1. Introduction
Rapid urbanisation and population growth have led to a significant surge in energy demand, prompting discussions about eco-friendly, affordable, and renewable energy production methods. Despite this, fossil fuels dominate global energy consumption, comprising around 80% [[1], [2], [3]]. Depleting fossil fuel reserves raises concerns about future energy supplies, especially with projections indicating a population of 10 billion by 2050, further straining energy demands. Fossil fuel combustion has severe environmental consequences, releasing carbon dioxide and contributing to global warming, with a 0.85 °C increase in Earth's atmospheric temperature over the past 130 years [4,5]. Climate change's impact is felt worldwide, affecting 3 billion people. Researchers are exploring eco-friendly energy production methods to address potential shortages as fossil fuel reserves decline. Global coal, oil, and gas reserves are estimated to last approximately 60, 200, and 40 years, respectively, highlighting the urgent need to transition to sustainable renewable energy sources for long-term development [[6], [7], [8]].
Hydrogen stands out as an appealing energy source due to its high calorific value and eco-friendly advantages, positioning it as a viable alternative to fossil fuels, particularly in the field of transportation. Its significance is magnified as fossil fuel reserves dwindle [[9], [10], [11]]. Due to its straightforward electrochemical conversion, low weight, and impressive energy density, hydrogen can efficiently transport energy over significant distances using liquid fuels, either through cargo ships or pipelines [12]. These characteristics position hydrogen as an optimal replacement for fossil fuels, leading to its widespread recognition as a highly viable and environmentally friendly alternative fuel[13,14].
Furthermore, 1.0 kg of hydrogen stores more energy than 2.75 kg of gasoline, making 1 L of hydrogen equivalent to 0.25 L of gasoline. Fossil fuels account for 96% of the world's hydrogen production, with naphtha reforming contributing to 30%, steam reforming of natural gas making up 48%, and coal gasificationrepresenting 18%. Nevertheless, these traditional approaches impose notable ecological consequences, compelling energy and environmental specialists to advocate for adopting sustainable methods for producing hydrogen through renewable sources.
Yue et al. in their work [15] provided an overview of the latest advancements in hydrogen-related technologies and their utilisation in power systems, encompassing hydrogen production, storage, and re-electrification. Dawood et al. [16] reviewed different hydrogen production methods, examining how these pathways are interconnected and their impact on various aspects of the hydrogen cycle. In a study conducted by Najjar [17] the focus was on evaluating the safety aspects of hydrogen throughout its production, transmission, and utilisation stages without delving into the diverse constraints related to the various hydrogen production technologies. As per their research findings, discussions regarding the safety concerns associated with hydrogen use primarily revolve around its ignition and combustion characteristics, which include attributes such as low ignition energy, rapid diffusion, relatively high flame velocity, and an extensive flammability range, among others. Parra et al. [18] assessed hydrogen production progress from a cost perspective, but their analysis did not include a comprehensive examination of the various trends in the advancement of different technologies in this field.
Hanley et al. [19] delivered a comprehensive overview of hydrogen production using low-carbon approaches, as observed through diverse integrated energy system models. Their primary goal was to pinpoint the policy scenarios and factors that promote the emergence of hydrogen as a preferable option compared to other low-carbon technologies. They determined that bioenergycould play a dual role, acting as both an enabler and a rival to hydrogen energy. This is in conjunction with scenarios featuring high levels of renewable electricity and greater electrification. Liu et al. [20] investigated the patterns and forthcoming hurdles associated with both hydrogen production and storage. Their research from 2004 to 2018 revealed that the most extensively studied hydrogen production method during this timeframe was the photocatalytic decomposition of water into hydrogen. Mengdi and Wang [21] have recently comprehensively examined technologies utilised in hydrogen production, encompassing both renewable and non-renewable sources. Furthermore, they conducted a comparative assessment of the life cycle environmental impact for these diverse technologies. Hosseini and Wahid [6] also explored different hydrogen production methodologies. Their research revealed that the primary obstacles to commercialising solar-based hydrogen production are the substantial cost of photovoltaic cells and their limited efficiency. Maggio et al. [22,23] examined the potential impact of producing hydrogen from renewable energy sources on the fuel markets. According to their research findings, generating hydrogen via electrolysis is expected to exert a substantial economic influence, particularly in transportation. This is likely to result in the establishment of new supply chains and market dynamics, ultimately altering the characteristics of the energy market.
Abe et al. [23] examined hydrogen as an energy carrier concisely. Their study pinpointed hydrogen storage as a significant obstacle to its advancement and offered suggestions for its improvement. El-Emam and Ozcan [24]evaluated the economic, technological, and environmental dimensions of hydrogen production. Their study noted that the affordability of electricity linked with geothermal and nuclear energy sources positions them as optimal choices for cost-effective hydrogen production. Lane et al. [25] predicted the distribution of renewable hydrogen production technologies in a different investigation. The study's results indicate that biomass gasifiers are the dominant technology in the market initially. Nevertheless, due to the electrolyser s' higher learning rate and the long-term trend of decreasing costs for renewable energy, it is anticipated that by the mid-term, there will be equal shares of new installations, and ultimately, electrolysers are expected to emerge as the leading technology for new facilities.
This article presents an all-encompassing examination of diverse clean hydrogen production technologies, encompassing those reliant on Geothermal, wind and solar, alongside biomass decomposition facilitated by chemical, microbial, and electrolytic methods. Apart from examining the technologies and their constraints, the paper also tackles the obstacles that impede the worldwide advancement of the hydrogen economy. Moreover, it highlights the significance of hydrogen in the worldwide energy industry, concurrently examining and contrasting the economic, technological, and environmental consequences of various methods for producing hydrogen.
2. Assessment of global hydrogen production status
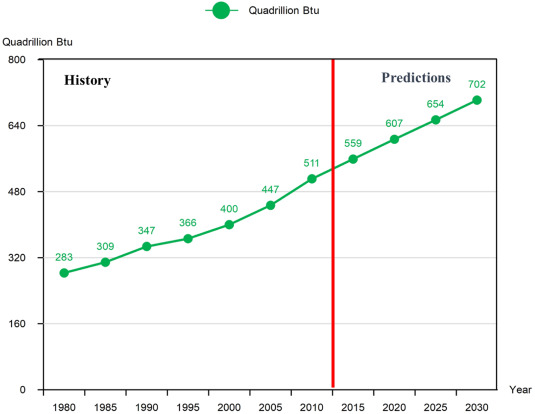
According to the International Energy Agency (IEA), hydrogen productiontechnologies demonstrated remarkable resilience in the face of the COVID-19 pandemic, maintaining robust progress throughout 2020. The IEA report highlights that 2020 witnessed unprecedented policy action and an increased focus on low-carbon production, with ten governments globally adopting hydrogen strategies. In the past year, approximately 70 MW capacity was installed for electrolysis, doubling the previous year's record. Furthermore, two hydrogen production plants are now operating with fossil fuels and employing Carbon Capture, Utilisation, and Storage technology. As a result, the production capacity has seen a rise of approximately 15%. As per the cited reports, the worldwide need for hydrogen has seen a remarkable expansion, with a growth rate of 27.2% over a seven-year period. The demand surged from 255.3 billion cubic meters in 2013 to around 324.8 billion cubic meters in 2020 [26,27]. Over the next decade, there is an anticipated increase in global energy consumption, as illustrated in Fig. 1.
 Fig. 1. Estimation of worldwide energy usage spanning from 1980 to 2030 [28].
Fig. 1. Estimation of worldwide energy usage spanning from 1980 to 2030 [28].3. Hydrogen production technologies
The heavy reliance on fossil fuels for hydrogen must shift to eco-friendly alternatives due to resource depletion, promoting renewable methods. This segment will investigate key techniques for generating renewable hydrogen, such as biomass, solar, wind, and electrolysis, and explore innovative methodologies incorporating nuclear energy and algae. Subsequent sections will address cost assessment and emissions, and Fig. 2 provides an overview of various hydrogen production techniques. Table 1 presents the Recent studies on the production of hydrogen [29].
 Fig. 2. Renewable hydrogen production methods.
Fig. 2. Renewable hydrogen production methods.Table 1. Recent studies on the production of hydrogen.
| Year | Approach | Primary source | Focus of the study | Key findings | References |
|---|---|---|---|---|---|
| 2010 | Thermodynamic evaluation or analysis | Natural gas | Creating a computational model for hydrogen production through natural gas steam reforming, including a coupled furnace model. | The study revealed that the SRP integration modelling approach offers greater flexibility compared to using separate CFD models. Moreover, it has the potential for extension to reformer tubes in industrial furnaces. | [30] |
| 2010 | Thermodynamic modelling and evaluation | Geothermal energy | Evaluating the thermodynamics of hydrogen production models using geothermal energy | The first model proposed utilising geothermal work in the electrolysis process, achieving an efficiency of 28.5%. The second model utilised a fraction of geothermal heat to preheat the water, resulting in 29.9% efficiency. The third model employed a portion of geothermal heat to preheat the water for a high-temperature electrolyser, significantly improving efficiency to 37.2%. Finally, the fourth model used a fraction of geothermal work for liquefaction and the remainder for electrolysis, yielding an efficiency of 16.1%. | [31] |
| 2011 | Photocatalytic electrochemistry | Solar PV | The research aims to examine the rate of hydrogen evolution in a photoelectrochemical setup comprising a platinum photoanode, a cathode, and anodized tubular TiO2, solar cell, along with seawater prepared using a nanofiltration membrane. | The hydrogen evolution rate was determined to be 270 mol/cm2 h. | [32] |
| 2014 | Analysis of real-time data for wind energy curtailment | Wind | Development of a wind-assisted hydrogen storage system to address substantial wind energy curtailment | A hydrogen storage system was designed to handle significant wind energy curtailment, taking into account real-time data with 10-min averages throughout the entire wind farm year. | [33] |
| 2014 | Evaluation of environmental impact, thermodynamic performance, exergetic analysis, and life cycle assessment (LCA). | Biomass | The process focuses on creating hydrogen through the gasification of lignocellulosic biomass. | The suggested gasification study of biomass (poplar) achieved an exergetic efficiency of 48%, with 4.6% of exergy losses attributed to flue gas streams and a 47% exergy destruction rate. | [34] |
| 2016 | Comparative evaluation of green energy carriers | Biomass | Hydrogen generation from sustainable and renewable energy sources | Supercritical water gasification of biomass was observed to produce hydrogen efficiently at high pressure with minimal energy input, reducing the formation of char and tar. Additionally, the study emphasized the cost and efficiency challenges related to photovoltaic cells. | [6] |
| 2017 | Thermo-electrochemical protonic membrane reformer for efficient production | Natural gas | To achieve exceptionally pure hydrogen through a one-step procedure with minimal energy wastage. | The research successfully attained a well-balanced thermal operating state, resulting in a modelled hydrogen plant with a total energy efficiency exceeding 87%. | [35] |
| 2018 | Thermodynamic evaluation or analysis | Nuclear | Hydrogen generation through water electrolysis | The study discovered that the proton exchange membrane electrolysis system was suitable for integrating renewable energy systems, while the alkaline electrolysis system was recommended for coupling with industrial stationary applications. Additionally, the solid oxide electrolysis system was suggested for utilising the excess energy production from nuclear power plants. | [36] |
| 2019 | Thermodynamic evaluation and life cycle analysis | Coal | Evaluating the thermodynamic and life cycle analysis of integrated coal gasification for hydrogen and power generation | The system's design achieved an efficiency of 53.3% at a temperature of 700 °C and with a coal concentration of 15%. The global warming potential (GWP), nitrogen oxides, and acidification potential were measured to be 0.058kgCO2eq/(kWh), 7.04 × 10−5NO2eq/(kWh), and 3.63 × 10−4 kg SO2 eq/(kWh) respectively. | [37] |
| 2019 | Cost evaluation, carbon emission analysis, and multi-objective Optimisation | Solar thermal | Investigation and evaluation of a solar energy-driven natural gas reforming system designed for hydrogen production. | The combined steam-autothermal reforming model exhibited significant energy enhancement, resulting in improved energy and exergy efficiencies of 59.1% and 31.1%, respectively. | [38] |
| 2019 | Performance assessment: economic, social, technical, environmental, and reliability aspects | Biomass, nuclear, geothermal, wind, solar, and hydro have been chosen as sources for hydrogen production. | An examination, of various hydrogen production methods for environmental improvement. | In this research, the relative performance of diverse hydrogen production methods was analysed, and it was observed that solar energy exhibited the highest environmental performance (8/10), while nuclear energy had the lowest environmental performance (3/10). Geothermal energy obtained the lowest overall average ranking (4/10). | [39] |
| 2019 | Assessment of solar-powered natural gas reforming system. | Solar and Natural gas | To evaluate the influence of combining steam methane reforming with carbon dioxide and steam-based autothermal reforming. | Both exergy and energy efficiencies exhibited enhancements. The exergy efficiency reached 31.1%, while the energy efficiency reached 59.1%. | [40] |
| 2020 | Preparation and characterization of a composite proton exchange membrane (PEM) with a focus on PBI/ZrP composite membrane. | Solar and Wind | To introduce a novel composite membrane of polybenzimidazole (PBI) by incorporating zirconium oxide (ZrO2) and subsequent phosphoric acid treatment. | The copper chloride (CuCl) electrolyser exhibited an efficiency ranging from 91% to 97%, indicating that the hybrid PBI/zirconium phosphide (ZrP) membrane could be a viable alternative to the Nafion membrane. | [41] |
| 2021 | Co-fermentation, dark fermentation, co-digestion, and co-culture systems for bio-hydrogen production. | Biomass | The study aims to investigate the syntrophic co-fermentation model to evaluate the evolution of microbial communities and the carbon transfer pathway within the co-fermentation system. | The maximum hydrogen production reached 165 mL/g, accompanied by an average hydrogen concentration of 52.3%. | [42] |
| 2022 | Investigate the effect of electrode spacing on hydrogen production by alkaline water electrolysis | Solar PV | Examining how the spacing between electrodes influences hydrogen production. | The research findings indicated that reducing the spacing between electrodes enhances the interaction between the immersed electrode and the ionic electrolyte. As a result, this leads to an increase in the rate of the electrochemical reaction, improved efficiency, and a higher production of hydrogen. | [43] |
| 2022 | Enthalpy-porosity approach to simulate the PCM (Phase Change Material) | Solar PV | The study's objective was to evaluate the performance of a PEMEC powered by a photovoltaic thermal (PVT) system. The investigation focused on analysing how the integration of a thermoelectric generator (TEG) and phase change materials (PCM) affected the hydrogen production process. | The PVT/TEG/PEMEC system demonstrated superior performance compared to alternative systems. On the other hand, the PVT/PEMEC/PCM system showed minimal impact on the overall process. | [44] |
| 2022 | Integrates both experimental and analytical methodologies to investigate the effective utilisation of corn stalks (CS) for biohydrogen production using PFHP. This approach places emphasis on optimizing various parameters and gaining insights into the structural modifications within the CS | Biomass | To investigate the influence of catalysts on increasing energy conversion efficiency and biohydrogen yield in photo-fermentation from corn stalks (CS) by enhancing beneficial metabolic products. | The hydrogen yield was increased by 15.93% when 0.2 g/g CS of kieselguhr was added to the liquid culture. | [45] |
| 2022 | Investigating the catalytic performance of Palladium–Nickel (PdNi) and Nickel oxide (NiO) materials for the oxidation of carbohydrate alkaline degradation products and comparing their potential requirements and current densities | Biomass | To suggest an effective approach for substituting the oxygen evolution reaction with partially oxidising degradation products derived from carbohydrates. | The findings suggest that utilising industrial waste streams has the potential for sustainable hydrogen production. | [46] |
| 2023 | Convective heat transfer at supercritical pressures | Natural gas | To study convective heat transfer under supercritical pressures for various substances, especially within the turbulent flow domain. | This research endeavours to improve the design and advancement of highly efficient heat engineering apparatus and devices. | [47] |
| 2023 | Hybrid optimisation model for electric renewables to perform the design optimisation of the green hydrogen production system | PV and Wind | Determine the optimal capacity of each component of the system and identify the best site for hydrogen production based on factors such as Net Present Cost (NPC), Levelized Cost of Hydrogen (LCOH), and type of storage used. | Combining photovoltaic panels and wind turbines can help produce low-cost hydrogen in Morocco, with the lowest cost of 2.54$/kg in Dakhla, | [48] |
| 2023 | Thermo-ecological cost methodology | Natural gas, Wind and Solar | Examines and contrasts the thermo-ecological cost values associated with hydrogen production using renewable and non-renewable methods, emphasizing the distinctions between them. | The primary findings of this study underscore the importance of utilising the thermo-ecological cost methodology for evaluating the energy-ecological efficiency of hydrogen production systems, particularly those integrating renewable energy sources. The methodology emphasizes the cumulative consumption of non-renewable primary exergy as a crucial factor. | [49] |
3.1. Geothermal to hydrogen
Geothermal energy is a dependable and consistent renewable energy sourcedue to its near-independence from surrounding environmental conditions. This renewable source is stored as thermal energy deep within the Earth's subsurface. In certain regions worldwide, gaining access to these subterranean formations poses challenges due to drilling costs and various constraints. However, this energy source has been harnessed for industrial purposes in countries with abundant geothermal resources and more cost-effective drilling options. A heat transfer medium is required to extract this natural resource from deep underground to the surface. This process can be achieved in two stages: initially, heat is conducted from the rocks, followed by convective heat transfer, where hot water is transported to the surface. A broad range of applications exists for utilising this energy source, encompassing heating, cooling, power generation, and energy storage. The specific application depends on whether it involves shallow or deep geothermal systems.
In 2010, 24 countries generated 10,715 MW of geothermal power, increasing by 20% since 2005. By 2015, the capacity had grown to 18,500 MW, a nearly 60% rise. Geothermal power plants extract heat from the Earth's crust to generate electricity, while heat pumps provide heating to buildings on the surface. Estimating hydrogen generation through electrolysis from sustainable energy sources is a well-established approach. Various researchers have already investigated hydrogen production through geothermal energy using high-temperature electrolysis (HTE). These studies have included thermodynamic analyses, consistently demonstrating energy efficiency exceeding 80% in connection with hydrogen production. Moreover, geothermal energy can also be harnessed for the liquefaction of hydrogen. This model can be further enhanced to incorporate geothermal energy in hydrogen liquefaction cycles, absorption cooling systems, and co-generation systems that provide both geothermal electricity and heat for the absorption system [50].
Most hydrogen production systems powered by geothermal energy employ PEMelectrolysers, breaking water down into oxygen and hydrogen. The water's temperature is a crucial factor in the electrolysis process, as it influences the system's production capacity, power requirements, and overall cost for hydrogen production. Elevated temperatures during the electrolysis process reduce the electricity demand, consequently lowering the overall cost of the hydrogen production system. Two commonly employed hydrogen production systems powered by geothermal resources include the geothermal power plant (GPP). In this system, geothermal fluid and residual heat are utilised to operate a system capable of preheating water and generating electricity. In the second system, a flash separator separates water and steam. The steam is directed to produce electricity through a steam turbine, whereas the heat extracted from hot water via a heat exchanger is utilised to generate supplementary electricity for hydrogen production. Diverse catalysts are employed to enhance the separation of oxygen and water. The exceptionally pure water can attain elevated temperatures, increasing process efficiency. Achieving a geothermal fluid temperature of 200 °C can result in a 19% reduction in the cost of hydrogen production. Recent advancements have enabled PEM electrolysers to operate effectively at temperatures as high as 150 °C, displaying commendable efficiency. Nonetheless, a persistent challenge in hydrogen production from geothermal sources is the management of impurities, such as H2S and other noxious gases, on the opposite side of the electrode [24,[51], [52], [53], [54], [55], [56], [57], [58], [59]]. Fig. 3 presents the process flow diagram for hydrogen production using geothermal energy. Table 2 displays geothermal-driven hydrogen production facilities along with their production capacities.
 Fig. 3. Geothermal-based hydrogen production.
Fig. 3. Geothermal-based hydrogen production.Table 2. Experimental geothermal-based hydrogen production installations and their production capacities [60,61].
| Location | Name of the Plant | H2 Production (MW/day) |
Operational Status |
|---|---|---|---|
| Reykjavik, Iceland | Blue Lagoon Geothermal Power Plant | 1 | Operational |
| Neskowin, Oregon, USA | Big Island Geothermal Project | 1 | Operational |
| Landau, Germany | GEOTHERMIE Park | 0.5 | Operational |
| Gemert, Netherlands | Weert Geothermal Power Plant | 0.25 | Operational |
| Vilaflor, Spain | Socas II Geothermal Power Plant | 0.1 | Operational |
| Florence, Italy | Enel Green Power Geothermal-to-Hydrogen Plant | 10 | Planned (2023) |
| Riga, Latvia | Latvenergo Geothermal-to-Hydrogen Plant | 10 | Planned (2024) |
| Reykjavik, Iceland | HS Orka Geothermal-to-Hydrogen Plant | 10 | Planned (2025) |
| Valle Nevado, Chile | Geoterma Andes Geothermal-to-Hydrogen Plant | 10 | Planned (2027) |
| Geysers, California, USA | First Hydrogen Geothermal-to-Hydrogen Plant | 10 | Planned (2028) |
3.2. Solar to hydrogen
Solar energy has emerged as a prominent renewable resource for clean hydrogen production, aided by decreasing costs in the renewable energy sector. The cost-effectiveness of solar energy can potentially drive the advancement of solar hydrogen as an alternative fuel source. Various methods, such as solar thermolysis, thermochemical cycles, gasification, and electrolysis, harness solar energy for hydrogen production [62]. While storage techniques address the intermittent nature of solar energy, converting it directly into hydrogen is considered a more reliable and efficient solution. Electricity generation from solar power involves photovoltaic (PV) and photothermal methods [63]. Solar-generated electricity powers water electrolysis technologies like Alkaline Water Electrolysis (AWE), PEM, and Solid Oxide Electrolysis Cells (SOE) for hydrogen production. PEM systems facilitate hydrogen and oxygen production through specific voltage applications in water-filled compartments [64]. Thermochemical water splitting methods, such as the UT-3 cycle and the sulphur iodine cycle, exhibit high efficiency in generating hydrogen [65].
Ongoing solar photonic-based hydrogen production developments aim to enhance the conversion efficiency of solar-to-hydrogen systems, with PV and concentrated solar photovoltaic (CSPV) systems achieving notable efficiencies [66,67]. Combining Concentrated Solar Power (CSP) with CSPV systems shows promise for cost-effective hydrogen production [68]. Low-temperature water electrolysis combined with PV power plants offers an early and cost-efficient approach to solar-based hydrogen production [69]. Despite lower efficiency, third-generation multi-junction solar cells (MJSC) and reflective mirrors maximise electricity generation. Commercial applications often employ two-step reduction water electrolysis for environmentally friendly hydrogen production [70]. Solar thermolysis necessitates high temperatures, while the thermochemical solar-to-hydrogen process operates at moderate temperatures using a solar thermochemical reactor and metal-metal oxide reactions. Although solar-based hydrogen production methods like Alkaline Water Electrolysis (AWE) and PEM show promise in decarbonising fossil fuels, they are still undergoing refinement [71]. Solar-based hydrogen production as shown in Fig. 4. Table 3 displays solar-driven hydrogen production facilities along with their production capacities.
 Fig. 4. Solar-based hydrogen production.
Fig. 4. Solar-based hydrogen production.Table 3. Experimental solar-based hydrogen production installations and their production capacities [[72], [73], [74], [75], [76], [77]].
| Location | Name of the Plant | H2 Production (MW/day) |
Operational Status |
|---|---|---|---|
| Toulouse, France | Hydrogen Refuelling Station | 0.3 | Operational |
| Stockholm, Sweden | PROTON II Plant | 0.2 | Operational |
| Osaka, Japan | Hydrogen Energy Station | 0.1 | Operational |
| Fukuoka, Japan | Hydrogen Station | 0.1 | Operational |
| Melbourne, Australia | Hydrogen Production Plant | 0.05 | Operational |
| Andalusia, Spain | Iberdrola Solar-to-Hydrogen Plant | 100 | Planned (2025) |
| Bavaria, Germany | Siemens Solar-to-Hydrogen Facility | 10 | Planned (2024) |
| California, United States | Bloom Energy Solar-to-Hydrogen Plant | 10 | Planned (2023) |
| Western Australia, Australia | Fortescue Future Industries Plant | 100 | Planned (2027) |
| Atacama Desert, Chile | H2Pro Solar-to-Hydrogen Facility | 100 | Planned (2028) |
3.3. Wind to hydrogen
The growth of renewable energy capacity is driven by cost reductions and increasing demand. Offshore wind power is crucial for sustainable electricity production. Offshore installations minimise visual and acoustic impacts and allow for larger deployment areas. A recent project aims to enhance offshore wind energy infrastructure in the Atlantic Canadian region. The produced hydrogen can be used in stationary or mobile fuel cells, and excess hydrogen can be stored for periods of low wind speed. This process is an efficient, clean, and sustainable method of energy production.
Incorporating energy storage systems with wind power dispatch helps avoid wastage [78,79] Methods like lithium-ion batteries, power-to-gas conversion, and power-to-hydrogen technologies are used for surplus electricity storage. Hydrogen conversion is increasingly preferred and recognised as a cleaner fuel with various applications [80,81]. Electrolysis of water stands out for hydrogen production from fossil fuels. Wind energy demonstrates superior performance in hydrogen production. An efficient hybrid system designed for harnessing wind energy is necessary to achieve direct hydrogen production from renewable sources. This procedure helps reduce greenhouse gas emissions linked to hydrogen production from fossil fuels. In Algeria, producing hydrogenfrom wind energy costs $1.214/kgH2, with a 3.1-year payback period and an annual profit of $2224 for the Manjil wind plant. Challenges include wind intermittency, as well as hydrogen fuel storage and transportation [[82], [83], [84]]. Wind-based hydrogen production as shown in Fig. 5. Table 4 displays wind-driven hydrogen production facilities along with their production capacities.
 Fig. 5. Wind-based hydrogen production.
Fig. 5. Wind-based hydrogen production.Table 4. Experimental wind to hydrogen production installations and their production capacities[62,[75], [77],85].
| Location | Name of the Plant | H2 Production (MW/day) |
Operational Status |
|---|---|---|---|
| Dundee, Scotland | Hywind Scotland Pilot Project | 1.2 | Operational |
| Ulsan, South Korea | Ulsan Hydrogen Demonstration Project | 0.7 | Operational |
| Fukushima, Japan | Fukushima Hydrogen Energy Research Field | 0.4 | Operational |
| Leicestershire, England | HyDeploy Project | 0.1 | Operational |
| Hindmarsh, Australia | ARENA Hydrogen Electrolyser Pilot Plant | 0.05 | Operational |
| Andalusia, Spain | Iberdrola Solar-to-Hydrogen Plant | 100 | Planned (2025) |
| Bavaria, Germany | Siemens Solar-to-Hydrogen Facility | 10 | Planned (2024) |
| California, United States | Bloom Energy Solar-to-Hydrogen Plant | 10 | Planned (2023) |
| Western Australia, Australia | Fortescue Future Industries Plant | 100 | Planned (2027) |
| Atacama Desert, Chile | H2Pro Solar-to-Hydrogen Facility | 100 | Planned (2028) |
3.4. Biomass to hydrogen
Biomass presents a hopeful substitute for hydrogen generation, thanks to its abundant reserves and uncomplicated oxidation procedure. It can be generated through various methods, including thermochemical conversion and photocatalysis of waste materials. Although biomass hydrogen production emits CO2, it balances with the CO2 absorbed during the organisms' lifespan. Moreover, biomass's higher hydrogen-to-carbon ratio reduces dependence on hydrocarbons and aids in stabilising the atmospheric CO2 balance through photosynthesis [86,87]. Biomass gasification turns biomass into syngas via fixed bed reactors heated to approximately 800 °C. Biomass pyrolysis converts biomass into bio-oil and syngas, typically utilising fluidised bed reactors at around 500 °C. Biomass reforming produces hydrogen through biomass reaction with steam in steam reformers, typically operated at 800 °C. Biomass-based hydrogen production as shown in Fig. 6. Table 5 displays biomass-driven hydrogen production facilities along with their production capacities.
 Fig. 6. Biomass-based hydrogen production.
Fig. 6. Biomass-based hydrogen production.Table 5. Experimental biomass to hydrogen production installations and their production capacities [[88], [89], [90], [91]].
| Location | Name of the Plant | H2 Production (MW/day) |
Operational Status |
|---|---|---|---|
| Porto, Portugal | H2Port Initiative | 1 | Operational |
| Stockholm, Sweden | Stockholm Exergi Plant | 0.8 | Operational |
| Osaka, Japan | Osaka Gas Plant | 0.5 | Operational |
| Uster, Switzerland | H2Uster Plant | 0.2 | Operational |
| Athens, Greece | H2City Athens Project | 0.1 | Operational |
| Beijing, China | Beijing Municipal Biomass-to-Hydrogen Plant | 100 | Planned (2025) |
| Stockholm, Sweden | Stockholm Exergi Biomass-to-Hydrogen Plant | 20 | Planned (2024) |
| Osaka, Japan | Osaka Gas Biomass-to-Hydrogen Plant | 10 | Planned (2025) |
| Green Hydrogen Systems | Biomass-to-Hydrogen Facility | 10 | Planned (2023) |
| Athens, Greece | H2City Athens Biomass-to-Hydrogen Plant | 10 | Planned (2025) |
3.4.1. Biological production
3.4.1.1. Dark fermentation process
Dark fermentation (DF) is regarded as the most favourable method for producing biohydrogen from biomass, boasting a net energy ratio of 1.9, in contrast to steam methane reforming, which has a ratio of 0.64 [92]. Hydrogen can be produced using anaerobic bacteria cultivated in carbohydrate-rich or dark substrates. The anaerobic metabolism of pyruvate, generated through the breakdown of diverse substrates, is responsible for most microbial hydrogen production. This process is catalysed by one of two enzyme systems [93]. In dark fermentation (DF), anaerobic bacteria cultivated without light transform substrates, as depicted in Fig. 7 Many anaerobic microorganisms primarily rely on hydrogen as a crucial substrate in their metabolism. When hydrogen-rich molecules are accessible, these microorganisms have the capacity to harness the energy within them by utilising the electrons obtained through hydrogen oxidation, resulting in energy production. Without external electron acceptors, organisms accumulate surplus electrons due to metabolic processes stemming from the reduction of protons that lead to the production of hydrogen molecules. The pivotal enzymes responsible for regulating hydrogen metabolism are known as hydrogenases. The decomposition of pyruvate is facilitated by one of two enzyme systems mentioned in equations (1), (2)) [92].
 Fig. 7. Metabolic pathways for substrate-to-hydrogen conversion in dark fermentation.
Fig. 7. Metabolic pathways for substrate-to-hydrogen conversion in dark fermentation.Pyruvate: formate lyase (PFL)(1)Pyruvate + CoA → acetyl -CoA + formate
Pyruvate: ferredoxin oxido reductase (PFOR)(2)Pyruvate + CoA + 2Fd(ox) → acetyl -CoA + CO2 + 2Fd(red)
3.4.1.2. Photo fermentation process
Photo fermentation is regarded as one of the potential routes for generating biological hydrogen.
During this process, anoxygenic photosynthetic bacteria, particularly the purple non-Sulphur (PNS) bacteria, have the capability to convert H+ ions into gaseous H2. They achieve this by utilising both the reduction power derived from the oxidation of organic compounds, such as low-molecular-weight fatty acids, and light energy. This process is considered promising due to the absence of oxygen-evolving reactions, the ability to harness a wide spectrum of sunlight, high conversion rates of substrates, and the possibility of integrating this method of hydrogen production with waste disposal. Rhodobacter, a PNS bacteria genus, is the most frequently employed microorganism for biohydrogen production. Purple non-sulphur bacteria have the ability to engage in photoheterotrophic growth by obtaining both their carbon and electron requirements from reduced fixed carbon compounds. They utilise CO2 as their sole carbon source and can employ H2, S2, or Fe2+ as electron donors. Additionally, certain species are capable of photolithoautotrophic growth. Depending on the specific species, PNS bacteria can utilise a diverse array of organic carbon compounds, including amino acids, alcohols, carbohydrates, acetate, various organic acids, and pyruvate. Different organisms have the ability to employ one-carbon atom-containing substances like formate and methanol, while some can thrive by consuming aromatic organic compounds, including chlorobenzoate, cinnamate, benzoate, phenol, and phenylacetate [94]. In Fig. 8, the diagram illustrates the generation of hydrogen via the process of photo-fermentation.
 Fig. 8. Photosynthetic bacteria pathways in a non-sulphur-deprived photosynthetic bacterium to hydrogen conversion in photo-fermentation.
Fig. 8. Photosynthetic bacteria pathways in a non-sulphur-deprived photosynthetic bacterium to hydrogen conversion in photo-fermentation.(3) depicts the reaction producing hydrogen through the photo-fermentative method utilising acetate. This reaction is non-spontaneous because it possesses positive free energy, necessitating external energy input in the form of light, whether from natural or artificial sources. Under suitable physicochemical conditions, generating 4 mol of hydrogen from 1 mol of acetate is theoretically possible [94].(3)2CH3COOH + 2H2O → 4H2 + 2CO2, ΔGo = +104 kJ
3.4.1.3. Bio-photolysis process
The biological process utilises photosynthesis principles from algae and plants, adapted for hydrogen gas production. While green plants can't produce hydrogen due to the lack of specific enzymes, certain algae can generate hydrogen under specific conditions. Bio-photolysis, occurring under illumination, can lead to direct and indirect H2 and O2 production. Chlamydomonas reinhardtii, a well-studied microalga, is actively examined for direct bio-photolysis involving photosystems (PSI and PSII) and hydrogenase enzymes. In direct bio-photolysis, green algae split water molecules during photosynthesis, creating oxygen and hydrogen ions, converted into H2 by the hydrogenase enzyme. However, oxygen sensitivity is a drawback, requiring maintenance below 0.1% [95]. Indirect bio-photolysis with cyanobacteria involves dark fermentation and the hydrogenase enzyme to convert CO2 into endogenous reserve carbohydrates before hydrogen production. The process includes reactions in Equations (4), (5) [96,97].(4)12H2O + 6CO2 → C6H12O6 + 6O2(5)C6H12O6 + 12H2O → 12H2 + 6CO2
3.4.2. Thermochemical production
The thermochemical approach transforms biomass into hydrogen-enriched gases, offering a promising solution for achieving zero greenhouse gas emissions and sustainable development. Gasification and pyrolysis stand as the principal conversion methods, resulting in the generation of CH4 and CO, alongside other gaseous substances. Conversely, liquefaction and combustion techniques are less favoured due to their lower hydrogen yields, the emission of byproducts, and the complexities associated with their operation [98].
3.4.2.1. Pyrolysis
Pyrolysis and co-pyrolysis are promising methods for hydrogen production by heating and gasifying raw organic material at temperatures of 500–900 °C and pressure of 0.1–0.5 MPa [99]. This procedure occurs in an oxygen-free environment, reducing emissions by avoiding dioxin and carbon oxide formation. However, when water or air is present, COx emissions may occur. The technology offers clean carbon byproducts, fuel flexibility, and a simple design [100,101]. Pyrolysis can be categorized into high, medium, and low-temperature types, where fast pyrolysis transforms organic matter into high-energy outputs. However, fouling from carbon formation is a challenge that can be addressed through appropriate design [102]. Equation (5a) illustrates the reaction to this mechanism [100].(5a)