| Metal-on-metal before the 1960s | |||||
| Philip Wiles | Stainless steels | Stainless steels | No line | Stainless steels | 1938 |
| Austin Moore | CoCr alloy (vitallium) | CoCr alloy (vitallium) | No line | No cup | 1950s |
| McKee–Farrar |
Stainless steels CoCrMo alloys |
Stainless steels CoCrMo alloys |
No line |
Stainless steels Co alloys |
1950s |
| Peter Ring |
Stainless steels CoCrMo alloys |
Stainless steels CoCrMo alloys |
No line |
Stainless steels CoCrMo alloys |
1950s |
| Metal-on-UHMEPE | |||||
| Charnley |
Stainless steels CoCrMo alloys Ti alloys |
Stainless steels CoCrMo alloys Ceramics (Al2O3 or ZrO2) |
UHMWPE |
Stainless steels CoCrMo alloys Ti alloys |
1960s |
| Metal-on-metal since the 1960s |
CoCrMo alloys Ti alloys |
CoCrMo alloys | No line | CoCrMo alloys | 1960s |
| Ceramic-on-ceramic |
CoCrMo alloys Ti alloys |
Ceramics (Al2O3 or ZrO2) | No line | Ceramics (Al2O3 or ZrO2) | 1980s |
| Ceramic-on-metal |
CoCrMo alloys Ti alloys |
Ceramics (Al2O3 or ZrO2) | No line | CoCrMo alloys | 2000s |
Biocompatible metal corrosion feature several distinct mechanisms that take place in the implant devices such as uniform, galvanic, crevice and stress cracking, fatigue and pitting corrosion [82], [83]. The degradation mechanisms induced the release of certain amounts of metal ions concentration in body fluids resulting biological response to the material released and subsequently shorten the functional lifespan of the implant devices [84], [85]. Although there are continuous recent developments in materials used for implants; clinical diagnostic routine reports confirms that these metallic materials are still susceptible to corrosion [85], [86]. However, the stability of oxide scales and modification near the oxide surface layer physically help to render the delay of corrosion responses [33], [87].
Therefore, it is essential for an implant material to undergo a corrosion screening test to identify its performance in various environments [22], [88], [89], [90] prior to the application implementation. The corrosion resistance of materials could be checked based on the American Society for Testing and Materials (ASTM) standards under different conditions. Several standard corrosion tests consensuses to the best currently available test are listed in Table 4. In certain cases, wear and fretting processes, such as “speeded up routes” ahead to tribiocorrosion method [91], [92]. The effects of fretting on the separation of the protective oxide layer are different from the effects on the sliding interfaces. It should be noted that there are still no available standards to investigate the tribiocorrosion performance of the metallic implant.
Table 4. Standards for corrosion resistance testing of biomaterials [48].
| ASTM standards | Specifications |
|---|---|
| ASTM G 61-86, and ASTM G 5-94 | Corrosion performance of metallic biomaterials |
| ASTM G71-81 | Galvanic corrosion in electrolytes |
| ASTM F746-87 | Pitting or Crevice Corrosion of metallic surgical implant materials |
| ASTM F2129-01 | Cyclic potentiodynamic polarization measurements |
2.1. Types of corrosion in metal implants
Electrochemical dissolution of metallic phenomena, mechanical wear or a synergistic amalgamation, are the two routes that significantly result in the degradation of biocompatible implant metal devices [93], [94]. In most cases, corrosion of metals takes place through the interaction with electrochemical cells that causes different forms of corrosion reactions [95], [96]. First, general (uniform) corrosion occurs when the entire surface of the metal are exposed to the cathodic reactants during the localized corrosion. Uniform corrosion mechanism theoretically involved two crucial phases; a) initial surface of metal bombardment via chemical solutions led to the formation of preliminary phase where the cathodic and anodic positions are nearly attached to one another, b) followed by the propagation of a corrosion nucleus around the surface region of the metal by means of the uniform corrosive engine [97]. The passivation of the protective film will be blocked once this phenomenon takes place. Passive films must have certain characteristics to limit further oxidation process. The characteristics are i) non-porous ii) high abrasion resistance and iii) structure that will restrict the electrons and ions migration through the oxide boundary-solution boundary [48], [49]. Uniform corrosion also refers to the corrosion that proceeds at approximately the same rate over the exposed metal surface.
Second, the pitting corrosion which is also known as a form of localized corrosion on the metal surface. Pitting attacks in the form of spots or pits on the surface. Fig. 2 shows the microscopic images at 100 × magnification from scanning electron microscope (SEM) analysis of implant samples after tests for (a) pitting corrosion (b) uniform corrosion. The initial spreading of pitting corrosion on metal takes place in the presence of chloride solution concerning to the electrochemical and corrosion phases. Normally, the basic pitting process can be separated into three distinct stages. Firstly, the formation of a number of new pit nucleation that is stable and prompts the development of a minor bare area, without becoming passive film surface on the metal. Secondly, the formation of a metastable pit nucleation that may have repassivated the prompts local dissolution of the tiny parts of the underlying metal [98]. The dissolved metal area is referred to as a pit embryo. The consequence of the dissolution number of pit embryo is either the development of a stable pit or the re-passivation of the embryo. Finally, the accumulation of damage through the development of a stable pit gives prompts repassivation of metastable pits.
 Fig. 2. SEM microscopic images (at 100 ×) of samples after tests. (a) Pitting corrosion. (b) Uniform corrosion [97].
Fig. 2. SEM microscopic images (at 100 ×) of samples after tests. (a) Pitting corrosion. (b) Uniform corrosion [97].Third form is the electrochemical and mechanical processes such as fretting, fatigue, stress corrosion cracking (SCC), corrosion and shielding forces that interact and encourage the accumulation of stresses, causing premature degradation. Thus, the conditions cause the structural change and alterations of mechanical properties by which accelerated the overall metal particles and ions losses. In the existence of a dynamic body environment, metallic bioimplants are considered to defy which is primarily due to fatigue in the human body that subjected to cyclic loading and high stress [4], [6], [26]. Formation of wear debris due to the growth of unwanted particles displacement caused by fatigue wear speeds up the simple mechanical fatigue process [99]. As the displacement of oxide layer interference and layer and incapability of the material to repassivate instantaneously during the process, the interference color of on the metal implant part becomes significant [100], [101]. The exposure areas of the metal to the corrosive environment lead to the stress corrosion cracking. Additionally, the occurrence of fretting among bone and implant also leads to the acceleration of fatigue, since the re-passivation becomes more challenging in the existence of fretting.
2.2. Corrosion: in vitro and in vivo studies
To be accepted as biomedical implants, the metals used or chosen to fabricate the implant must be compatible with the human body environment [53], [105]. In vitro and in vivo testing are two types of experimental studies performed to investigate the behavior and compatibility of the implants prior approval for the real life application. In vitro is the method where the studies conducted through a given procedure in a controlled environment outside of a living organism or cells [106], [107], [108]. The major weakness of the in vitro studies is the difficulty to replicate the precise internal environment of an organism or cells. Where else, the in vivo studies refer to the technique applied directly to the living organism. This testing widely applied on the animals for the clinical trials [106], [107], [108]. Therefore, in vivo are the most preferred technique.
Presently, in “in vitro” test, investigators studied the corrosion behavior of the implants through simulating the human body environment using simulated body fluid (SBF), Ringer's solution or Hank's solution. Here, the temperatures of the environment are controlled to approximately 37 °C and pH values around 7.35–7.45, to simulate the human body environment. The components of these solutions are listed in Table 5 [102], [103]. Whereas, for dental implant materials, the corrosion performances are assessed using artificial saliva with its own reagents have been summarized in Table 6 [48], [104]. The findings from these different solutions give an overview of the behavior of the material for approval as an implant [109]. From literature, the acceptable corrosion rate for biomaterial implant is around 0.13 mm/year. However, for the metallic implant, the corrosion rate was reported around 2.5 × 10− 4 mm/year [49], [110]. On the other hand, the “in vivo” studies are typically conducted using animal models [108]. It evaluates the real characteristic of the implant in exact condition and environment. At the same time, the tests are also necessary for the approval by the Food and Drug Administration (FDA), USA [111].
Table 5. Composition of SBF's, Rigger's and Hank's solution [102], [103]
| Reagents | SBF's solution | Ringer's solution | Hank's solution |
|---|---|---|---|
| Amount (g l−1) | Amount (g l−1) | Amount (g l−1) | |
| NaCl | 8.035 | 8.69 | 8.0 |
| KCl | 0.225 | 0.30 | 0.4 |
| CaCl2 | 0.292 | 0.48 | – |
| NaHCO3 | 0.355 | – | 0.35 |
| Na2SO4 | 0.072 | – | – |
| K2·HPO4·3H2O | 0.292 | – | – |
| MgCl2·6H2O | 0.311 | – | – |
| 1 M HCl | 39 ml | – | – |
| Tris | 6.118 | – | – |
| 1 M HCl | 0–5 ml | – | – |
| NaH2PO4·H2O | – | – | 0.25 |
| Na2HPO4·2H2O | – | – | 0.06 |
| MgCl2 | – | – | 0.19 |
| MgSO4·7H2O | – | – | 0.06 |
| CaCl2·2H2O | – | – | 0.19 |
| Glucose | – | – | 1.0 |
| pH | – | 6.4 | 6.9 |
Table 6. Composition of different artificial saliva [48], [104]
| Reagents | Artificial saliva | ||
|---|---|---|---|
| Xialine1 (g l−1) | Xialine2 (g l−1) | Saliveze (g l−1) | |
| Xanthan gum | 0.92 | 0.18 | – |
| Sodium chloride | 0.85 | 0.85 | 0.87 |
| Potassium chloride | 1.2 | 1.2 | 0.62 |
| Sodium carboxymethylcellulose | – | – | 10 |
| Calcium chloride | 0.13 | 0.13 | 0.17 |
| Magnesium chloride | 0.05 | 0.05 | 0.06 |
| Di-potassium hydrogen orthophosphate | 0.13 | 0.13 | 0.80 |
| Potassium di-hydrogen orthophosphate | – | – | 0.30 |
| Sodium fluoride | – | – | 0.0044 |
| Sorbitol | – | – | 29.95 |
| Methyl p-hydroxybenzoate | 0.35 | 0.35 | 1.00 |
| pH | Neutral | Neutral | Neutral |
2.3. Effect of corrosion to human body
The corroded implants in the human body cause excessive harmful and toxic metal ions such as Fe, Cr, Ni, Co and Ti [73], [112] released to body fluid. Metal ions released from the implant have been acknowledged as the core reason for clinical failure and allergic reactions [113]. Matusiewicz [114] reported that the higher concentrations of metals for instance Cr, Ni, Co, Ti, and V in the blood of the patients' wearing the implant causing significant health problems. Initially, these major trace elements in metallic implant would not be harmful by the ions released. However, when the implants start to corrode, these trace elements would aggressively diffuse to the body. The release of these excessive harmful metal ions could cause adverse effects to the human body. For example, the huge contents of Fe released from the implant can cause a higher level of Fe in the blood. Even though Fe is a necessary element for all living organisms, ranging from primitive bacteria to humans. For a human, Fe is an essential component of hemoglobin, which helps to carry oxygen from lungs to the tissues. However, the presence of significant amount of Fe in the blood leads to the damage of protein, DNA, lipids and other cellular components [115]. Excessive Fe content typically damages cells in the liver and heart, which can cause critical effect including shock, liver failure, coma coagulopathy, long-term organ damage and even death if left without treated [114], [116].
Where else, Cr another trace elements in the body, acts a cofactor in the regulation of sugar levels in the blood. Once in the body, the Cr (IV) is reduced to Cr (III) before it enters the cells. Higher concentration of Cr (III) in cells results in hemolysis which could damage the DNA, kidneys, liver and blood cells [73], [112]. In Ni and Co based implants, the toxicity of the implant discovered after 4 to 5 years implantation, contact-allergy related dermatitis, red skin, and itchiness was reported for Ni-based implants, while pathology of systemic and neurological symptoms for Co based implants [116]. Apart from that, the higher level of Co in the human body has also reported causing extreme muscle fatigue and cramping, dyspnea, inability to perform simple motor tasks, the decline in cognitive function, memory difficulties, severe headaches and anorexia [12], [112], [114].
3. Surface modification to improve corrosion resistance
Surface modification technique has been identified as a potential approach to overcome and improve the implantable challenges and performance owing to highly internal corrosive human body surroundings. Researchers around the world have focused on improving various surface engineering tools to impart well with biofunctional-, corrosion- and tribiocorrosion-resistant of the implants for superior in-vitro bioactive surfaces and mechanical properties. The techniques used for this purpose include deposition of a thin uniform coating, development of stable passivation oxide layer, ion beam processing for surface modification and surface texturing.
3.1. Deposition of coatings
Depositions of coating onto the metal surfaces are one of the best solutions to improve the osseointegration for in vivo performance and blood cytocompatibility. Theoretically, the surface coating is a formation of the outer layer to improve or prevent metal from corrosion or exposure to oxidation [117], [118], [119]. The addition of a coating on top of the implant surface helps to prevent the release of harmful metal ions into the human body surrounding.
For biocompatible metals, bioactive coatings such as hydroxyapatite (HAp) or HAp-based widely applied since HAp has similar chemical composition, structural and biological aspects as human bone and teeth. Moreover, it has superior mechanical properties that can further improve the strength of the implant. Therefore, with the presence of HAp as a coating layer; the corrosion resistance and strength of the implants expected to improve. Robin et al. [120]have reported in detail regarding the electrochemical deposition of different composition of HAp coating (5, 20 and 50 wt% of HAp) onto 316L SS to improve corrosion properties of the metal. The corrosion performance from the various open-circuit potential (OCP) with sintering time of 316L SS and HAp coated 316L SS in Ringer's solution at room temperature are shown in Fig. 3. However, the studies showed that introducing HAp as a coating to 316L SS did not improve the corrosion resistance behavior of the 316L SS.
 Fig. 3. Variation of open-circuit potential with time for sintered 316L SS and HAp-316L composites in Ringer's solution at room temperature [120].
Fig. 3. Variation of open-circuit potential with time for sintered 316L SS and HAp-316L composites in Ringer's solution at room temperature [120].On the other hand, T.M. Sridhar et al. [121] in his work showed the improvement of corrosion performance of HAp coated onto 316L SS as shown in Fig. 4. The OCP of HAp coated 316L SS were found to be more corrosion resistant compared to uncoated 316L SS. The OCP of uncoated 316L SS shifted towards the active direction which could be due to the dissolution that could occur at the alloy surface.
 Fig. 4. OCP–time measurements in Hank's solution of uncoated and HAp coated type 316L SS obtained with different coating potentials at a constant time of 3 min [121].
Fig. 4. OCP–time measurements in Hank's solution of uncoated and HAp coated type 316L SS obtained with different coating potentials at a constant time of 3 min [121].Recently, the advanced research on the interest ranging from single bioactive coatings to bioactive hybrid coatings has drawn much attention to chemist, physicist, and scientist. The hybrid organic-inorganic coatings processing usually carry out through hydrolysis and condensation reactions in the presence of organically modified silanes with traditional alkoxide precursors using sol–gel method. Many reported works have proved that by combining organic and inorganic materials for coatings could provide an excellent and with more superior corrosion resistance layer for metal substrates. Garcia et al. [122]developed and compared silica containing hydroxyapatite, bioactive glass, and glass–ceramic particles coatings onto 316L SS. The coated 316L SS substrates were immersed for ten days in simulated body fluid (SBF) for “in vitro” study. The electrochemical experiments were estimated by potentiodynamic polarization assay using SBF as the electrolyte. The presence of the coating shown improves the corrosion resistance of 316L SS substrate after immersion in SBF as shown in Fig. 5, signified obstruction of electrolyte coating from reaching the metal device surfaces. Fig. 5, demonstrated the coated substrates that have higher corrosion resistance than the bare substrate. The potentiodynamic data showed a reduction of the passivation current density (ipass). The current density of coated substrates remains passive, and a shift towards positive potentials of the breakdown potential (Eb) compared to the bare substrate.
 Fig. 5. Anodic polarization curve for coatings; glass, glass–ceramic and hydroxyapatite, compared with the bare substrate after immersion in SBF for 24 h [122].
Fig. 5. Anodic polarization curve for coatings; glass, glass–ceramic and hydroxyapatite, compared with the bare substrate after immersion in SBF for 24 h [122].S.M. Hosseinalipour et al. [15] in counterpart have deposited silica-based organic–inorganic hybrid coatings onto 316L SS by sol–gel method. The 3-methacryloxypropyltrimethoxysilane (TMSM) and tetraethylorthosilicate(TEOS) was used as a hybrid coating in the experimental work. The corrosion analysis of the composite coatings on 316L SS was performed by varying the molar ratios of TMSM: TEOS (10:1, 3:1, 1:1, 1:3, and 1:10) in Ringer's solution. Fig. 6 showed the best corrosion protection obtained at TMSM: TEOS molar ratio of 1:1. All coated substrates inhibit lower current density as compared to the uncoated substrate. The increment of the molar ratio of TMSM: TEOS from 1:10 to 10:1 decreases the current density. However, the amount of decrement from 1:1 to 10:1 was not significant. Enhancement of corrosion resistance of hybrid coatings at higher TMSM content might be due to the higher flexibility of such coatings. These findings further supported by the morphological appearance of the cells on sol–gel coated substrates as shown in Fig. 7. The micrographs demonstrated that the cells on sol-gel coated substrates were similar to the cells on uncoated substrates. Hence, sol–gel coatings for L929 cells found to be non-toxic.
 Fig. 6. Polarization curves for coated steel samples at different molar ratios of TMSM: TEOS. Uncoated steel curve included for comparison [15].
Fig. 6. Polarization curves for coated steel samples at different molar ratios of TMSM: TEOS. Uncoated steel curve included for comparison [15]. Fig. 7. Light microscopy investigation of cell morphology on (a) sol–gel coated substrates; and (b) substrates without coating [15].
Fig. 7. Light microscopy investigation of cell morphology on (a) sol–gel coated substrates; and (b) substrates without coating [15].The research on these bioactive hybrid coatings has been vigorously studied in these recent years. Several investigators approached various materials to discover the best suited hybrid coatings for biocompatible implants. A. Janković et al. [123] introduced a combination of graphene-based silver/hydroxyapatite/graphene (Ag/HAp/Gr) composite coatings onto titanium. The coatings were produced via an electrophoretic deposition (EPD) technique, and the finding shows improvement in the corrosion stability in simulated body fluid (SBF). Additionally, Y. Huang et al. [124] compared the bond strength and corrosion behavior for HAp/CaSiO3 coating and HAp coating on metal substrates. Formation of HAp/CaSiO3 coating showed to improve the corrosion resistance and the bond strength of titanium.
The development of additional bioactive materials such as Hap, graphene, and CaSiO3 as surface coatings proven to enhanced the corrosion resistance of biocompatible metals. There also have been numerous efforts by some scientists to use others bioactive materials as coatings onto biocompatible metals for implantation purpose. These are because of bioactive materials were shown to improve osseointegration besides being able to improve corrosion behavior in the human body.
3.2. Passivation-oxide layer
Hydroxyapatite surface coating on the metal surface starts to degrade by way of time evolution. This condition affects the corrosion behavior of essential biocompatible metals. Therefore, before the development of a coating, surface treatment of a biocompatible metal become important to prevent the corrosion and to prolong the implants life in the human body. A stable surface oxide layer on the passivated metal surface plays a significant role in rendering its corrosion resistance including the changes of the surface oxide through the release of metal ions [125], [126], [127]. The surface oxide layer changes in composition due to the responses among the surfaces of living tissues and metallic materials. Moreover, the surface oxide layer on metallic implants also plays a vital role for biocompatibility of the tissues [40], [80].
Low concentration of oxygen, proteins, cells and dissolved inorganic ions expeditates harmful ion diffusion [48]. Also, active oxygen species have also been reported to cause the dissolution of the surface oxide layer [128]. This passivity can be improved by modifying the thickness, morphology, or chemical composition of the surface oxide layer using different treatments [125], [126]. The passivation for enhancing the surface properties of biocompatible metals can be performed either thermally, or electrochemically, or passivation in nitric acid [129], [130], [131]. Table 7 shows the possibility methods for passivation and oxide layers that would form after undergoing passivation process on three widely used biocompatible metals for implants. Their excellent corrosion resistance ascends from the protective passive oxide layers which consist of mainly titanium oxide enriched parts (TiO2) for titanium [132], [133], [134]; chromium oxide enriched parts (Cr2O3) for Cobalt-based alloy [18], [135], [136]; and 316L SS and iron oxide-enriched parts (Fe2O3) for 316L SS [102], [103], [137], [138]. Several authors have supported that the passive layer on stainless steel exhibits a duplex property; the inner layer is a chromium oxide-enriched part while the outer layer is an iron oxide-enriched part [139], [140], [141].
Table 7. Possible surface oxide layers on various biocompatible metals.
| Biocompatible metal | Possible oxide layer formed | Methods to form passivation oxide layer | References |
|---|---|---|---|
| 316L stainless steel (316L SS) | Cr2O3, Fe2O3 |
|
[30], [31], [33], [34] |
| Titanium and its alloy | TiO2 | [35], [36], [37] | |
| CoCrMo alloy | Cr2O3 | [6], [38], [39] |
D. Gopi et al. [142] developed passivation layer on 316L SS in borate buffer solution before HAp coating. Fig. 8 shown the SEM images which support 316L SS with a protective oxide layer with HAp coating was found to have highest corrosion resistance compared to other treated conditions variations; a) no protective layer over the surface, b) un-passivated-HAp coated 316L SS samples and c) borate passivated 316L SS surface. The pit formation still can be observed in un-passivated-HAp coated SS sample (Fig. 8b) that revealed the suspension of metal ions from this sample. However, in borate passivated SS surface (Fig. 8c), the pit growth has virtually stopped due to the development of a surface oxide layer. Finally, Fig. 8d shows uniform HAp coating on the passivated 316L SS surface suspends completely the pit formation on the sample proving the improved corrosion resistance behavior in Ringer's solution.
 Fig. 8. SEM images of (a) bare SS in Ringer's solution (b) SS surface coated in un-passivated-HAp (c) surface of borate passivated in Ringer's solution (d) borate passivated-HAp coated surface in Ringer's solution [142].
Fig. 8. SEM images of (a) bare SS in Ringer's solution (b) SS surface coated in un-passivated-HAp (c) surface of borate passivated in Ringer's solution (d) borate passivated-HAp coated surface in Ringer's solution [142].Albayrak et al. [117] reported the introduction of HAp coating after formation of TiO2 coatings on titanium by EPD. Coating potentials tested at 10–50 V and the effects of TiO2 inner layers was studied. Additionally, Sankara Narayanan et al. [143] developed nano-HAp/TiO2 coatings on titanium alloy by EPD. Furthermore, Sridhar et al. [144] developed a corrosion-resistant HAp coating by EPD deposition method with inner oxide (TiO2) on titanium followed by sintering the coatings at 800 °C for 1 h in air. The TiO2 has been developed as the oxide layer in the middle of the bioceramic coatings and metal surface at elevated temperatures. These add to the overall improvement in the corrosion resistance and allowed for better behavior of the implantable devices as soon as it inserted into the body.
When the oxide layer on the metal is disrupted, corrosion occurs, and a metal ion is released. The outer layer is then repassivated in a process known as ‘regenerated’. The contact stuck between the material and the physiological medium act as a critical role in improving the cyclic oxidation resistance layer within the similar time taken. The regeneration time or repassivation time of the surface oxide layer is dissimilar for several materials applied. The corrosion rate and some metal ions release are highly dependent on regeneration time [58], [145]. Regeneration time for several alloys was observed based on surface oxide layers formation as shown in Fig. 9. From the observation of these alloys, the regeneration time for SS is longer compared to CoCrMo and Ti-6Al-4V alloys. Fig. 9 indicates the SS released a larger number of ions compared to CoCrMo and Ti-6Al-4V alloys, which highlights one of the superior behavior of SS.
 Fig. 9. Time taken for regeneration of surface oxide layers for several alloys [58].
Fig. 9. Time taken for regeneration of surface oxide layers for several alloys [58].There has been a constant attempt to enhance the surface of metallic materials to overcome the failure of implants due to the weak adhesion strength and the loss of harmful metal ions. Therefore, development of passivated oxide layer on the biometallic surface is the most frequent performed to enhanced corrosion and wear resistance. Besides that, biocompatibility of the materials also may be improved by introducing these treatments [55], [146].