1. Introduction
The recent emergence of plasmonics, the science and technology of metallic nanostructures interacting with light, is based upon the surface plasmonpolariton (SPP) modes in planar surfaces and localized surface plasmon resonance (LSPR) in metallic nanoparticles [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]], two unique phenomena that manifest exclusively at the nanoscale. As a result, plasmonics is one of the most characteristic examples of what is called ‘nanotechnology’ nowadays, despite being manifested since antiquity and theoretically explained since the early twentieth century [15,16]. Thus, plasmonics promise radical breakthroughs in electronic devices [[17], [18], [19], [20], [21], [22], [23], [24]], biosensing [[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]], catalysis and photochemistry [[36], [37], [38], [39], [40], [41], [42], [43], [44], [45]], solar energy harvesting [[46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64]], photodedection [[65], [66], [67], [68], [69], [70], [71], [72], [73], [74]], optical storage of information [[75], [76], [77], [78], [79], [80], [81], [82], [83], [84]], telecommunications [[85], [86], [87], [88], [89], [90], [91], [92], [93], [94]] and metamaterials [[95], [96], [97], [98], [99]]. These applications are, in their turn, based on the high spatial resolution of SPP and LSPR, which is below the diffraction limit of light [7,82,[100], [101], [102], [103]], the ultra-fast response of plasmonic systems [[104], [105], [106], [107]], the maximized absorption and scattering of light [1,108], the elevation of the metal’s electrons to a hot state [[109], [110], [111], [112], [113]], and more importantly the creation of extreme near-fields [[114], [115], [116], [117], [118], [119]] that result in enhanced Raman and fluorescence signals of adjacent molecules [28,[120], [121], [122], [123], [124], [125], [126], [127], [128]] and the increased electron field emission probability [129,130] at the resonance wavelengths.
The most popular plasmonic metals are gold and silver due to their high conductivity and low dielectric losses (especially for Ag); the former due to its chemical inertness and stability and its facile surface functionalization by thiolates [131] and the later due to the absence of interband transitions in the visible spectral range rendering stronger and tunable LSPR throughout the visible spectral range, yet in expense of the feature size resolution (i.e. for Ag nanoparticles with LSPR in red the particles should exceed 100 nm [132]). However, their spectral tunability is limited (LSPR of Au or Ag nanoparticles cannot be extended to UV, and extension to IR would dictate size and shape compromises), their melting point is low, especially when in nanoparticle form [133,134], making them unstable for photothermal and hot electron devices, their conduction electron mobility is very low, and consequently they exhibit high conduction electron losses [[135], [136], [137]] and they both exhibit relatively high work function [138] minimizing the electron emission probabilities. Copper combines the drawbacks of gold (interband absorption and dielectric losses in the visible) and silver (reactivity), its plasmonic behavior is inferior [139] and, consequently, studies on its plasmonic response are less frequent [[140], [141], [142], [143]].
In order to tackle the aforementioned obstacles of gold, silver and copper, a quest for alternative plasmonic conductors is taking place recently [136,137,[144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175], [176]]. Group-III metals (aluminum, gallium and indium) have been investigated for extending the plasmonic devices to the UV range [[148], [149], [150], [151], [152], [153], [154], [155], [156], [157]]; however they suffer from fast oxidation resulting in a metal/oxide core/shell structures, which eventually operate in the visible spectral range [148]. In addition, they have exceptionally low melting temperatures (Ga nanoparticles melt even below room temperature) and high diffusivities that compromise their long-term stability. On the other hand, for extending the operation of plasmonic devices to IR, transparent conductors, such as indium tin oxide (ITO), aluminum-doped zinc oxide (AZO) and gallium-doped zinc oxide (GZO) have been implemented [[158], [159], [160], [161], [162], [163], [164], [165], [166], [167], [168], [169]].
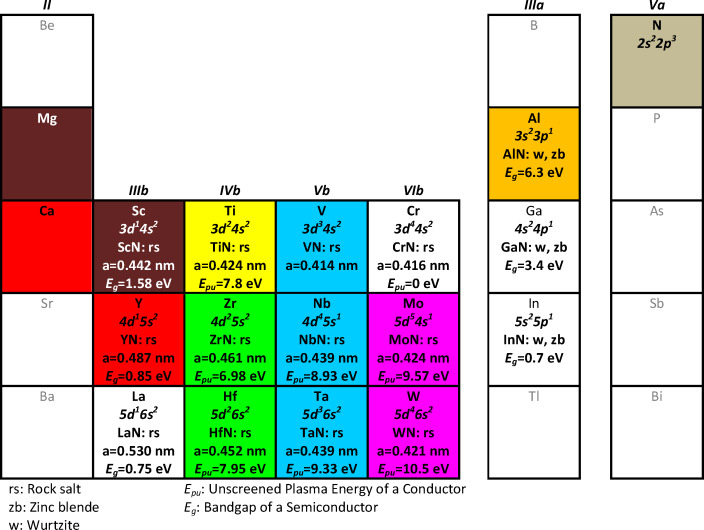
An emerging category of alternative plasmonic materials is the conductive transition metal nitrides (TMN), such as TiN, ZrN and TaN [[169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182], [183]]. Going beyond the three aforementioned nitrides, of significant technological importance are all the nitrides of the transition metals of the group IVb-Vb-VIb (4–6 IUPAC) of the periodic table of elements, as shown in the reduced periodic table of elements in Fig. 1 (data taken from [[184], [185], [186], [187]]); these nitrides can form cubic rocksalt-type crystals (B1-structure, Fm3m symmetry) and constitute a category of very important technological materials due to their exceptional mechanical properties, high melting point, refractory character and chemical stability over hostile environments. Thus, TMN are widely studied and used for a variety of applications, such as decorative coatings, protective and anti-corrosive coatings, in cutting tools and machining equipment; therefore, the works dealing with comparisons on their growth and properties are numerous and well-established [184,[188], [189], [190], [191], [192], [193], [194], [195], [196], [197], [198], [199], [200]]. They also exhibit electronic conductivity due to the partially filled valence d orbitals that are not completely hybridized with the N-2p electrons, as we will show below. The unique combination of their electronic properties with their stability and refractory character resulted in TMN being used also for applications in electronics, such as diffusion barriers [[201], [202], [203], [204], [205], [206], [207], [208], [209], [210]], Schottky contacts [[211], [212], [213]], superconducting devices [[214], [215], [216], [217], [218], [219]], conductive growth templates for wide bandgap semiconductors [[220], [221], [222]], field emission cathodes [223], and Ohmic contacts for optoelectronic devices [176,184,224] or other types of metallizations [[225], [226], [227], [228], [229], [230], [231]]. Finally, an exceptional asset is their compatibility with CMOS technology, due to their high electron mobility and refractory character [[232], [233], [234], [235]], which enables their easy integration and upscaling in realistic, mainstream electronic devices. In this respect, TMN would boost the integration of plasmonics into CMOS technology for optical communications by replacing the incompatible Au [236] and Ag [237]. Alloying elements to form conductive ternary compounds in the B1 rocksalt structure include the elements of the groups II, IIIa, and IIIb (mostly Al).
 Fig. 1. An excerpt of the periodic table of elements where the usual constituents of the various conductive binary and ternary transition metal nitrides are shown (blue and green are the usual constituent of the conductive nitrides of B1 structure, magenta are the elements that can be potentially used as dopantsin order to control the conductivity and the LSPR spectral position and red are elements that are not recommended for electronic applications); data taken from [[184], [185], [186], [187]]. The colors of the cells are a graphic indication of the color of light where LSPR can occur for nanoparticles of a ternary nitride of the form B1-TixE1-xN, where E is the corresponding element (dark wine color stands for infrared). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 1. An excerpt of the periodic table of elements where the usual constituents of the various conductive binary and ternary transition metal nitrides are shown (blue and green are the usual constituent of the conductive nitrides of B1 structure, magenta are the elements that can be potentially used as dopantsin order to control the conductivity and the LSPR spectral position and red are elements that are not recommended for electronic applications); data taken from [[184], [185], [186], [187]]. The colors of the cells are a graphic indication of the color of light where LSPR can occur for nanoparticles of a ternary nitride of the form B1-TixE1-xN, where E is the corresponding element (dark wine color stands for infrared). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)In this work we review the fundamental properties (phases, microstructure, growth techniques) of the TMN, as well as their electronic structure via detailed ab-initio and semi-empirical computational methods, their dielectric function spectra extracted from an extended literature survey of reported ellipsometry and optical reflectivity spectra. As a result, we critically compare the reported electronic properties (such as resistivity/conductivity, conduction electron density-via the unscreened plasma energy, the spectral position of their interband transitions and their work-function). We also consider the effect of grain size to the optical and electronic properties of TMN.
Furthermore we consider the successful alloying of TMN to form ternary TMN (or more accurately pseudobinary nitrides, because the B1 structure is retained in the entire compositional range and the nitrogen sublattice of the B1 structure remains intact during alloying) with tuned electronic properties [[238], [239], [240], [241], [242], [243], [244], [245], [246], [247], [248], [249], [250], [251], [252], [253]]. We demonstrate that these ternary systems provide exceptional flexibility, vast range for the values of the main electronic properties, thus offering immense potential for applications in plasmonics.
Finally and most importantly, we evaluate the potential use of binary and ternary TMN as core components for plasmonics by providing the LSPR performance of TMN nanoparticles through detailed Maxwell-Garnett effective medium approximation calculations and accurate Finite Difference Time Domain (FTDT) calculations. We demonstrate that the plasmonic response of nanoparticles of TMN is equivalent of that of nanoparticles of noble metals. In addition, we show that TMN can be active and stable plasmonic materials with LSPR ranging from the UV (using TaN and Ta-rich TixTa1-xN) to IR (using ternary TixSc1-xN and TixAl1-xN) and combining additional assets such as refractory character, which makes them suitable for high temperature/high power plasmonics [182,183,254], photothermal applications [255], and low work function that makes them suitable for plasmon-enhanced electron emitters.
2. TMN continuous films
2.1. Fundamental features and growth
2.1.1. Fundamental features
2.1.1.1. Nitrides of group IVb elements (TiN, ZrN, HfN)
The metals of the group IVb of the periodic table of elements have four valence electrons with a configuration of d2s2 for all the three metals ([Ar]3d24s2, [Kr]4d25s2 and [Xe]4f145d26s2 for Ti, Zr, and Hf, respectively) and they form bonds with N atoms (valence electronic configuration 2s22p3). While various nitride phases such as the Me2N [256,257] and Me3N4 (Me = Ti, Zr, Hf) [258,259] were reported, the most stable and durable nitrides of these metals are those in the cubic rocksalt B1-MeN arrangement or also called δ-MeN (Me = Ti, Zr, Hf). The B1 TiN, ZrN and HfN are characterized by gold-like yellow color (bright yellow for TiN and becoming gradually paler for ZrN and HfN [184,190]), high hardness [188,196], electrical conductivity [184,226] and refractory character [198].
B1-TiN is the archetypical example of the conductive nitrides. It has been studied since the early 1930s and B1-TiN films have been systematically grown and studied since the mid-1970s [260]. B1-TiN is considered nowadays as one of the most technologically important materials. A wide variety of fabrication techniques have been used for the growth of B1-TiN films, such as Magnetron Sputtering (MS) [[261], [262], [263], [264], [265], [266], [267], [268]], Cathodic Vacuum Arc (CVA) [[269], [270], [271]], Chemical Vapor Deposition (CVD) [272,273], Atomic Layer Deposition (ALD) [189], Pulsed Laser Deposition (PLD) [184,274], Ion Beam Assisted Deposition (IBAD) [275], Dual Ion Beam Sputtering (DIBS) [276], and High Power Impulse Magnetron Sputtering (HIPIMS) [277]. The numerous works on the growth of B1-TiN led to an unprecedented understanding and control of its microstructural features, such as the grain size, orientation, columnar or globular type of growth, etc [276,[278], [279], [280], [281], [282]], and epitaxial growth of TiN on MgO, GaN, AlN, and Si has been achieved [283], even at low temperature [284], as shown in the high-resolution transmission electron microscopy images of Fig. 2 for the cases of MgO (Fig. 2a) and GaN (Fig. 2b) substrates images from Refs. [224,284]. Therefore, it is evident that with the appropriate substrate selection both TiN(100) and TiN(111) films can be grown.
 Fig. 2. High-resolution transmission electron microscopy images of epitaxial TiN grown by magnetron sputtering on (a) MgO, and (b) GaN/Al2O3. The horizontal black lines indicate the interfaces; lettering in (b) defines the stacking of cystal planes, which is different for the hexagonal w-GaN and the cubic B1-TiN according to [224]; the scale bar applies to both images. Images from [224,284].
Fig. 2. High-resolution transmission electron microscopy images of epitaxial TiN grown by magnetron sputtering on (a) MgO, and (b) GaN/Al2O3. The horizontal black lines indicate the interfaces; lettering in (b) defines the stacking of cystal planes, which is different for the hexagonal w-GaN and the cubic B1-TiN according to [224]; the scale bar applies to both images. Images from [224,284].The optical properties of B1-TiN, which are relevant to plasmonic applications, have been also a subject of intense experimental research [170,[172], [173], [174],176,184,190,195,200,224,226,239,246,249,251,[285], [286], [287], [288], [289], [290], [291], [292], [293], [294], [295]]. The optical properties and the electronic structure reported in the aforementioned references [170,[172], [173], [174],176,184,190,195,200,224,226,239,246,249,251,[285], [286], [287], [288], [289], [290], [291], [292], [293], [294], [295]] will be thoroughly reviewed in the following paragraphs.
B1-ZrN is another widely studied material, which finds a wide spectrum of applications such as superconductors [296], protective coatings [297], diffusion barrier layers [206], and IR reflectors [298]. The most popular technique for the growth of B1-ZrN is MS [229,297,[299], [300], [301], [302], [303]], however, CVA [[304], [305], [306]], ALD [307], PLD [296], and IBAD [308] were also successfully implemented. ZrN usually contains a higher amount of intrinsic stress and structural defects than TiN grown with similar conditions, mostly for kinetic reasons [309,310]. Consequently, the heteroepitaxy of ZrN on Si was not successful so far, as only local epitaxial domains were achieved [311], and the ZrN heteroepitaxy on MgO was achieved much later than for TiN and HfN [312].
Finally, B1-HfN is the less frequently studied compound of the group-IVb nitrides, possibly due to the scarcity of Hf and the difficulty of purifying metallic Hf from Zr impurities, despite of B1-HfN films’ receiving attention since the early 1970s [313]. Sputter deposition is the most widely used deposition method for B1-HfN [[314], [315], [316], [317]], as well, while alternative deposition techniques include ALD [318], CVD [319], IBAD [320] and Molecular Beam Epitaxy (MBE) [321]. The heteroepitaxy of HfN films was achieved on Si and MgO [322,323].
The dielectric function spectra and the optical reflectivity spectra of B1-ZrN [184,190,195,200,244,251,[285], [286], [287],308,311,[324], [325], [326]] and B1-HfN [184,190,200,[285], [286], [287],[327], [328], [329]] were studied and reported by many groups and are critically compared to those of B1-TiN in this work.
2.1.1.2. Nitrides of group Vb elements (VN, NbN, TaN)
V, Nb and Ta have one more valence electron than Ti, Zr, and Hf, respectively, consequently they have five valence electrons and their valence electron configurations are [Ar]3d34s2, [Kr]4d45s1, and [Xe]5d36s2, respectively. VN and NbN can form easily the B1 nitride phase while B1-TaN is metastable. Several techniques were used for their growth, such as reactive sputter deposition (VN [[330], [331], [332], [333]], NbN [334], TaN [[335], [336], [337], [338], [339], [340]]), CVA (NbN [[341], [342], [343]], TaN [344]), PLD (VN [345], NbN [184,200,346,347], TaN [184,200,274]), ALD (VN [348,349], NbN [188,350], TaN [188,351,352]), CVD (VN [353], NbN [191], TaN [191,353]), and IBAD (VN [354], NbN [355], TaN [356]). The epitaxial growth of B1-VN, B1-NbN and less frequently of B1-TaN, mostly on MgO, was also achieved successfully [333,[357], [358], [359], [360]].
According to Papaconstantopoulos et al. [361] B1-VN, B1-NbN, B1-TaN are paramagnetic, while they all have been reported to be superconductors [347,362]. Especially NbN is a well known superconductor whose superconducting properties have been under investigation since the early 1980s [[216], [217], [218],[363], [364], [365], [366], [367], [368]]. B1-VN, B1-NbN, B1-TaN have also remarkable mechanical properties and stability and were studied accordingly [188,[369], [370], [371], [372], [373], [374], [375], [376]], though not so intensively as B1-TiN and B1-ZrN.
Among them, TaN was more intensively studied, especially regarding its electrical properties, which are relevant to applications as diffusion barrier [[202], [203], [204], [205],209,377] and metallizations [196,225,228,233,378,379] where TaN is becoming an industry-standard material. B1-TaN has a colorless grayish appearance and its optical properties have been thoroughly investigated [168,169,174,184,200,242,246,248,251,[380], [381], [382], [383]] and can be tuned by alloying with TiN, [184,246,248,251] or ZrN [242]; due to its remarkable optical properties, it was proposed as a promising alternative plasmonic material [168,169,174]. Note however, that TaN is very hardly stabilized in the B1 phase [376,384], although high quality B1-TaN has been achieved by few authors [381,385,386], and it has been recently suggested that the point defects, which are undesirable for the electrical conductivity, are playing a crucial role towards the stability of B1-TaN [387].
Studies on the optical properties of VN [238,328,380,388] and NbN do exist [184,200,[389], [390], [391]] but they are far more rare.
2.1.1.3. Nitrides of group VIb elements (CrN, MoN, WN)
Cr, Mo and W have one more electron than V, Nb, and Ta, respectively, consequently they have six valence electrons and their valence electron configurations are [Ar]3d44s2, [Kr]4d55s1, and [Xe]5d46s2, respectively. Cr can easily form the B1 nitride phase by conventional sputtering [[392], [393], [394], [395]], HIPIMS [277,396,397], MBE [398], and CVD [399]. The attention that B1-CrN received was due to its superior wear and anti-corrosion performance compared to B1-TiN [[400], [401], [402], [403], [404], [405], [406], [407], [408]]. The magnetic properties of CrN are unique among all the TMN, as implied since the pioneering work of Papaconstantopoulos [361] where the paramagnetic state of CrN was questioned, and clearly proven by Filipetti et al. who performed spin polarized calculations and predicted its semiconductive character when in anti-ferromagnetic state [409]. The interplay between the magnetic properties of CrN and its semiconductive behavior was experimentally confirmed by several groups [408,[410], [411], [412]] and disregard it as a candidate plasmonic conductor; therefore, the study of its electronic structure and dielectric function spectra is excluded from this work.
B1-MoN and B1-WN are the less studied among all TMN. MoN has been studied since early 1980s [413]. It is hardly stabilized in the B1 structure and competitive hexagonal MoN phases exist [414,415]. MoN is also paramagnetic [361] and superconducting [[416], [417], [418]]. B1-MoN has been reported to be grown by sputtering [414,419], PLD [184,200], CVA [420], IBAD [421], CVD [422,423], and ALD [424]. WN was considered mostly as a diffusion barrier [425] and despite of being desposited by a variety of techniques, such as sputtering [211,370,426,427], CVD [422,428], and ALD [429,430] its B1 phase in epitaxial form on MgO and sapphire has been very recently achieved [187]. Consequently, the studies of their optical properties are extremely rare [184,200,431], while until recently [184,432] the optical properties of B1-WN were missing from the literature, since most of the reported works dealing with WN refered to other phases.
Both MoN and WN are unstable in the B1 cubic structure [186,387,433] and it was recently revealed that the existence of point defects (metal and ligand vacancies) play a crucial role for their stabilization [186,387,433].
2.1.1.4. Ternary conductive nitrides
Alloying the aforementioned TMN to form ternary (or more accurately pseudo-binary) films is an effective pathway to controlling their mechanical, optical and electronic properties. The similar crystal structure (rocksalt), local symmetry (octahedral) and the similar bonding to N (via hybridization of the metal’s delectrons with the nitrogen’s 2p electrons) [187], make all the nitrides of the group IVb, Vb, and VIb metals completelty soluble to each other in the B1 phase and in the entire compositional range [184,249,250]; in fact, such alloying may result in more stable films with less structural defects compared to some metastable binary nitrides, with the most prominent example being the stable Ta-rich B1-TixTa1-xN and the metastable B1-TaN [376]. The most widely used alloying phase for electronic applications is the B1-TiN, which is implemented as the basis for the formation of TixZr1-xN [251,305,376], TixHf1-xN [184,249], TixV1-xN [238,247,253], TixNb1-xN [184,249,250], TixTa1-xN [376,434], TixCr1-xN [241], TixMo1-xN [184,239,241,249,250,435,436], and TixW1-xN [184,437,438]. Electronics-relevant Ta-based (TaxZr1-xN and TaxW1-xN) [184,242,244,249,250,439], Zr-based (ZrxHf1-xN, ZrxNb1-xN, ZrxCr1-xN, ZrxY1-xN,) [243,245,440,441], and V-based (VxW1-xN) [370] ternary nitrides were also reported.
Apart from the structural stability, the driving force for alloying and forming ternary conductive nitrides is the tailoring of key properties, such as the resistance against oxidation that enhances the stability and lifetime of the films [442], the diffusion barrier capability [443], the electrical resistivity [248,251,444], the work function [184,445], the superconductivity [446,447], the optical reflectivity edge, which defines the color and brightness of optical films [184,448], and more recently the conduction electron density, which defines the plasmonic response of nanoparticles of these nitrides [439].
Ti, Zr, and Hf share the same valence electron configuration of the constituent metal (d2s2) and they form bonds with nitrogen atoms (valence electron configuration 2s22p3). Their electrical conductivity is due to the excess of delectrons of the metal. By substituting any of these metal atoms by atoms of a group Vb (V, Nb, Ta) or group VIb (Mo, W) element, the cubic ternary structure is enriched with conduction electrons [184], thus increasing the resistivity [248], and blueshifting the reflectivity edge [184,200,434] and the localized surface plasmon resonance of the corresponding nanoparticles [439].
It is clear that such alloying cannot reduce the conduction electron density below that of B1-TiN, B1-ZrN, and B1-HfN. In order to reduce their conduction electron density, and shift their optical and plasmonic performance towards the infrared, B1-TiN and B1-ZrN should be alloyed with group III or II elements. Among the group IIIa elements the only efficient candidate for alloying with B1-TiN and B1-ZrN is Al (valence electron configuration 3s23p1). Despite of AlN being mostly crystallized in the wurtzite-type hexagonal phase w-AlN and being a well-known extremely wide band gap semiconductor (Eg = 6.2 eV) [[449], [450], [451], [452]], Al can be incorporated as an alloying element into Ti substitutional positions in B1-TiN and form the pseudobinary alloy B1-TixAl1-xN [198,439,[453], [454], [455], [456], [457]]. The B1-TixAl1-xN phase is electrically conductive and stable only in the range 0.55 < x < 1; its electrical and optical properties are apparently varying with x [453,[457], [458], [459], [460]]. Electronic and photonic applications of the TixAl1-xN system include decorative colored coatings [453], plasmonic conductors operating in the deep red [439] and solar selective absorbers [461].
For the time being, the rest of the group IIIa elements are not efficient in alloying with B1-TiN. Ti-B-N films tend to be nanocomposite, and not ternary or pseudo-binary compounds, due to the competition of the rocksalt TiN and the hexagonal TiB2 phases [462,463] and resemble more the properties of the Ti-Si-N nanocomposites [464]. TixGa1-xN is extremely scarce [461] due to the incompatibility of the sputtering process used for the TiN growth and the MBE process used for the GaN growth [465]; note that gallium targets cannot endure the plasma in the sputtering process and liquefy due the exceptional low melting point of gallium [466]; the emergence of the ALD technology for the growth of both TiN and GaN [467,468] can overcome the process incompatibility and might enable in the future the elaboration and the more careful investigation of this compound. Finally, indium combines the drawbacks of gallium in alloying with B1-TiN with the large mismatch of the atomic radii of titanium and indium, which are expected to induce severe stress and a strong driving force for decomposition; consequently, there is no report for the formation of TixIn1-xN.
As Al is not soluble in B1-TiN in the entire compositional range, other candidate substitution elements with three or two valence electrons, such as the rare earth elements (Sc, Y, La) of group IIIb and the alkaline earth elements (Mg, Ca) of group II have been considered, and TixSc1-xN, TixY1-xN, ZrxY1-xN, Ti-La-N (amorphous), TixMg1-xN and TixCa1-xN films were reported [441,[469], [470], [471], [472]]. Among them, the most promising in terms of its electronic and optical properties, as well as of its stability, is TixSc1-xN [441,469,471]. This is mostly due to the valence electron configuration (3d14s2) of Sc, and the B1 crystal structure being the most stable phase of ScN, which is, however, an intermediate bandgap semiconductor (Eg = 1.30–1.58 eV) [[473], [474], [475], [476]]. As a direct consequence of the similar crystal structure (B1) and valence electron configuration (partially filled d-band add filled s-band) of ScN and TiN, the pseudobinary B1-TixSc1-xN is stable in the B1 structure in the entire compositional range (0 < x < 1) and films of exceptionally high crystalinity and outstanding optical performance can be achieved [441,[469], [470], [471]].
2.1.2. Growth mechanisms, structural defects and stress, strain development
The high melting point of TMN has the direct consequence of their usual fine crystal structure and the ability of growing high crystalline quality TMN has been a subject of intense research for several years. In addition, it has been recently suggested [439,441] that the crystallinity of such films is of paramount importance for their plasmonic behavior. As a result, reviewing the growth mechanisms, microstructure and stress development during physical vapor deposition (PVD) of TMN thin films is very important for the understanding of TMN and for their critical evaluation for realistic applications in photonics and plasmonics. A special emphasis is given to TiN, which is the most widely studied binary compound among conductive TMN. We discuss the origin of stress evolution in binary and ternary compounds, based on recent kinetic stress models, as well as the growth-induced structural defects due to energetic particles impingement of the film surface/subsurface that takes place under energetic deposition conditions, such as MS, DIBS, CVA, and HiPIMS. We will also illustrate the interplay between growth morphology, phase formation, texture and stress development for the case of ternary nitrides, for which a large variety of microstructures can be formed. Such microstructural attributes, in terms of grain size, preferential orientation and stress levels can deeply influence the TMN optical properties. The knowledge of these ‘extrinsic’ effects is as crucial as the ground electronic features of TMN for the quest in the design of conductive TMN for photonic and plasmonic applications.
2.1.2.1. Microstructural development during TiN film growth
A general development of film microstructure with respect to various growth conditions is commonly represented by diagrams called structure zone models (SZM) [477], which tend to relate the effects of the process parameters on the resulting microstructure of polycrystalline thin film materials. These diagrams are usually compiled as a function of the homologous temperature, Th = Ts/TM, where Ts is the substrate temperature and TM is the melting point of the deposited material (TM = 3222 K for TiN). For the case of sputter-deposition, it also necessary to take into account the working pressure [478], and more generally the energy deposited per incident particle, Ed [479]. Typical deposition conditions to obtain stoichiometric TiN films with a relatively dense microstructure require sufficient adatom mobility, i.e. operating at Ts in the 550–800 K range, resulting in Th ∼ 0.17–0.25, which corresponds to microstructures of ‘Zone T’-type of SZM [480]. The microstructure of polycrystalline TiN films grown under Zone T consists of V-shaped columns [184,481] see Fig. 3a, characteristic of an evolutionary microstructure throughout the film thickness, i.e. the columns become larger at larger film thickness; such features are limited for films thinner than 100 nm, which are usually employed in photonic and plasmonic devices [224]. The bright contrast between columns in the cross-sectional TEM image of Fig. 3a corresponds to voided grain boundaries, and this effect is also more pronounced with increasing film thickness. These columns emerge at the surface forming facets, corresponding to crystallographic planes of low surface energy γ, resulting in rough surface morphologies. The planes of lowest γ correspond to the planes with the largest lateral growth rate, in other words to the lowest perpendicular crystallographic growth rate.
 Fig. 3. a) Bright-field cross-section TEM micrograph of a TiN sputter-deposited film at 573 K and Ptot = 0.66 Pa and ion energy Ei = 20 eV, from Ref. [481] b) and c) Plan-view SEM surface morphology of TiN films sputter-deposited at 5 (b) and 20 sccm N2 at, characteristic of Zone T and Zone II regions of SZM, respectively, from Ref. [477], d) the evolution of the competitive of the (111) and (200) crystals of TiN-based on sketch from Ref. [482], the differences are mostly due to the perpendicular and lateral surface diffusion of the deposited species.
Fig. 3. a) Bright-field cross-section TEM micrograph of a TiN sputter-deposited film at 573 K and Ptot = 0.66 Pa and ion energy Ei = 20 eV, from Ref. [481] b) and c) Plan-view SEM surface morphology of TiN films sputter-deposited at 5 (b) and 20 sccm N2 at, characteristic of Zone T and Zone II regions of SZM, respectively, from Ref. [477], d) the evolution of the competitive of the (111) and (200) crystals of TiN-based on sketch from Ref. [482], the differences are mostly due to the perpendicular and lateral surface diffusion of the deposited species.The formation of V-shaped columns is the result of a kinetically-limited competitive columnar growth, due to anisotropy in adatom surface diffusion: after random nucleation of grains [480], some column/grain orientations survive, while other disappear, and the film microstructure shown in Fig. 3a testifies that overgrowth has occurred during film thickening. Mahieu et al. [477] have shown that the preferred orientation of TiN films grown under Zone-T corresponds to the geometrically fastest growing direction perpendicular to the substrate, i.e. the texture that develops corresponds to the [hkl]-oriented columns that gradually envelop and overgrow the other out-of-plane oriented grains. In this temperature range, the mobility is high enough to allow for surface diffusion as well as intergrain diffusion (mass transport from one grain to another), but not for bulk diffusion (grain boundary are immobile) so that restructuring is prohibited. The preferred orientation depends on the nature of the reactive gas species (either atomic N or N2 molecule) and incoming adparticles (either Ti or TiN), which dictates the crystallographic orientation which forms the facets [480]. For conditions at which the formed facets are {100}, the fastest geometric growth direction is [111], and TiN films will have a [111] out-of-plane preferred orientation. The corresponding surface morphology consists of nicely faceted grains of tetrahedron shape, pointing with a corner upward (see Fig. 3b), typically obtained for MS at low N2 partial pressure for which the state of reactive gas is molecular and the incoming flux is composed of Ti atoms. However, in the presence of atomic N, which is the case of MS at higher N2 flow, faceting results in {111} crystal habits, so that the preferential out-of-plane orientation is along [001]. In this case, the characteristic surface morphology consists of square-base pyramids (shown in Fig. 3d [482]). Therefore, for certain applications (i.e. plasmonic nanoparticles) that are based on isolated self-assembed TiN islands the different growth behavior of the [001] and [111] oriented grains, depicted in Fig. 3d, should be taken into account.
Let us mention that TiN films grown under Zone T conditions can develop also an in-plane preferential orientation in addition to their out-of-plane orientation, often referred as a biaxial alignment, this is typically the case when the substrates are tilted with respect to the incoming material flux [477]. This is called glancing angle deposition (GLAD). Anisotropic in-plane growth rates (in terms of 2D capture length of diffusing particles) also govern the development of in-plane alignment similarly to the mechanism responsible for the out-of-plane orientation.
At higher homologous temperatures, typically for Th > 0.3, TiN films can develop a Zone II-type microstructure, consisting of straight columns with constant column diameter throughout the entire film thickness. The column diameter in Zone II is much larger than that of Zone T, and the columns are not faceted, but rather exhibit a smooth morphology. This is illustrated in Fig. 3c, where it can be clearly seen that the TiN surface is more compact and grains have a rather rounded aspect; consequently, we might propose as a rule of thumb to select these conditions for the growth of TiN for plasmonic and photonic applications. The resulting out-of-plane preferred orientation corresponds to the plane of lowest surface energy. For TiN, the computed values of γ evidence a strong anisotropy: the planes of lowest surface energy being (001), with γ001 ∼ 1.3 J m−2, much lower than the values for the (110) and (111) surfaces, with γ110 ∼ 2.6–2.9 J m−2 and γ111 ∼ 4.5–5.0 J m−2, respectively [281]. Note that the (111) surface of TiN is polar, so that the energy of N- and Ti-terminated (111) surfaces differ significantly [483].