1. Introduction
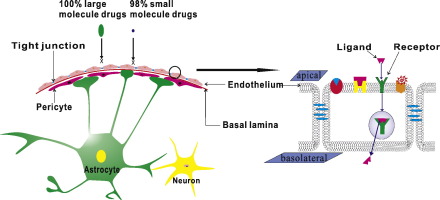
Brain diseases such as Parkinson disease (PD), Alzheimer's disease (AD) and brain tumor are serious threats to human health. Many drugs with considerable promises have been developed to treat brain disorders every year. However, the blood brain barrier (BBB), heavily restricts the passage of therapeutic agents from the blood to the brain. The BBB regulates ionic and molecular traffic, which results in essential blocking 100% of large molecule drugs and more than 98% of small molecule drugs [1]. As shown in Fig. 1, the BBB is composed of brain capillary endothelial cells (BCECs), tight junction (TJ), basal lamina, astrocyte end-feet and pericytes [2]. The BCECs are tightly closed by the TJ and covered by basal lamina. In addition, the pericytes partially envelope the basal lamina, and the astrocyte end-feet almost 100% encircling of the microvessel wall.
 Fig. 1. The diagrammatic sketch of the BBB structure.
Fig. 1. The diagrammatic sketch of the BBB structure.The BCECs, differing from other vessel cell membranes, consist of a lipid bilayerwhich is lack of fenestrations and pinocytotic vesicles [3]. The high concentration of sterols and lipids, up to 20% and 50% respectively [4], might increase the packing density and decrease the permeability of brain endothelium. In addition, the substance traffic is further limited by the catabolic enzymes and efflux transporter such as P-glycoprotein (Pgp), multidrug resistance related proteins (MRPs) and breast cancer resistance protein (BCRP) [5]. Moreover, TJ seals the paracellular cleft of epithelial cells to form a ‘physical’ barrier. Occludin, claudin, junction adhesion molecules and a series of cytoplasmic accessory proteins represent the major integral components of bicellular TJ [6]. The basal lamina is approximately 30–40 nm thick, and located between the endothelial cells and astrocyte, consisting of laminin, fibronectin, tenascin, collagen, and proteoglycan [7]. The pericytes cover about 20%–30% of the outer microvascular circumference. The proliferation of brain blood endothelium might be influenced by the pericytes via selective inhibition of endothelial cell growth [8]. Astrocytes enclose more than 99% of the basal capillary membrane and play an indispensable role in the induction and maintenance of BBB integrity [7]. The basal lamina, pericytes, and astrocytes as a physical barrier might limit the transport of substances into brain tissues.
Many drugs cannot penetrate BBB and present therapeutic effect due to the specific structure and function of the BBB. Therefore, more promising strategies have been studied for efficient drug delivery to the brain. At present, these methods can be classified into two types, invasive and noninvasive approaches.
1.1. Invasive approaches
Invasive approaches, which allows the direct transfer of the drug into the lesions on the brain, include CED (convection enhanced delivery) [9], [10], [11], [12], [13], ICV (intracerebroventricular) [14], intracerebral injection and disruption of the BBB using osmotic disruption, ultrasound [15], [16], and bradykinin analogs. Although invasive approaches could deliver drugs into the brain, these methods present some risks, such as lack of targeting, traumatic and some of surgical complications. In addition, because these methods require sophisticated device and professional technique, the widespread application of these methods is limited.
1.2. Non-invasive approaches
The noninvasive approaches mainly include carrier mediated transport (CMT), adsorption mediated transport (AMT), receptor mediated transport (RMT) and so on. The schematic of presentation various mediated transport systems is shown in Fig. 2. The CMT mechanism can mediate the entry of major nutrients such as glucose, amino acid, monocarboxylic acid, vitamin, and nucleotides into the brain. Some small hydrophilic molecules might across the BBB by use of CMT systems via conjugate with the natural CMT ligands, such as amino acid transporter, hexose transporter and monocarboxylic acid transporter [17]. AMT is a vesicle transport system. The cationic drugs bind to the negatively charged domains of luminal surface via electrostatic attraction, and further trigger the endocytosis of intracellular vesicles containing the drug. Then, the drug is transported by vesicles within the endothelial cells and eventually cross the abluminal surface into the brain parenchyma [18]. In addition, unlike the delivery mechanism of intravenous administration, the intranasal administration is also an effective non-invasive approach which allows some proteins, peptides and other drugs to bypass the BBB and access the CNS regions. Many drugs can be transported into the brain via olfactory nerves to the olfactory bulbs and rostral brain regions or via trigeminal nerves to the brainstem and spinal cord region [19], [20], [21].
 Fig. 2. The schematic representation of mechanisms of different mediated transport system.
Fig. 2. The schematic representation of mechanisms of different mediated transport system.RMT involves the vesicular trafficking system of the brain endothelium. A circulating ligand binds to a cognate transmembrane receptor, such as transferrin receptor or insulin receptor, which is expressed on the apical plasma membrane (blood side) of the endothelial cell. The process of endocytosis takes place and membrane invagination leads to the formation vesicle that contains receptor-ligand conjugates. Subsequently, the conjugates are dissociated, and the vesicles of receptor return to the apical. However, the vesicles of ligand can be trafficked to the basolateral, where the ligand fuse with the membrane and are released into the brain parenchyma [22], [23].
In recent years, the RMT-based drug delivery system becomes a hot research topic for overcoming the BBB. A large number of RMT approaches have been developed. In this article, we will review the RMT systems that have been used for brain disorders and discuss the efficiency of each RMT systems.
2. Drug delivery to the brain by RMT
The ligand coupled drug or drug-carrier might specifically bind to the receptors on the BCECs and realize targeted delivery to the brain parenchyma by RMT. We will briefly discuss various RMT systems, including transferrin receptor, insulin receptor, LDL receptor, LDL receptor related protein, Lactoferrin receptor, SR-B1, leptin receptor, nAch (Nicotinic acetylcholine) receptor, tetanus virus receptor, diphtheria toxin receptor, AAV9 (Adeno associated virus serotype 9) receptor, and sdAb (Single domain llama antibody) receptor (shown in Fig. 3). In addition, the delivery characteristics of the various BBB RMT systems have been shown in Table 1 to facilitate comparison.
 Fig. 3. Various RMT systems of the BBB.
Fig. 3. Various RMT systems of the BBB.Table 1. Outline of RMT systems for drug delivery overcoming BBB.
| Receptor-ligand | Drug | Vector | Model | Ref. |
|---|---|---|---|---|
| Tf R-Tf | Temozolomide [31]; docetaxel [32]; paclitaxel [33], [34]; taxotere [38] | PEG-PLGA NPs [31]; vitamin E TPGS micelles [32]; micelles [33], [34]; PEG-PCL polymersome [35]; magneto-LPs [36]; magnetic NPs [37]; 3-diaminobutyric polypropylenimine dendrimer [38] | bEnd.3 cells; HBMECs/HAs; C6 glioma cell; healthy rats; F98 tumor rats; female BALB/c mice; albino rats; U-87 MG glioma bearing mice; Charles Foster rats; male Wistar rats | [31], [32], [33], [34], [35], [36], [37], [38] |
| TfR-OX26 |
Z-DEVDFMK [42]; dalargin [43]; α-cobratoxin [45]; hTERTC27 [46]; vasopressin fragment [48] EPO |
Chitosan-PEG-BIO-SA NPs [42]; EM–HPG–PLGA NPs [43]; PEG-PLA-NPs [45]; CTX-PEG-LIPs [46]; PEG-LIPs [47]; PEG-PCL PO [48] | BCECs [41]; albino mice [42]; BMVECs [46]; rat [40], [43], [47], [48] | [40], [41], [42], [43]; [45], [46], [47], [48] |
| TfR-8D3 | Loperamide | Fusion protein | Mice | [50] |
| TfR-R17217 | NHS-PEG-MAL-5000 NPs | Mice | [51] | |
| IR-29B4 | Loperamide | HSA NPs | ICR (CD-1) mice | [59] |
| IR-83-14 | SQV [62]; BCNU [63] | Polymersome [61]; SLNs [62], [63] | Rhesus monkey [60]; HBMECs/HAs; hcMEC/D3 [61] | [60], [61], [62], [63] |
| IR-HIRMAb | IDUA [66]; sulfamidase [67]; arylsulfatase-A [68]; GDNF [69], [70], [71]; TNFR [72]; EPO [73], [74]; paraoxonase-1 [75] | Fusion protein | Rhesus monkey | [66], [67], [68], [69], [70], [71], [72], [73], [74], [75] |
| LDLR-APOE/B | Dalargin [81], [82]; loperamide [81] | PBCA-NPs [81], [82], [86]; SLNs [88] | Albino mice; HCMEC/D3 cells; PBCECs | [81], [82]; [86]; [88] |
| LRP-Mtf (P97) | Adriamycin [92]; Ad5 [93] | – | BBMVECs; C6 glioma mice; ZR-75-1 mammary tumor mice | [92], [93] |
| LRP-Ang-2 | Temozolomide [97]; DOX [98], [101]; AmB [99]; paclitaxel [102] | PEG-PCL NPs [96]; PAMAM-PEG NPs [97]; PEG-AuNPs [98]; micelles [99], [103]; PEG-PLA micelles [100]; nanotubes [101] | BBCECs; immunosuppressive murine; CF-1 mice; U87 Glioblastoma mice; NuNu mice; metastases mice | [96], [97], [98], [99], [100], [101], [102], [103] |
| Lf R-Lf | Senktide [109]; etoposide [115]; DNA [110], [116], [117]; curcumin [116]; docetaxel [117] | Polymersomes [108]; LIPs [109]; β-cyclodextrin [111]; NPs [113], [114], [115], [116], [117]; NLC [118]; SLNs [119] | BCECs; PBCECs; U87MG cells; SD rats; bEnd.3cells; KM mice; HBMEC/HA; Parkinson's disease rat; Swiss albino mice | [108], [109], [110], [111]; [113], [114], [115], [116], [117], [118], [119] |
| SRB-1-Apo A-I | ΑTocH [122]; DOX [123]; loperamide [125] | NPs [123], [124], [125] | BCEC; Wistar rats; CD mice | [122], [123], [124], [125] |
| ObR-Leptin | DNA [130] | PEG-LPs [129]; DGL-PEG NPs [130] | MBEC4; BCECs | [129], [130] |
| nAchR- RVG29 | DNA [133], [134] | PAMAM NPs [133]; exosomes [135] | Neuro 2a cells; BCECs; KM mice | [133], [134], [135] |
| GT1b-TTC | – | PLGA-PEG NPs [138] | N18-RE-105 Neuroblastoma | [138] |
| HB-EGF-CRM197 | Loperamide [141]; HRP [142]; zidovudine [143] | PLGA NPs [140]; PBCA NPs [142] | BCECs; HBMECs; guinea pigs; CD1 mice | [141], [142], [143] |
| LamR-AAV9 | EPO [147] | – | C57Bl/6 littermates | [147] |
| TMEM30A-sdAb | – | – | SV-ARBEC; Wistar rats | [153] |
2.1. Transferrin receptor
The transferrin receptor (TfR) might be the most well-known RMT system, which could mediate the delivery of iron to the brain via bonding to the transferrin (Tf) [24], [25]. Human TfR is a transmembrane glycoprotein, consisting of two identical monomers with 90-kDa linked by intermolecular disulfide bonds [26].
2.1.1. Transferrin
The TfR is highly expressed on the BCECs, and has a high affinity toward the Tf [27]. Plenty of studies have shown that the TfR-Tf conjugated mechanism could mediate the targeting delivery of drug to the brain. For example, Jiang Chang and his colleagues used a co-culture of the Tf modified PLGA NPs (nanoparticles) labeled by DiI (derivatives of indocarbocyanine iodide) and BCECs for 1 h at 37 °C [28]. Fluorescence microscopy demonstrated that cellular endocytosis of Tf-NPs was about 20-fold greater than blank NPs and 2-fold greater than BSA-NPs. Furthermore, they also found that the endocytosis was a temperature-dependent process and the endocytosis at 4 °C was much lower than that at 37 °C. The another study shown that the DiI accumulation in cortex released by Tf-NPs was 2 folds higher than BSA-NPs in healthy rats while both NPs entered massively within F98 tumor-bearing rats brain [29]. It is worth noting that the DiI accumulation within the brain of tumor-bearing rats was improved compared with the healthy. This observation indicated that the tumor might result in the disruption of the BBB structure. The Tf-coupled NPs could evade reticuloendothelial system (RES) uptake via the surface modification with PEG (polyethylene glycol) [30]. For example, the Tf-appended PEGylated NPs could mediated the delivery of rhodamine 6G to the cortex, striatum and the third ventricle in albino rats and showed more than 70% growth inhibition against cancer cell lines when loaded with temozolomide [31]. Numerous micelle carrier systems are being investigated to improve drug solubility and stability for brain delivery [32], [33], [34]. The impressive thing is that the BCECs uptake of Tf anchored micelle loaded with c[RGDfK]-paclitaxel conjugates (c[RGDfK] is a peptide that specifically bonds to the integrin) was increased significantly when compared with unadorned micelle [34]. In addition, the mean survival time of mice bearing intracranial U-87 MG glioma treated with Tf-micelle (42.8 days) was significantly longer than nude paclitaxel (33.6 days).
The biodegradable polymersome is also a promising carrier for drug transport across BBB. Tf coupled polymersome shows 2.3 folds higher percentage of ID/g brain (injected dose per gram of brain) than the undecorated polymersome [35]. In order to further improve the efficiency of brain targeting delivery, the Tf embedded magnetic nanocarriers have been developed [36], [37]. The vitro BBB transmigration study showed that approximately 50% increase of Tf-magneto-liposome in the presence of external magnetic force, compared with the magnetic force or Tf RMT alone [36]. In recent years, gene drugs show great potential to treat the brain diseases, but many gene drugs cannot cross the BBB. Tf-modified polypropylenimine dendrimer has been used to transport the plasmid DNA to the brain [38]. After 2 h incubation with Tf-targeted dendrimer, the DNA uptake by bEnd.3 cells increased 1.4-fold and 2.3-fold compared to that of non-targeted dendrimer and naked DNA. In vivo, the gene expression in brain was more than 2 folds higher than that of the undecorated dendrimer.
2.1.2. Transferrin receptor monoclonal antibody
Tf modified nanocarrier could mediate the transport of the drug into brain via binding to the Tf receptor. However, the mechanism of Tf and Tf-receptor binding is a saturable process, and the transcytosis of Tf-NPs might be inhibited by natural Tf [28]. In comparison, Tf receptor monoclonal antibodies (TfRmAbs) could overcome this deficiency, because the binding site on the TfR of the TfRmAb is spatially removed from endogenous Tf [39]. Rat anti-transferrin receptor IgG2a (OX26) has been widely used to for delivery into the brain. After 5 h injection of the OX26 antibody or mouse immunoglobulin G2a into the rats, the distribution of brain volume of OX26 was 18-fold higher than the mouse IG2a group [40]. Another study showed that the brain uptake of OX26 was approximately 0.4% ID, but the non-immune IgG2a group was 0.03% ID [41]. Hence, OX26 is often applied to modify the nanocarrier, such as nanoparticle (Fig. 4A) [42], [43], [44], [45], liposome [46], [47] and polymersome [48]. The peptide Z-DEVD-FMK could protect the neuronal cell via inhibiting the activity of caspase-3, but Z-DEVD-FMK cannot cross the BBB. The chitosan (CS) NPs, adorned with OX26, conjugated with PEG have been used to mediate the delivery of Z-DEVD-FMK into brain. Fluorescence micrographs demonstrated that functional NPs labeled with FITC (fluorescein isothiocyanate) could penetrate into the brain whereas the free NPs could not, 2 h after intravenous injection (Fig. 4B–C) [42]. In another similar study, the TfRmAb CS NPs coupled with Z-DEVD-FMK and bFGF (fibroblast growth factor, an anti-apoptotic peptides) respectively to treat the stroke mice. The mean infarct volume of treated mice (37 ± 3 mm3 and 33 ± 1 mm3) was significantly lower than that of the control group (51 ± 1 mm3) [44].
 Fig. 4. (A) Schematic representation of the formation of TfRmAb conjugated NPswith encapsulation of drug. (B) and (C) represent the fluorescence micrographs of OX-26 conjugated NPs and OX-26 free NPs, respectively [42]. (D) The brain uptake of enzyme-8D3 conjugate was 10-fold higher than control [49]. (E) Maximally possible anti-nociceptive effect (MPE) of loperamide in female ICR (CD-1) mice after injection of nanoparticles with covalently attached R17217, OX26, IgG2a and negative control [51].
Fig. 4. (A) Schematic representation of the formation of TfRmAb conjugated NPswith encapsulation of drug. (B) and (C) represent the fluorescence micrographs of OX-26 conjugated NPs and OX-26 free NPs, respectively [42]. (D) The brain uptake of enzyme-8D3 conjugate was 10-fold higher than control [49]. (E) Maximally possible anti-nociceptive effect (MPE) of loperamide in female ICR (CD-1) mice after injection of nanoparticles with covalently attached R17217, OX26, IgG2a and negative control [51].Intranasal administration as an efficient approach for drug delivery could transport the OX26 NPs into the brain. The OX26 modified PEG-PLA NPs loaded with α-CT (α-cobrotoxin, an analgesic peptide) were intranasally (i.n.) or intramuscularly (i.m.) administered to rats. The brain transport results indicated that the corresponding absolute bioavailability of i.n. OX26–αCT–NPs were about 125 and 155% with i.n. αCT–NPs and i.m. OX26–αCT–NPs [45]. These nanocarriers could easily traverse the BBB and carry the gene medicines into the brain. For example, the PEGylated liposome modified with OX26 and chlorotoxin (CTX) loaded with hTERTC27 (a peptide of human telomerase reverse transcriptase) could treat brain glioma via the inhibition of the tumor cell growth [46]. The MST (median survival time) of OX26/CTX bispecific PEG liposome (46 days) was significantly longer than the PEG liposome (21 days) and OX26 PEG liposome (29 days). It is notable that the CTX could enhance the therapeutic effects of drug loaded OX26 liposome due to the CTX actively targeting to the glioma cells.
OX26 antibody against the rat TfR cannot cross the mouse BBB, whereas the monoclonal antibody 8D3 could against the mouse TfR [39]. Yun Zhang and his colleagues employed the 8D3 antibody to transport the β-Galactosidase (a candidate for enzyme replacement therapy of lysosomal storage disorder) across the mice BBB [49]. The enzyme activity analysis indicated that the brain uptake of 8D3 β-Galactosidase conjugates was 10-fold higher than control (Fig. 4D). More than 90% of 8D3 β-Galactosidase conjugates into the brain tissue, as measured by capillary depletion technique. The 8D3 antibody as a promising ligand also could be used to construct fusion protein via re-engineering technique. EPO (erythropoietin) is a potential drug for the therapy of multiple brain diseases via protection of the neuronal cells, but it could not cross the BBB. For enhance the therapy efficiency, a new fusion protein cTfRmAb-EPO has been fabricated based on 8D3 antibody. The capillary depletion analysis showed that the 69% of cTfRmAb-EPO fusion protein had crossed the BBB, and brain uptake of fusion protein was 2.0 ± 0.1% ID/g of brain after i.v. injection [50]. The mouse monoclonal antibody R17-217 also could cross the BBB via transcytosis [39]. The R17-217 modified nanocarrier could mediate the delivery of medicine compounds into the brain. Ulbrich, K and his colleague use HSA (Human serum albumin) NPs linked with R17-217 antibody to deliver the loperamide to the mice brain for anti-nociceptive [51]. The results showed that the R17-217-NPs conjugates (7 mg loperamide/kg) induced 100% of MPE (maximally possible anti-nociceptive effect), but the IgG2a group and negative control induced less than 20% and 40% MPE, respectively (Fig. 4E). In addition, the R17-217 antibody possess higher efficiency of targeting delivery into brain than other peripheral organs [52].
TfR as a target for the BBB possesses great potential to mediate the delivery of drug into brain. But there are also some drawbacks. Firstly, the TfR presences not only in BBB, but also in other peripheral organs [26]. Secondly, some ligands might impact each other if they share the same binding site of TfR. In addition, the high-affinity antibody could induce the TfR degeneration and significantly decrease the transcytosis efficiency of the TfR [53]. So, chronic treatment strategies that using the high affinity antibody for drug delivery might result in decreased antibody and physiological iron uptake in the brain. Finally, some antibodies remain in the BCECs body instead of crossing the basolateral membranes to enter the brain parenchyma probably due to the strong bond between the antibody and the TfR [54].
2.2. Insulin receptor
Insulin receptor (IR) has been extensively studied as a part of RMT system. It could mediate the transport of blood-borne insulin into the brain parenchyma [55]. IR is a transmembrane glycosylated protein, which consists of two α and two β chains linked by disulfide bonds [56]. The so-called insulin molecular pocket is formed by the two α subunits. This results in an increase in tyrosine phosphorylation of the β subunit, and induces a conformational change of the insulin receptor to form a channel (that could allow transmembrane transport of molecule) [56], [57].
The application of insulin as an RMT-targeting vector is limited in vivo, due to the short serum half-life of insulin (10 min) and hypoglycemia caused by exogenous administration of insulin [58]. As an alternative, the murine insulin receptor monoclonal antibody mAb83-14, mAb83-7 and mAb29B4, which bind the different epitopes of human insulin receptor α subunits, can induce the delivery into the brain via the transcytosis process [56]. The mAb29B4 modified HSA NPs could cross the BBB to exert significant antinociceptive effects [59]. However, the mAb83-14 is more widely used for delivery into brain than other antibodies. After 3 h of intravenous injection to the rhesus monkey, the brain uptake of mAb83-14 was 3.8 ± 0.4% ID of per 100 g brain, but there was no brain uptake of mouse IgG2a isotype antibody [60]. The mAb83-14 modified carries can also be used for drug delivery. Le-Ha Dieu and his colleagues constructed a PDMS-b-PMOXA-based polymersome anchored with mAb83-14 [61]. Functionalized polymersome incubated with hCMEC/D3 cells at 37 °C in vitro, the median fluorescence intensity (MFI) value was increased from negative control of 2.6 to 5.6 after 1 h and 10.8 after 2 h. The mAb83-14 modified solid lipid nanoparticles (SLNs) could transport saquinavir (SQV) to the brain via improved the BBB permeability. The results showed that the permeability of the BBB was positively correlated with concentration of mAb83-14 on the SLNs [62]. Furthermore, the antineoplastic agents carmustine could be trafficked into brain by taking advantage of the carrier of mAb83-14 grafted SLNs [63].
However, the murine 83-14 mAbs might induce severe immune reaction when injecting it into human body. Therefore, the humanized HIRMAb was produced by grafting the complementarity determining region (CDR) on the framework regions (FR) of the human IgG [64]. The HIRMAb usually have been used for delivery of biologic drugs into the brain via genetic engineering to construct an antibody-drug fusion protein [65]. For instance, mucopolysaccharidosis type I (MPSI) is an inherited metabolic disease caused by a deficiency of alpha-l-iduronidase (IDUA), but the IDUA cannot cross the BBB [66]. The HIRMAb-IDUA fusion protein was employed to carry the IDUA into brain. The hurler fibroblasts incubated with HIRMAb-IDUA fusion protein for 48, the accumulation of glycosaminoglycan in hurler fibroblasts was decreased by 70% [66]. Furthermore, the brain VD (volume of distribution) and % of injected dose (ID) in rhesus monkey brain are approximately 270 μL/g and 2% ID, after 2 h i.v. injection, respectively [66]. In another study, enzyme replacement therapy with HIRMAb-N-sulfoglucosamine sulfohydrolase (SGSH) fusion protein is a new noninvasive treatment of the brain in mucopolysaccharidosis type IIIA (MPS IIIA) [67]. The lysosomal glycosaminoglycan labeled with sulfate in MPS-IIIA fibroblasts is reduced 72–83% after 48 h of incubation with 0.25–0.5 μg/mL HIRMAb-SGSH fusion protein [67]. In addition, the adult rhesus monkey brain uptake of fusion protein, radiolabeled with the [125I]-Bolton-Hunter reagent, was ~ 1% ID/brain after 140 min i.v. injection [67].
Numerous other macromolecular biopharmaceuticals that do not cross BBB could fuse to HIRMAb and form a fusion protein. For example, the HIRMAb-arylsulfatase A (HIRMAb-ASA) fusion protein has been used to treat MLD (metachromatic leukodystrophy) [68]. The brain uptake of HIRMAb-ASA fusion protein in rhesus monkey was 1.1 ± 0.1% ID/100 g brain, after 2 h of i.v. injection [68]. Other biopharmaceuticals, such as GDNF (glial cell-derived neurotrophic factor) [69], [70], [71], TNFR (tumor necrosis factor receptor) [72], EPO [73], [74], and paraoxonase-1 [75] also have been transported into the brain by taking advantage of fusion protein technique.
2.3. LDL receptor
The low density lipoprotein receptor (LDLR) expressed on the capillary endothelial cells of brain and other peripheral tissues [76]. The LDL such as cholesterol [77], tocopherol [78], and apolipoprotein (Apo) could bind to the LDLR. The LDLR, which located in the caveolae membrane fraction [79], could mediate the transport of LDL into the brain tissue via RMT process [80]. Therefore, it is a promising method that uses RMT system based on LDLR for drug delivery into brain.
The apolipoprotein B or E (ApoB or ApoE) coated poly(butyl cyanoacrylate) nanoparticles (PBCA NPs) and loaded with hexapeptide dalargin or loperamide were able to cross the BBB and achieve an antinociceptive effect [81]. The most analgesic effect can be achieved when the drug loaded PBCA NPs pre-coated with polysorbate 80 (PS80) and then coated with apolipoprotein [81]. Another study subsequently supports this result, where the multi-coated PBCA NPs can not only increase the antinociceptive effect, but also prolong the time of this effect [82]. The PS80 coated NPs also could transport into the brain, because the PS80 promotes the serum ApoB and/or ApoE to absorb on the surface of the NPs. Then, the LDLR recognize it and uptake by the brain capillary endothelial cells [83], [84]. The ApoE has been widely used to transfer drug-carrier into the brain because of the human ApoE receptor 2 predominantly expressed on brain tissue [85]. For example, the ApoE-3 modified PS80-coated PBCA-NPs could increase the permeability of porcine BCEC monolayer. Result shows that the number of NPs across the porcine BCEC increased almost 80% when compared with the PS80-coated PBCA-NPs [86]. However, the PBCA-NPs has several drawbacks, such as low drug loading capacity and low biodegradation. As an alternative, the biodegradable and biocompatible solid lipid nanoparticles (SLNs) have been produced as a drug carrier [87]. The SLNs modified with ApoE showed that the capacity of the penetration for endothelial cells membranes, the hCMEC/D3 permeability coefficient of functionalized SLNs was 1.5 times higher than the non-functionalized SLNs [88].
2.4. LDL receptor related protein
Low density lipoprotein receptor related protein 1 (LRP1, alpha-2-macroglobulin receptor) and 2 (LRP2, Megalin), which are members of the LDL receptor family, are expressed on the cerebral vascular epithelial cells. LRP is an endocytic receptor that could mediate the transport of the other ligands of the endothelial cells, including receptor-associated protein (RAP) [89], melanotransferrin (P97) and angiopep-2.
The ligands P97 and angiopep-2 are widely studied for the delivery of drugs into brain via the process of LPR mediate tanscytosis. P97 is a glycosylated protein that reversibly binds iron and closely related to Tf and lactoferrin (Lf) [90]. Although the content of P97 in normal serum is very low, but the transport efficiency of P97 across BBB is higher than other proteins. The VD of [125I]-P97 in the mice brain parenchyma is 11.4 mL/100 g, which is 5.7-fold higher than [125I]-transferrin with 2.0 mL/100 g, after 1 h i.v. injection [91]. In addition, the efficiency of transcytosis through the bovine BCEC monolayers of P97 was 14-fold higher than bovine holo-transferrin [91]. P97 also has a capability to enhance the brain target ability of drugs by conjugated with the drug or drug carrier. One hour after the injection of the conjugates of P97-ADR (adriamycin) through tail vein in mice, the brain uptake of P97-ADR conjugates was approximately 10-fold higher than that of BSA and Lf modified ones [92]. Moreover, mice intracranial ZR-75-1 mammary tumors or C6 gliomas were treated with P97-ADR conjugates, and the MST was increased by 20.8% and 44% compared with the PBS group, respectively [92]. In another report, an adaptor protein sCAR-MTf, containing the extracellular domain of the coxsackie-adenovirus receptor (CAR) and P97, has been employed for gene therapy [93]. The sCAR-MTf could re-direct the Ad5 (adenovirus serotype 5, a gene delivery vector) to significantly traverse the BBB model, with an efficiency of 40–50 folds higher than control [93]. Recently, melanotransferrin modified etoposide entrapped SLNs could cross the BBB for brain tumor therapy [94].
Angiopep-2 is a peptide with 2.4 kDa against the LRP1, and belongs to the family of Kunitz domain derived peptides, It demonstrated higher transcytosis capability and parenchymal accumulation than transferrin, lactoferrin, and avidin [95]. Angiopep-2 could be exploited to modify the carrier such as NPs [96], [97], [98], micelle [99], [100] and carbon nanotube [101] for drug delivery into the brain. Au-NPs surface couple with the PEG, DOX (doxorubicin) and angiopep-2 respectively forms an Ang-PEG-DOX-AuNPs conjugate [98]. The functional NPs could deliver the DOX across the BBB to treat the mice with brain glioma, and shows a significant MST value, of 2.89-fold higher than the control group [98]. AmB (amphotericin B, a hydrophobic antibiotic) is commonly used in the treatment of cryptococcal meningitis of the CNS. Kun Shao and his colleagues developed a brain targeting preparation to improve the penetration rate of the AmB across BBB into the brain [99]. The study results demonstrate that the AmB loaded polymeric micelles modified with angiopep-2 could effectively deliver AmB into the brain tissues and achieve the highest AmB level than other treated groups. The angiopep-2 as drug vector could couple with the PTX (paclitaxel) molecule form a novel derivative, named ANG1005 [102]. After ANG1005 in situ brain perfusion, the BBB transfer coefficient (Kin) of ANG1005 was 86-fold higher than free PTX. In addition, the brain uptake of ANG1005 was more than 10-fold higher than free PTX in mice bearing brain metastases of breast cancer. Recently studies that the retro-inverso isomer of traditional Langiopep-2, called Dangiopep-2, showed lower binding affinity with the LRP1 [103]. However, the Dangiopep-2 could avoid the proteolysis in rat blood serum, in contrast, more than 85% of Langiopep-2 disappeared within 2 h. These results indicate that the Dangiopep-2 might be a desirable alternative for brain targeting vector.
2.5. Lactoferrin receptor
Lactoferrin (Lf) receptor (105 kDa) has been identified in many cells, including monocytes, lymphocytes, liver cells, and mammary epithelial cells [104]. Besides, the Lf receptor is also expressed on the brain BCECs and exhibited two types of binding sites (high affinity: dissociation constant (Kd) = 6.77 nM, low affinity: Kd = 4815 nM) for Lf transport [105]. Lf is an iron binding cationic glycoprotein with 80 kDa, which belongs to the transferrin family. Lf would be transported through the BBB via endocytosis process mediated by Lf receptor, and LRP (Low density lipoprotein receptor related protein) might also involve in this process [106]. As a potential brain targeting ligand, Lf has been widely studied in recent decades. Bin Ji found that the brain uptake of Lf was much higher than Tf and transferrin receptor antibody OX26 at 60 min after i.v. injection (Fig. 5A) [107]. However, the situation becomes unpredictable and confused when Lf was modified on nanocarriers. For example, the uptake of Tf-polymersome (Tf-PS) by bEnd.3 cells is significantly greater than that of Lf-polymersome (Fig. 5B–C) [108], while the brain level of senktide (a selective NK3 receptor agonist) loaded by Lf liposome is lower than OX26 modified ones (Fig. 5D) [109]. However, the gene expression of Lf-modified NPs in brain was about 2.3-fold than that of Tf-modified NPs (Fig. 5E) when the Lf or Tf decorated on the NPs loaded gene [110]. In addition, the Lf coupled β-cyclodextrin showed 6.8-fold higher uptake than the control, while just only 3.5-fold for the Tf conjugates [111]. All of these results indicate that different nanocarriers modified with Lf might result in different efficiency of brain targeting delivery.
 Fig. 5. Transport efficiency comparison of Lf and other ligands: (A) The brain uptake of Lf higher than Tf and OX26 [107]. (B) The brain uptake of Tf-polymersome is greater than Lf-polymersome [108]. (C) The bEnd.3 cells uptake of Lf-polymersome and Tf-polymersome [108]. (D) The brain level of Lf-liposome is lower than OX26-liposome [109]. (E) The brain gene expression of Lf-NPs was about 2.3-fold than Tf-NPs [110]. (F) Whole body fluorescence images of SD mouse after intravenous injection NLC and LfH-NLC [118].
Fig. 5. Transport efficiency comparison of Lf and other ligands: (A) The brain uptake of Lf higher than Tf and OX26 [107]. (B) The brain uptake of Tf-polymersome is greater than Lf-polymersome [108]. (C) The bEnd.3 cells uptake of Lf-polymersome and Tf-polymersome [108]. (D) The brain level of Lf-liposome is lower than OX26-liposome [109]. (E) The brain gene expression of Lf-NPs was about 2.3-fold than Tf-NPs [110]. (F) Whole body fluorescence images of SD mouse after intravenous injection NLC and LfH-NLC [118].As a brain targeting vector, Lf could mediate the transport of many drug-carriers into the brain, and the most widely studied is NPs [112], [113], [114], [115]. Yung-Chih Kuo and his colleague employed the PLGA (poly(lactic-co-glycolic acid)) NPs as a carrier, which was anchored with Lf and folic acid (FA), loaded with etoposide to treat brain glioblastoma [115]. In vitro, the permeability coefficient for etoposide across the BBB by using LF/FA NPs was increased 2-fold when compared with PLGA NPs. Besides, the survival rate of U87MG cells after treating with Lf/FA/PLGA NPs is just half of the PLGA NPs group. The dendrimer-based NPs modified with the Lf usually is used to deliver the plasmid DNA to the brain [116], [117]. A novel Lf conjugated LDL-mimic nanostructured lipid carrier (NLC) has been fabricated to transport curcumin for the treatment of the AD (Fig. 5F) [118]. The cellular uptake study indicated that the uptake of LfH-mNLC in BCECs was 1.39-fold higher than NLC and the vivo imaging results revealed that the level of Lf-mNLC was 2.78 times greater than NLC. In addition, the Lf modified SLNs (solid lipid nanoparticles) also possess the potential for the targeting delivery into brain [119].
2.6. Scavenger receptor class B type 1
Scavenger receptor class B type 1 (SR-B1, a plasma membrane glycoprotein) is a primary receptor that expressed on the apical membrane of BCECs against high density lipoprotein (HDL) [120], [121]. Zoltan Balazs and his colleagues found that SR-B1 could mediate the transcytosis of HDL-associated αTocH across the BBB [122]. Apo A-I (apolipoprotein A-I) is a plasma ligand for SR-B1, which belongs to the HDL family. One study demonstrated that the DOX loaded PBCA NPs coated with PS80 could deliver DOX into the brain by absorbing Apo A-I [123]. As a targeting ligand, the Apo A-I has been employed to modify the nanocarrier for drug delivery. In the in vitro BBB model, the Apo A-I coated protamine oligonucleotide NPs shown that the uptake by astrocytes was increased 2-fold when compared with the uncoated ones [124]. In another study, the Apo A-I conjugated with HSA NPs could increase the content of loperamide in the mice brain and induce a significant antinociceptive effect [125]. All of these indicate that the SR-B1 might be a promising target on the BBB for drug delivery when coupled with Apo A-I.
2.7. Leptin receptor-ObR
Leptin receptor (ObR, regulate the body weight) is a saturable transporter which is expressed on the endothelial cells or epithelia of the BBB [126]. ObR has two subtypes including the short isoform ObRa and the long isoform ObRb. The ObRa is highly expressed on the brain capillary endothelium, and hence a major receptor subtype for leptin [127]. Leptin, a 16-kD peptide secreted by adipocyte, could permeate across the BBB to reach the brain via ObR mediated transcytosis [128]. This process demonstrated a saturable transport and temperature dependent manner. For example, 65% of radiolabeled leptin was bound per milligram of capillary protein at 37 °C, but the value is just 23% per milligram at 4 °C [128]. Leptin is a potential candidate that modify the nanocarrier for drug delivery. A leptin derived peptide (Lep70–89) as a ligand coupled with the liposome could transport across the brain-derived endothelial cells [129]. Furthermore, a 30 amino acid leptin derived peptide modified Dendrigraft polyl-lysine (DGL) carrier could mediate the transport of DNA into the brain [130]. In vitro gene transfection results showed that the luciferase activity of the leptin30 vector was 4-folds than that of unretouched vector. Overall, the leptin and leptin derived peptide possess the characteristic to penetrate the BBB. It is necessary to further study the leptin-ObR system for brain targeting delivery.
2.8. Nicotinic acetylcholine receptor
Nicotinic acetylcholine receptor (nAchR) is an ion channel that is expressed in different regions of the CNS and the peripheral nervous system [131]. Earlier studies revealed that the nAchR exists two subclasses. One class that bind the nicotine with high affinity called nAchR, the another bind alpha bungarotoxin (α Bgtx) with high affinity called α Bgtx nAchRs [132]. The nAchR as a channel could mediate the transport of the rabies virus glycoprotein (RVG, a 505 amino acid) into the neuron cells [131].
A 29 amino acid peptide derived from RVG (rabies virus glycoprotein), named RVG29, was employed to deliver the drug to the brain. RVG29 modified PAMAM (polyamidoamine) NPs shows higher BBB crossing efficiency than the conventional NPs in vitro BBB model [133]. Subsequently, the RVG29 decorated on PAMAM NPs through PEG and loaded with pGL2 (PAMAM–PEG–RVG29/pGL2 NPs) were injected into the mice. After 48 h, the luciferase expression of PAMAM–PEG–RVG29/pGL2 NPs in the brain was approximately 1 fold higher than PAMAM/pGL2 NPs and PAMAM-PEG/pGL2 NPs. In addition, the luciferase expression of PAMAM–PEG–RVG29/pGL2 NPs in the lung, liver and kidney was decreased compared with other kinds of NPs (show in Fig. 6A–B) [133]. In another study, Cheng Gong and his colleagues constructed a non-virus drug delivery system, called RVG29-9rR peptide, for brain-targeting gene delivery [134]. The synthesized RVG29-9rR/pDNA conjugates were transfect into Neuro 2a cells, and the transgene expressions were significantly higher than other controls (show in Fig. 6C–D). Furthermore, the in vivo studies showed that the luciferase expression mediated by the RVG29-9rR/pGL3 complex was much higher than control group (approximately 3-fold) [134]. However, above mentioned delivery techniques also have immune response more or less. Recently, a nonimmunogenic drug vector, exosomes, has been used for brain targeting delivery. The exosomes produced by self-derived dendritic cells, fused with the RVG peptide through the Lamp2b (a protein expressed in the exosome membrane) and loaded with anti-BACE1 siRNA via electroporation. When treated with the wild-type mice AD model, the levels of Aβ (amyloid β-protein) was significantly decreased [135].