1. Introduction
Unconventional energy resources have become very important in recent years due to the decline of conventional hydrocarbon reservoirs. Shale gas reservoirs and natural gas hydrates are the most common ones of the unconventional reservoirs in the world (Chong et al., 2016; Ritts, 2017). Feasible gas production from shale gas reservoirs is currently possible with the technological advancements in horizontal drilling and hydraulic fracturing operations. However, gas production from another unconventional gas reservoir, which is gas hydrate, is still immature and is in the stage of development (SBC, 2015; Max and Johnson, 2016).
Water (host molecules) and molecules of gases or liquids (guest molecules) form ice-like structures together at high pressure and low temperature conditions (Sloan and Koh, 2008; Liu, 2013). These ice-like structures are called “gas hydrates” if the guest molecules are in gaseous state (Carroll, 2009). Approximately 130 compounds form hydrates (Sloan and Koh, 2008). Methane (CH4), ethane (C2H6), propane (C3H8), carbon dioxide (CO2), nitrogen (N2), hydrogen sulfide (H2S), etc. can create their own gas hydrates or mixed hydrates when appropriate pressure and temperature conditions are met (Giavarini and Hester, 2011). Depending on the molecular diameter of hydrate former (guest molecule), different gas hydrate structures form: mainly structure I (sI) and structure II (sII). Structure H (sH) rarely exists in nature. Different types of cages compose each gas hydrate structure. These cages are pentagonal dodecahedron (512), tetrakaidecahedron (51262), hexakaidecahedron (51264), irregular dodecahedron (435663) and icosahedron (51268). For example, pentagonal dodecahedron (nm = 512) is formed from 12 (m = 12) pentagonal (n = 5) faces. Hence, n and m numbers are used to define the shape of different cages of hydrates. One sI hydrate structure consists of 2 pentagonal dodecahedron cages (small cages: 512) and 6 tetrakaidecahedron cages (large cages: 51262). sII hydrate structure is larger than sI hydrate structure and they are composed of 16 small cages (512) and 8 large cages (hexakaidecahedron: 51264). Finally, sH hydrate consists of 3 small (512), 2 mediums (435663) and 1 large cages (51268) (Sloan, 2003; Zou, 2013). Approximately 46, 136 and 34 water (H2O) molecules are essential for sI, sII and sH hydrates, respectively. CH4, C2H6, CO2, and H2S establish sI hydrates and C3H8 and N2 form sII hydrates (Sloan and Koh, 2008). In nature, approximately, 99% of gas hydrates is CH4 hydrate (Kvenvolden, 1995; Max et al., 2013). Moreover, at least 95% of gas hydrates in nature are found in marine environment while the rest are found in permafrost regions (Max and Johnson, 2016).
There is a controversy about the amount of CH4 hydrate in the world but it is thought that even the most conservative estimates of the total quantity of CH4in CH4 hydrates are much larger than the conventional gas resources (404 tcm) and shale gas (204 tcm–456 tcm) (Johnson, 2011; Chong et al., 2016). The magnitude of this resource can make hydrate reservoirs a substantial future energy resource. Gas production from the coarse sands saturated with gas hydrates might be feasible with current technology because porosity and permeability are high in coarse sands (Boswell, 2014; Max and Johnson, 2016; Heeschen et al., 2016; Boswell et al., 2017).
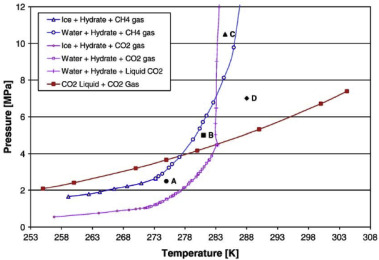
Currently, there are mainly four production methods from gas hydrate reservoirs: depressurization, thermal injection, chemical injection and CH4-CO2replacement (Moridis et al., 2013; Xu and Li, 2015; Chong et al., 2016). Depressurization method is applied by reducing reservoir pressure below hydrate equilibrium pressure. It is considered that this method is the most economically viable method because no external energy is used. However, its disadvantages include a slow rate of production and the risk of reservoir subsidence (Konno et al., 2010; Huang et al., 2016). In thermal injection, mainly external heat is introduced to gas hydrate reservoirs by injecting hot water, steam or microwaves so gas hydrate equilibrium is disturbed (Liang et al., 2008; Xu and Li, 2015). The disadvantages of this method are low injection rate and low energy efficiency. Hence, for different gas hydrate reservoirs, it is suggested to combine this method with depressurization method or chemical injection method for feasible gas production (Yuan et al., 2013; Feng et al., 2015; Minagawa et al., 2015). Among four gas hydrate production methods, chemical injection method is the least preferred approach. Although chemical injection shifts gas hydrate equilibrium curve to higher pressures and lower temperatures for gas hydrate dissociation, its disadvantages are low injection rate (mostly due to low effective permeability of gas hydrates), low-cost efficiency and environmentally harmful (Moridis et al., 2013; Chong et al., 2016; Max and Johnson, 2016). CH4-CO2 replacement or CO2 injection method is quite different from depressurization method, thermal injection method and chemical injection method (Ohgaki et al., 1996). Below 283.2 K, CO2 hydrate is much more stable than CH4 hydrate as shown in Fig. 1 (Goel, 2006). When CO2is injected into CH4 hydrate reservoirs, CH4 molecules leave their hydrate cages and CO2 molecules fill these empty cages due to the difference in chemical potentials of both systems. CO2 has high chemical potential in gas phase and zero in gas hydrate but CH4 shows the opposite. This difference and initially very steep gradients drive the replacement (Xu and Li, 2015). CH4-CO2replacement method in gas hydrates is advantageous due to CO2 sequestration, low water production and low geomechanical risks (Hyodo et al., 2014).
 Fig. 1. Hydrate equilibrium curves of CH4 and CO2 (Goel, 2006).
Fig. 1. Hydrate equilibrium curves of CH4 and CO2 (Goel, 2006).Table 1 summarizes the advantages and disadvantages of CH4-CO2 replacement. The recovery of CH4 might be up to 64% with CO2 injection to CH4 hydrates (Nago and Nieto, 2011). CH4 hydrate and CO2 hydrate are sI type of gas hydrates (2 large cages and 6 small cages). The ratio of molecular diameter of CO2 to cavity diameter of sI hydrate structure is 1.0 for small cages and 0.834 for large cages but CH4 easily fills both small and large cages of sI hydrate (Sloan and Koh, 2008). Consequently, CH4-CO2 replacement is extremely low in small cages and mostly CH4 molecules stay in the small cages of sI hydrate (Yuan et al., 2012). Furthermore, the content of small cages remains largely immobile due to very low probability of a molecule from small cage to jump into the large cage and then reach the gas hydrate surface. The exchange has its preferred paths namely interconnected large cages. Small cages are just sinks or dead ends. After the production of approximately 64% of CH4 from CH4 hydrate via CO2injection, the mixed CH4-CO2 hydrate in the sediments keep the sediments geomechanically much more stable compared to other production methods (Geng et al., 2009; Hyodo et al., 2014; Liu et al., 2016). This study reviews the application of CH4-CO2 replacement method, the first field tests of CH4-CO2replacement in Ignik Sikumi field in 2012, laboratory studies in micro scale and pore network modelling studies. However, it is also essential to review some macro-model studies and their deficits to indicate why micro-scale and pore network modelling studies are needful.
Table 1. Advantages and disadvantages of CH4-CO2 replacement (Geng et al., 2009; Zhao et al., 2012; Hyodo et al., 2014; Abbasov et al., 2016; Liu et al., 2016).
| Advantages | Disadvantages |
|---|---|
| A good alternative for CO2sequestration and gas production method | Operational difficulties for CO2 injection (CO2hydrate formation) and extra cost of CO2 injection |
| Less geomechanical risks in sediments | Slow injection rate due to low effective permeability of gas hydrates and slow replacement rate |
| Less water production | Low recovery of CH4 |
2. Field study of CH4-CO2 replacement
The injection of CO2 through gas hydrate reservoirs is generally a major problem because of low permeability. When high CO2 injection pressures are observed in impermeable or low permeable gas-hydrate bearing sediments, CO2 phase changes easily from gaseous state to liquid or supercritical state (Goel, 2006). High CO2 injection pressure increases the possibility of formation of pure CO2hydrate when there is free water in the pores. As pure CO2 hydrate is constituted instead of the CH4-CO2 hydrate replacement, the permeability of hydrate reservoir decreases further. In order to increase the effectiveness of CO2injection and to avoid the CO2 injection problem at high pressures, 77% N2 and 23% CO2 mixture injection to CH4 hydrates was suggested by University of Bergen and it was proved experimentally and also in Ignik Sikumi field pilot project (Schoderbek et al., 2013; Kvamme, 2015, 2016). During replacement processes in experimental studies, it was observed that large cages of sI hydrate filled by mostly CO2 and small cages are filled by N2. Thus, CH4 recovery was increased from 64% to 85% by injecting the mixture of CO2 and N2. This also avoids the injection problem of CO2 and reduces the chance of pure CO2 hydrate formation because N2 concentration is high. Moreover, N2 can only create its pure sII hydrate at very high pressure (i.e. ∼8.94 MPa at 281.15 K) (Sloan and Koh, 2008; Carroll, 2009). It also increases the recovery of CH4 by replacing CH4in small cages with injected N2. In another studies of Kang et al. (2014) and Ahn et al. (2015), air and CO2 injection (flue gas) was suggested for gas production from CH4 hydrates with the replacement mechanism because it is feasible to obtain flue gas rather than preparing CO2/N2 gas mixture. The efficiency of CH4-CO2 (20%)/air (80%) replacement in the study of Kang et al. (2014) and Ahn et al. (2015) exceeded approximately 85%.
Different from the previous pilot projects (i.e. depressurization tests) on gas hydrate reservoirs, CO2/N2 mixture was injected into the Ignik Sikumi gas hydrate field on the Alaska North Slope. It is a permafrost consisting gas hydrates. In 2012, this method was applied to the Ignik Sikumi field by injecting 77.5% N2 and 22.5% CO2 (167. Mscf of N2 and 48.6 Mscf of CO2) mixture. Then, 855 Mscf CH4 was produced during the total production period including a 6 weeks of flow back of gas. With CH4, N2 and CO2 were also produced. Approximately 70% of injected N2 was produced but only 40% of CO2 was recovered. It was considered that the rest of CO2 was stored in the mixed hydrate (CH4-CO2-N2 hydrate) after the replacement (White and Lee, 2014; Kvamme, 2015). Although it is considered that CH4-CO2 replacement occurred in this well, a direct evidence is still missing (Boswell et al., 2017). Besides, there are still many studies needed to prove the effectiveness of this method (i.e. improved well logging techniques to prove the existence of CH4-CO2 mixed hydrate in the reservoir after the replacement) (Boswell et al., 2017; Lim et al., 2017).
3. Laboratory studies
Depressurization method in gas hydrates is commonly selected both in laboratory and in field tests (Konno et al., 2010, 2014; Li et al., 2014; Zhao et al., 2015). The main reason of this is that it is easy to apply and this method is thought to be feasible (Huang et al., 2016; Boswell et al., 2017). However, the duration of all depressurization field tests is less than two months so the effect of depressurization method on gas hydrate reservoirs in long term production is still questioned. Especially, gas hydrates in marine environment are deposited in loose sediments so geomechanical risks might be high after applying depressurization, thermal injection and chemical injection methods (Hyodo et al., 2009, 2014b). Currently, the only gas hydrate production method with low geomechanical risks is CH4-CO2 replacement. Accordingly, many experimental studies and numerical studies are required to investigate and to improve CH4-CO2 replacement method. In this study, investigations related to CH4-CO2replacement are focused especially in microscale using micromodel, 3D tomography and other visualization methods, which are helpful to understand and to improve CH4-CO2 replacement mechanism.
3.1. Micromodel studies
Hydrate Morphology Using Micromodels. The morphology of gas hydrate reservoirs is crucial to make necessary exploration designs (i.e. drilling and coring) and to develop effective production strategies (Yoneda et al., 2016). Even though the origin of CH4 source for CH4 hydrates is mainly biogenic, there are also thermogenic gas hydrates deposited in the vicinity of thermogenic sources (Hester and Brewer, 2009). During the migration of gas from source rock to gas hydrate stability zone, gas might enter into gas hydrate stability zone as free gas or gas dissolved in water (Spangenberg et al., 2015). It is considered that gas hydrates in marine sediments are likely to be formed from gas dissolved in water but gas hydrates in permafrost are likely to be formed from free gas (Collett et al., 2009; Schindler et al., 2015). Although gas hydrate experiments with gas invasion is common, there are only a few experimental studies with gas hydrates formed in sediments from gas dissolved in water (Priegnitz et al., 2013, 2015; Heeschen et al., 2016). The reason of this is that gas invasion experiments are easy to be conducted compared to gas dissolved in water experiments. However, this might avoid mimicking gas hydrates in sediments.
Depending on sediment type, grain size, invasion type (gas invasion or gas dissolved in water), gas hydrates in nature occur in different ways: veined, fine-grained, pore-filling, mounds near sea floor, disseminated (Fig. 2) (Beaudoin et al., 2014; Rogers, 2015). Generally, pore-filling gas hydrates occur from gas dissolved in water whereas grain-cementing gas hydrates form from free gas (Schindler et al., 2015). The morphology of gas hydrates is decisive because it affects permeability and relative permeability of gas hydrate reservoirs (Boswell, 2009; Haeckel et al., 2015), which are used to decide whether the gas hydrate reservoir is feasible or not and to conduct the numerical simulation (Konno et al., 2015; Joseph et al., 2016). In the pore-scale study of Dai and Seol (2014), pore-filling hydrate reduces the water permeability a lot compared to grain-coating because more surface area is created by pore-filling hydrate, which reduces the fluid velocity frictionally. Consequently, the morphology of gas hydrates should be determined from well logs and core samples (Max and Johnson, 2016; Rajput and Thakur, 2016).
 Fig. 2. Gas hydrate (GH) occurrences in nature: Thin (A) and thickly veined (B) sediment-displacing GH in fine-grained sediment; (C) pore-filling GH in sand; (D) GH mounds on the sea floor; (E) Disseminated GH in fine-grained sediment; (F) GH in coarse sands (Beaudoin et al., 2014).
Fig. 2. Gas hydrate (GH) occurrences in nature: Thin (A) and thickly veined (B) sediment-displacing GH in fine-grained sediment; (C) pore-filling GH in sand; (D) GH mounds on the sea floor; (E) Disseminated GH in fine-grained sediment; (F) GH in coarse sands (Beaudoin et al., 2014).Most of recent studies focus on gas hydrates from macro-scale. Mainly, gas hydrates are deposited in coarse sands within high pressure reactors and then gas is produced from these reactors via depressurization, thermal injection, chemical injection or CH4-CO2 replacement. With these macro-scale experiments, the efficiencies of gas hydrate production methods at different reservoir conditions are found (Schicks et al., 2011; Fitzgerald and Castaldi, 2013; Konno et al., 2014; Cheng et al., 2015; Feng et al., 2015; Heeschen et al., 2016b; Merey and Sinayuc, 2016). Nonetheless, in macro-scale experiments, the interaction of gas, water, ice, hydrate, and grains cannot be investigated. Additionally, the movement of fines in porous media cannot be monitored in macro-scale experiments while applying gas hydrate production methods. All of these skipped in macro-scale studies can be successfully done with micro-scale studies. Accordingly, the micro-scale gas hydrate studies are as much important as macro-scale studies but there are not many microscale gas hydrate studies compared to those in macroscale.
Micromodel experiments are widely conducted in hydrocarbon recovery such as particle retention, water flooding, foam flooding, surfactant flooding, polymer flooding, and CO2 sequestration (Xu et al., 2017). For the studies using the micromodel in gas hydrates, the hydrates of THF (tetrahydrofuran, C4H8O-a soluble liquid sII hydrate former), CH4 (free gas) and CO2 (dissolved in water) were created in micromodels and the formation processes of hydrates were visually observed using a high resolution camera in the study of Tohidi et al. (2001) and the experimental set-up is similar to the one depicted in Fig. 3. In the study of Tohidi et al. (2001), CO2 dissolved in water was injected into the micromodel until 6.2 MPa at 9 °C temperature (grain size range: 0.090–0.500 mm ∼ very fine–medium sand). Results show that hydrate always formed in the center of pore space and thin water layer existed on grains because the grains of the micromodels were made up of water-wet silica glass. The study of Tohidi et al. (2001) reveals that higher gas hydrate saturations in porous media is essential for the cementation of grains by gas hydrates. For this reason, water permeability is likely to be high in Tohidi et al. (2001)'s study due to poor cementing of grains. Similarly, in the micromodel (silica glass grains) study of Yang et al. (2004), the cementation behavior of THF hydrate, CH4 hydrate (from dissolved gas at 6.5 MPa and 0.2 °C), natural gas mixture (from dissolved gas at 7.6 MPa and 1 °C) and CO2 hydrate (from dissolved gas) was investigated. It was observed that hydrates are likely to be deposited in the center of pores and cementation could only be observed at very high hydrate saturation. Moreover, Mahabadi (2016) formed THF hydrate (90.5 wt % water and 9.5 wt % THF resulting in hydrate saturation (Sh): 0.5) in micromodel and similarly THF hydrate emerged in the center of porous media.
 Fig. 3. (a) Schematic diagram of the experimental set-up. (b) The high-pressure micromodel chip (Cao et al., 2016).
Fig. 3. (a) Schematic diagram of the experimental set-up. (b) The high-pressure micromodel chip (Cao et al., 2016).After the studies of Tohidi et al. (2001) and Jadhawar et al. (2006), the number of micromodel studies in gas hydrates increased but not as much as macroscale studies. Katsuki et al. (2006) formed CO2 hydrates (from CO2 dissolved in water and gaseous CO2 + distilled water) in micromodel at 3.3 MPa. Furthermore, Katsuki et al. (2007, 2008) conducted micromodel experiments (pore space of 1.0 × 102 μm) from CH4 saturated water. Different from the study of Tohidi et al. (2001), in the study of Katsuki et al. (2006, 2007, 2008), hydrate crystals grew and bridged the pores without any thin layer of water on grains. The main reason of this is due to adequate subcooling (when the subcoolings were equal or smaller than 6.7 K (Katsuki et al., 2007)). Different from previous studies, in the study of Muraoka and Yamamoto (2017) for the first time, both hydrate morphology and permeability measurements were conducted within specially designed thin micromodels. CH4 hydrate occurred along gas-water interface and grew in the center of porous media (mainly as disseminated). It was observed that even if there are same gas hydrate saturations, gas hydrate morphology affects the effective permeability enormously (Muraoka and Yamamoto, 2017).
In the study of Hauge et al. (2016), 2D high-pressure silicon micromodels (water-wet due to the silicon dioxide film coating the surfaces as a result of the bonding process) with realistic rock structures (real pore shapes and sizes) were constructed according to the structure of Berea sandstone. CH4 hydrates (from gaseous CH4 at 8 MPa and 0.5 °C) and CO2 hydrates (from gaseous CO2 at 5.8 MPa and 0.5 °C) were created inside this micromodel. Because of the slow formation of gas hydrate in the micromodel, by decreasing temperature and the agitation of micromodels, the time of gas hydrate formation was lowered. Fig. 4shows CO2 and CH4 hydrates deposited inside the specially designed micromodel in the study of Hauge et al. (2016). These hydrates were dissociated by increasing temperature and then CH4 hydrate and CO2 hydrate were composed again but this time, they formed faster compared to first hydrates. This is because of the memory effect in gas hydrates. In the study of Hauge et al. (2016), similar to the study of Tohidi et al. (2001), a thin hydrate layer started to appear at gas-water interface and a thicker hydrate layer occurred along the pore wall and towards the pore center.
 Fig. 4. a) Hydrate formation in gas-filled pores (red) and water-filled pores (green), whereas several pores in the center remained water-filled without hydrates forming. b) The distribution of a coarse hydrate texture (red) and a more transparent hydrate (blue) (Hauge et al., 2016). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4. a) Hydrate formation in gas-filled pores (red) and water-filled pores (green), whereas several pores in the center remained water-filled without hydrates forming. b) The distribution of a coarse hydrate texture (red) and a more transparent hydrate (blue) (Hauge et al., 2016). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)CH4-CO2 replacement using micromodels. Jadhawar et al. (2006) investigated the effects of CO2 injection into CH4 hydrate formed in micromodels when there is excess water in the micromodel (similar to marine gas hydrate reservoirs), which was the first micromodel study of CH4-CO2 replacement. Tohidi et al. (2001) and Jadhawar et al. (2006) used methyl blue to increase the contrast of visualization in the study. CH4 hydrate appeared inside micromodel at high pressure and low temperature (10.8 °C) conditions where pure CO2 hydrate cannot form and liquid CO2 was injected into the micromodel. After the injection of liquid CO2 up to a prescribed pressure, the micromodel was kept closed to allow time for CH4-CO2 replacement. During this period, CO2surrounded CH4 hydrate crystals and then CH4-CO2 replacement was observed (CH4-CO2 replacement in Fig. 1 region C). This is because Point C in Fig. 1presents (1) out of CO2 hydrate formation boundary, and (2) inside CH4 hydrate formation boundary, which means that CH4-CO2 replacement can occur. CH4gas was released from the cages of sI hydrate after CH4-CO2 replacement but then, this CH4 was hindered quickly due to the dissolution of CH4 in liquid CO2and excess water in the micromodel. Then, CH4-CO2 mixed hydrate formed in the porous media of the micromodel because the temperature and pressure conditions in the micromodel were appropriate for the formation of mixed hydrate of CH4-CO2 so this mixed gas hydrate emerged in the porous media of micromodel. According to Jadhawar et al. (2006), in the experimental study, the temperature was constant but it was stated in the real field conditions, the heat release after CO2 formation and/or CH4-CO2 hydrate replacement can have the contribution to the dissociation of further CH4 hydrates.
Jang (2011) composed different CH4 hydrates, CO2 hydrates and their mixtures in different types of micromodels (all water-wet). The photos of these hydrates in micromodels are shown in Fig. 5. Fig. 5-a indicates the interaction of CH4 gas and water. Water covers the grains because the grains of micromodel is water-wet. Fig. 5-b shows the micromodel including ice and CH4 gas. Fig. 5-c shows CH4 hydrate formed in the micromodel from CH4 and water including green indicator to create contrasts. The interaction of CO2 gas-CO2 hydrate and CH4gas-CH4 hydrate in Fig. 5-d and Fig. 5-e, respectively. Liquid CO2-CH4 hydrate in the micromodel is shown in Fig. 5-f 30 min after CH4 hydrate is submerged into liquid CO2. The micromodels are very useful to visualize the interaction between different phases and their movement in the porous media.
 Fig. 5. Micromodel images: (a) CH4 gas-water (b) CH4 gas-ice (c) Water-CH4hydrate (d) CO2 gas-CO2 hydrate. (e) CH4 gas-CH4 hydrate. (f) Liquid CO2-CH4hydrate 30 min after CH4 hydrate is submerged into liquid CO2 (Jang, 2011).
Fig. 5. Micromodel images: (a) CH4 gas-water (b) CH4 gas-ice (c) Water-CH4hydrate (d) CO2 gas-CO2 hydrate. (e) CH4 gas-CH4 hydrate. (f) Liquid CO2-CH4hydrate 30 min after CH4 hydrate is submerged into liquid CO2 (Jang, 2011).Hydrate Dissociation Using Micromodels. Similar to the experiments of Hauge et al. (2016), Almenningen et al. (2016) conducted CH4 hydrate formation and dissociation experiments (with depressurization or thermal heating) in the micromodels mimicking Berea sandstone. It was observed that hydrate dissociation started at slightly higher pressure than theoretical dissociation pressure. Higher temperature values increase hydrate dissociation rate. Mass transport of CH4 gas dissociated from CH4 hydrate is very important. In order to form gas hydrate inside micromodels, turbulence or agitation is essential (Flatlandsmo, 2015).
3.2. 3D tomography
X-ray computed tomography (X-ray CT) is a technology that uses computer processed stacks of absorption radiographs to produce 3D visualization of investigated objects (Mees and Jacobs, 2003). Although X-ray tomography is mostly known from its clinical applications, this family of techniques became increasingly popular in fundamental and applied science. Recently, it is commonly used for pore network modelling, multiphase flow, CO2sequestration, gas hydrate studies etc. (Al-Kharusi and Blunt, 2008; Dong and Blunt, 2009; Yang et al., 2015; Wang et al., 2015; Mahabadi, 2016).
X-ray CT might be used in gas hydrate studies due to their advantages for several reasons (Jin et al., 2006; Jones et al., 2007; Holland and Schulthesis, 2014; Wang et al., 2015; Wang et al., 2015b; Mahabadi, 2016):
-
1)
The real gas hydrate core plugs might be scanned and the distribution of gas hydrates, gas and water molecules might be observed.
-
2)
It is a nondestructive method so real core samples might be used for different experiments as well.
-
3)
The distribution of gas hydrates in high pressure reactors is observed during hydrate formation and hydrate dissociation (by different production techniques, mainly depressurization or thermal heating).
-
4)
Effective permeability, relative permeability and capillary pressureparameters are difficult to find with experimental studies so pore network modelling is pretty essential to estimate these parameters. CT images are helpful (grain structure data, gas hydrate distribution in porous media, pores and throat data) for pore network modelling.
Due to the many advantages of CT images above, many gas hydrate studies were conducted by using CT images. Schindler et al. (2015) formed THF hydrate (considered as a proxy of CH4 hydrate) inside a Torlon (polyamide-imide) high pressure cell. Steel high pressure cells cannot be used in X ray CTs because they are not X ray transparent. During THF hydrate formation, many CT images were taken so the change in CT number (a normalized value of the calculated X-ray absorption coefficient of a pixel (picture element) in a computed tomogram, expressed in Hounsfield units) or density was used to detect THF hydrate distribution in porous media. Similar to micromodel experiments, THF hydrate formed in the center of pores and barium chloride brine in water precipitated on grains because hydrate formation excludes brines. In the another study of Kneafsey and Moridis (2011), instead of synthetically formed gas hydrate, the real core samples of the Mount Elbert gas hydrate well were scanned with X ray CT. The high pressure cell was made up of Aluminum alloy. Hydrate saturation and porosity of the core sample including gas hydrate and sandstone were calculated by using CT images. Choi et al. (2011) conducted X ray CT experiments to understand the effects of grain size (silica sand (median size of 180 μm), silica silt (1.7 μm), and kaolin clay (1.0 μm) inside X-ray transparent tri-axial pressure vessel) on gas production from gas hydrates via depressurization method. As expected, it was found that grain sizes have positive effect on gas production because coarse sands have good reservoir properties (high porosity and high permeability). Similar CT experiments were conducted by Seol and Kneafsey (2008) and Zhao et al. (2015b) by using CT number (a normalized value of the calculated X-ray absorption coefficient of a pixel in a computed tomogram, expressed in Hounsfield units, where the CT number of air is −1000 and that of water is 0) change or density changes via X ray CT. Gas hydrate occurrences in silica sands (inside an X-ray transparent high pressure cell made up of polyamide) were monitored in the study of Zhao et al. (2015b) via microfocus X-ray CT. Gas appears in images as dark color because it has lower absorption compared to other components such as hydrate and rock grains. CH4hydrate was in the center of pores in the study of Zhao et al. (2015b). The construction of 3D images by using CT images is very challenging and sensitive. The densities of water, water ice and CH4 hydrate are 1.0 g/cm3, 0.917 g/cm3, and 0.91 g/cm3 respectively. CT numbers of air, sediment minerals, water-ice, dry ice, and hydrate are −1000, 1000, −65, 400, −100 to −250, respectively (Matsumoto et al., 2000). Therefore, by evaluating these values, it is easy to distinguish gas, gas hydrate, water, brine and other components in porous media in CT images. With the help of density contrasts or CT number contrasts, gas hydrates, grains, water, gas, and fines might be detected. For this reason, X ray CT is quite important tool to understand the distribution of gas hydrates in real gas hydrate core samples taken by specially designed high pressure core holders. Different from previous gas hydrate studies with 2D or 3D images via CT, Lee et al. (2017) monitored particle migration by using X ray CT imaging. Gas hydrates are deposited in unconsolidated sands so during gas production after the start of gas hydrate dissociation, sand migration and sand production are likely (Max and Johnson, 2016). In the pressure, temperature and porous media conditions of Lee et al. (2017), particle migration during gas production from gas hydrate-bearing sands with approximately 10% of fine contents was monitored and mainly density differences between different phases from X ray CT measurements were used to investigate particle migration. During gas production from gas hydrates, fines are moving at the interface of gas-water and if fine content is high and cause pore clogging, and then with time, gas-driven fractures and vugs are formed (Jung et al., 2012). Thus, fine migration should be checked during all gas hydrate production methods.
Most of the previous gas hydrate studies with 2D and 3D imaging via CTs mainly focus on CH4 hydrate formation and dissociation with depressurization method or thermal heating, the effect of grain size, the identification of gas hydrate distribution in real field gas hydrate core samples, etc. However, there are a few studies about CH4-CO2 replacement production method from gas hydrate reservoirs by using X ray CTs. X ray CT studies in porous media are also essential for CH4-CO2 or CO2/N2 replacement with different gas hydrate saturation, gas mixture, temperature, pressure, salinity, and grain size. Schindler et al. (2015b) used X ray CT to monitor gas hydrate saturation and its distribution in porous media (sand-pack) for CH4-CO2/N2 replacement and together with X ray CT, gas chromatography (GC) was used to measure the composition of produced gas and to understand the efficiency of CH4-CO2/N2replacement. In the experimental study of Schindler et al. (2015b), similar pressure-temperature conditions and similar 23% CO2/77% N2 composition of Ignik Sikumi well were chosen. CH4 hydrate was formed in the sand pack at 4.83 MPa and 3.9 °C (within CH4 hydrate stability region) when water saturation is 50% and then 23% CO2/77% N2 composition was injected into the high pressure reactor until 9.6 MPa. After the injection of CO2-N2 mixture, CT images were collected with 195 μm and then, CT images were calibrated with density so density changes provided the information of gas hydrate distribution in porous media. However, the densities of CH4 hydrates and CO2/N2 hydrates are almost equal to each other so it is not possible to distinguish CH4 hydrate and CH4-CO2-N2 hydrate or CO2-N2 hydrate from CT images. It is important to test CH4-CO2/N2 replacement when there is no free water in the porous media. This might allow investigating this replacement after avoiding CO2/N2 hydrate formation.
Although there are many advantages of X-ray CT techniques, there are also some disadvantages. The experimental set-up preparation, sample preparation and measurement of CT data are very sensitive to other external effects such as equipment accuracy and operator dependency. Small changes of these parameters might cause discretations and imaging artefacts. Numerous studies employing standard medical CT scanners lack a proper resolution to visualize detailed microstructures of gas hydrate systems (Cnudde and Boone, 2013; Dong et al., 2018). High-resolution synchrotron radiation X-ray tomography (SRXCT) is advised for very good image data from gas hydrates in micro-scale porous media (Falenty et al., 2015; Sell et al., 2016). One of the greatest restriction of this approach is a very limited access and high complicity of the data treatment (Sell et al., 2016).