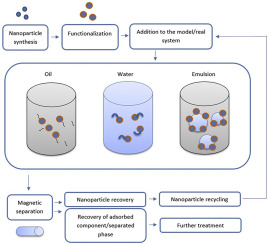
1. Introduction
Nanoparticle research is a rapidly developing scientific area due to the enormous potential of nanoparticle application in a wide range of biological, environmental, and technological fields. The tendency is largely related to the special properties that nanoparticles can offer, and how they can be modified to fit the desired applications. The current article presents an overview of scientific publications focusing on man-made nanoparticles exhibiting magnetic properties, and their possible applications in the oil and gas production sector. The magnetic properties of nanoparticles are of interest within industrial applications due to the possibility of using magnetic fields to recover or purify them. The properties and performance of magnetic nanoparticles mainly depends on the type of material, composition and dimensions of 1–100 nm. A number of materials can be used as base for the nanoparticle synthesis, including iron oxides Fe2O3/Fe3O4, pure metals Fe and Co, and spinel type ferromagnets MgFe2O4, MnFe2O4, or CoFe2O4 (An-Hui Lu and Ferdi, 2007). One of the special features of the nanoparticles is that their physical, structural, thermal, electrical, and magnetic properties differ from the properties of the bulk material and are directly related to their dimensions. In some cases, magnetic properties of the nanoparticles can be varied/affected by the type of the synthesis method and protective coating, but no general correlations have yet been established. One of the major drawbacks associated with the use of nano-scale particles is their limited intrinsic stability, where particle agglomeration can lead to loss of properties. Also, the unprotected freshly synthesized metal nanoparticles are highly active chemically and may easily oxidize in air. To prevent this, certain stabilization/protection strategies must be used. One of the most convenient ways is coating of the nanoparticle surface with a layer that can isolate of the core against the environment. Surface coatings with polymers, surfactants, or inorganic compounds may not only protect the nanoparticles, but also be used for further functionalization (An-Hui Lu and Ferdi, 2007). The requirements for the functionalization agents are mostly related to the properties they can introduce, environmental impact and the costs of the modification process. The use of biocompatible, long lasting, and abundant materials are prioritized.
Magnetite, or magnetic iron oxide Fe3O4, is one of the materials that have attracted the largest interest due to its favorable properties. It has low degree of cytotoxicity, even at considerably high loadings, making it a useful biocompatible material, especially in combination with surface coatings (Narayanan et al., 2011; Malvindi et al., 2014). Magnetite particles are inexpensive, chemically stable once formed, and are assumed to be non-porous (Cornell and Schwertmann, 1996). However, the synthesis of crystalline Fe3O4 is highly sensitive to pH and redox conditions and may lead to formation of non-magnetic hydrated Fe (II) and Fe (III) oxides (Jolivet et al., 1992).
Another interesting, and perhaps the most application-valuable, property of the magnetite is its superparamagnetic behavior. This means that the nanoparticles are strongly magnetic in the presence of a magnetic field, which do not show any residual magnetism when the field is removed and they can be immediately re-dispersed in solution. Superparamagnetic nano-scale aggregates made of iron oxides are also naturally present in paraffin wax deposits, and their presence can be used to control the physicochemical properties of crude oils (Lesin et al., 2010).
Transmission electron microscope (TEM) images of nanoparticles made of iron oxide Fe3O4 and silica coated iron oxide Fe3O4/SiO2 are shown in Fig. 1. The size of the bare Fe3O4 nanoparticles is in the range from 10 to 20 nm (Fig. 1A), and a typical core-shell structure for the silica coated Fe3O4/SiO2 nanoparticles can be observed from Fig. 1B.
 Fig. 1. TEM images of iron oxide Fe3O4 (A) and silica coated iron oxide Fe3O4/SiO2 (B) nanoparticles.
Fig. 1. TEM images of iron oxide Fe3O4 (A) and silica coated iron oxide Fe3O4/SiO2 (B) nanoparticles.The magnetic nanoparticles can be functionalized to target very specific applications and can be used in multiple fields, such as biomedicine (Chourpa et al., 2005; Gupta and Gupta, 2005; Jain et al., 2005), environmental separation (Kaur and Gupta, 2009; Reddy et al., 2016; Ngomsik et al., 2005; Leun and Sengupta, 2000), catalysis (Hermanek et al., 2007; Zahmakıran, 2011; Gross et al., 2012; Kirichenka et al., 2016), and even data storage (Galloway et al., 2013; Yurkov et al., 2002). It must also be mentioned that the introduced coating will change the nanoparticle size and affect certain properties, for example, magnetic response of nanoparticles with magnetic core. In case of petroleum production and processing, most of the attention has so far been given to the use of functionalized nanoparticles for reservoir mapping and magnetic nanofluids that can be used in drilling, completion and enhanced oil recovery (Cocuzza et al., 2012; Kapusta et al., 2011).
There is a large body of literature presenting detailed overviews of the properties, synthesis, and functionalization mechanisms of the magnetic nanoparticles and their applications in a wide variety of areas (An-Hui Lu and Ferdi, 2007; Gupta and Gupta, 2005; Reddy et al., 2016). The use of magnetic nanoparticles may contribute to solving certain production challenges by replacing, or being used in combination with the existing processes. In this review we will present the current status of nanoparticle-based separation solutions directly aimed at the petroleum industry. The article will also provide an overview of the magnetically driven nanoparticle recovery techniques, which are centrally important for applications on industrial scale in terms of time, costs and environmental implications. A short overview on the current knowledge regarding safe manipulation of the nanoparticles in work environments and potential risks related to iron oxide nanoparticles in particular is given in the last section.
2. Potential applications
Magnetically driven separation is one of the major potential application areas for nanoparticles and has received considerable attention in the past decades (Oberteuffer, 1974; Moeser et al., 2004). The majority of the separation approaches for magnetic nanoparticles follow a similar pattern and are schematically illustrated in Fig. 2. First, the nanoparticles are dispersed in a medium. Then, the targeted chemical, ion, surface, or functional group is adsorbed onto the nanoparticle surface. Finally, a magnetic field is introduced, and the nanoparticles, with the adsorbed material, are removed from the medium. The process is generally followed by recycling of the nanoparticles for further use.
 Fig. 2. Generalized scheme illustrating potential separation applications.
Fig. 2. Generalized scheme illustrating potential separation applications.In the following sections, the potential use of the magnetic nanoparticles for isolation of selected crude oil components, produced water treatment, emulsion destabilization, and other processes are presented. All of the presented applications, or processes, are related and can be driven in combination with each other. However, installation of additional equipment into existing production lines will be required.
2.1. Emulsion separation
Crude oils can exist in the form of a water-in-oil (w/o) or oil-in-water (o/w) emulsions. The type of emulsion depends among other things, on the ratio of the produced oil and water. These emulsions can be very stable due to the presence of interfacially active compounds, such as asphaltenes, resins, naphthenic acids, and solid particles (Kilpatrick et al., 2001). High stability of the emulsions may pose several problems both in upstream and downstream processes. Currently, several techniques, alone or in combination with each other, are used to separate water from crude oil. The methods vary in efficiency and costs and include thermal treatment, gravitational separation, filtration, membrane separation, chemical demulsification, and electrostatic destabilization (Abdurahman and a.M.N., 2010; Rodionova et al., 2014; Issaka et al., 2015). One of the most efficient methods is chemical demulsification. The principle is conventionally based on addition of surface active compounds that change the interfacial properties, such as interfacial tension, mechanical strength, elasticity, and thickness of the interfacial films, leading to enhanced droplet flocculation and/or coalescence in the emulsion. On the other hand, if magnetic nanoparticles are added to an emulsion, the demulsifying effect occurs due to droplets being covered by inerfacially active nanoparticles creating so-called Pickering emulsions (Wang et al., 2015; Józefczak and Wlazlo, 2015). Subsequently, an applied external magnetic field contributes to the droplet accumulation, coalescence, and separation. One of the most important properties for the nanoparticles in this application would be their interfacial activity, which is typically introduced by specific functionalization mechanisms.
The use of magnetic demulsifiers to separate water droplets from a diluted bitumen emulsion was demonstrated by Peng and co-workers (Peng et al., 2012a). Ethyl-cellulose-grafted nanoparticles (M-EC) were initially prepared using interfacially active ethyl cellulose surface modified nanoparticles. The M-EC were added into the naphtha-diluted bitumen emulsion and removed by a magnetic field. It was shown that addition of 1.5 wt % M-EC separated 2.5–25 wt % of the water, which corresponded to up to 93% water removal. The settling time was significantly shortened as a result of the magnetic field application. After 10 recycling cycles, the M-EC showed stable chemical structure and no significant drop in efficiency. In another study by Peng et al. (2012b) interfacially active bromoesterified ethyl cellulose was grafted onto the surface of amino-functionalized magnetite nanoparticles (M-EC) and used for separation of w/o asphaltene/toluene emulsions. It was shown that the obtained M-EC, unlike bare nanoparticles, could be well dispersed in the organic media as a result of the steric repulsive barrier created by the surface coating. The coating also resulted in 11% reduction of the nanoparticle saturation magnetization, but still left the M-EC sufficiently responsive to the magnetic field. The M-EC contributed to rapid phase separation of the emulsified water from the organic oil phase. Ali et al. (2015) also studied the possibility of using magnetic particles for dewatering heavy crude oil emulsions. An interfacially active poly(methylmethacrylate-acrylicacid-divinylbenzene) and composite were synthesized by soap free emulsion polymerization followed by a solvothermal process. The magnetic composite nanoparticles were introduced into the water/oil mixture and later separated by a magnetic field at 60 °C. The strong magnetic response of the nanoparticles at the oil/water interface enhanced the coalescence of the emulsified water, and a separation efficiency of 98% was achieved after 1 h. The nanoparticles were later recycled by solvent washing, and no change in the separation efficiency was registered after 5 cycles.
Another emulsion separation process is the removal of small amounts of oil dispersed in the water phase produced together with crude oils. Large amounts of produced water are generated as a result of the oil recovery, and it will only increase with time. The produced water is a complex mixture of highly dispersed micron-scale oil droplets, dissolved organic compounds, solids, gasses and salts (Neff et al., 2011). The produced water needs to be treated before it can be safely discharged or reused. Application of magnetic nanomaterials for waste water treatment from various chemical industries is widely discussed in the literature (Tiwari et al., 2008; Reddy and Lee, 2013; Dave and Chopda, 2014; Shen et al., 2009). Magnetic nanoparticles can potentially be applied for the removal of unwanted substances and oil droplets. The physico-chemical affinity of the nanoparticle surface to specific compounds, achieved via functionalization of the large available surface area, is an absolute advantage allowing to create a variety of multifunctional magnetic materials. However, a challenge of separating the nanoparticles from the aqueous solution may occur. According to Mandel and Hutter (2012), proper dispersion of the nanoparticles and avoiding their agglomeration are decisive for successful adsorption of the unwanted compounds from contaminated waters. However, the presence of ions in aqueous systems may prevent electrostatic stabilization of the nanoparticles even in well-dispersed solutions. For effective separation, strong enough magnetic fields also have to be used to overcome the Brownian forces often originated in the nano-dispersions. Kotsmar et al. (2010) investigated the stability of iron oxide nanoclusters in high salinity aqueous dispersions. The nanoparticle clusters were stabilized by citrate ions providing electrostatic repulsion. The obtained dispersions exhibited high stability at salt concentration 680 mM and pH values of 6 and 8. In a similar study, sulfonated copolymers were grafted on the surface of iron oxide nanoparticles to introduce steric stabilization of their dispersions. Here a long-term stability was achieved at both high salinity and temperatures up to 90 °C (Bagaria et al., 2013), conditions that can be encountered in subsurface reservoirs.
Most of the attention in this application of magnetic nanoparticles has been given to the removal of dispersed oil. A method to remove oil droplets from water by cationic surface-coated magnetic nanoparticles has been developed by Ko et al. (2014). Batch-scale adsorption experiments with 5 wt % decane-in-water emulsions were carried out. The reported removal efficiency of the decane droplets in the range between 85 and 99.99%. Modeling of the corresponding droplet settling process revealed that, for example, a 1 μm size droplet with 50% nanoparticle surface coverage in a field of 0.4 T sedimented with the velocity within the order of mm/s. One of the main factors affecting the settling velocity of the droplets was the distance to the magnet and the corresponding strength of the magnetic field. A continuation study by Ko et al. (2016) presented optimal operational conditions for oil droplet separation and suggested a possible mechanism of nanoparticle attachment to the oil droplets. Two types of nanoparticles were used for the treatment of 0.25 wt% o/w emulsions: amine-functionalized magnetic nanoparticles (A-MNPs) and polyacrylic acid (PAA) grafted amine-functionalized nanoparticles (PAA-MNPs). Positively charged A-MNPs was successfully attracted to the negatively charged oil droplets, and the oil removal ranged from 96.4 to 99.5%. The increase in the removal corresponded with the decrease in total acid number (TAN) of the dispersed oil droplets. The negatively charged PAA-MNPs did not contribute to the removal of oil droplets from the water phase, confirming that electrostatic attraction was the main mechanism behind the nanoparticle-droplet interaction. Modeling revealed that the effect of the magnetic field on the separation velocity depended on the nanoparticle size, with smaller size contributing to faster separation.
A process of separating oil droplets from the water phase was explored by Liang et al. (2015). Single-layer oleic acid-coated magnetite nanoparticles (@OA) were used as demulsifier for cyclohexane-diluted crude o/w emulsion. The concentration dependency, wettability of the @OA, and pH was studied as a function of the demulsification efficiency measured by the residual oil content. The increased concentration of nanoparticles in the sample contributed greatly to the demulsification efficiency and reached as high as 97%. The maximum demulsification efficiency was obtained for the nanoparticles with a contact angle of about 90°. No significant change in the demulsification efficiency was registered for the pH range of 4.0–7.5. However, a gradual decrease appeared in the pH interval between 8.0 and 11.0. As in the previous study, the nanoparticles were recycled 5 times with no loss of the original properties. pH-responsive hybrid magnetic nanoparticles for separation of diesel droplets from water were developed by another group of researchers (Wang et al., 2015). The four-step nanoparticle synthesis included silica and poly (2-dimethylaminoethyl methacrylate) (PDMAEMA) coating of the core creating a multilayer hybrid structure. The pH-responsiveness for the different batches of nanoparticles was introduced by the PDMAEMA and varied according to the length of the polymer chains. The nanoparticles coated with the longest polymer chains exhibited slower magnetic response in the optimal pH range.
2.2. Oil spill collection
Oil spills represent major environmental danger to our society and need to be contained quickly. The containment process can be difficult and depends on many factors, including the type of crude oil, the surroundings, and environmental conditions. The most common quick response methods for bulk recovery clean-up are barriers that concentrate the oil spills and skimming vessels designed for collecting the oil. The leftover contamination is typically low oil concentration appearing as 0.04–50 μm film on the water surface (Authority, 2014). To remove the traces of oil, inorganic, synthetic organic and natural organic adsorbents, or bioremediation are typically used (Adebajo et al., 2003; Hoff, 1993). Some water will unavoidably be taken out as a part of the oil collection process. This water is separated onboard of ships, purified, and then discharged into the ocean. This also requires additional post-treatment technology and equipment installation. Most of the typically used oil collection methods have limitations due to high costs, low separation efficiency, and a significant amount of time required for clean-up (Barron, 2012). Several research groups have investigated development of effective processes for oil spill cleanup involving functionalized magnetic nanoparticles or nanomaterials exhibiting increased oil sorption capacity. The hydrophobicity of the nanomaterial plays important role for this application as they have to be selectively distributed into the oil phase.
The first attempt to test ferromagnetic sorbents for containing the oil spills in laboratory conditions was made by Turbeville back in 1973 (Turbeville, 1973) and revealed high oil recovery rates as well as high-speed separation. More recently, Pavia-Sanders and co-workers designed hybrid magnetic shell cross-linked knedel-like nanoparticles (MSCKs) with hydrodynamic diameters of around 70 nm (Pavia-Sanders et al., 2013). The MSCKs were used for extraction of crude oil from aqueous environment and showed sorption capacity of 10-fold their dry weight. The reusability was also tested, and the nanoparticles were found highly effective even after undergoing chemical changes during the recycling process. The MSCKs also proved to be more than twice as efficient in terms of the loading capacity compared to the materials currently used for removal of oil contaminations. Atta et al. (2015) synthesized and characterized magnetite nanoparticles coated with rosin amidoxime. The nanoparticles exhibited antimicrobial activity and were tested for collecting a heavy crude oil sample spread over sea water at laboratory conditions. The best results showed 90% oil removal without nanoparticle precipitation in water, at a magnetite/oil ratio of 1/5. In another study by Yu et al. (2015) magnetic porous silica-coated submicroparticles (MPSS) with average diameter of 200 nm were used for adsorption of several oil samples. The MPSS showed fast magnetic response, superoleophobicity, and temperature stability for oil removal from the water surface with absorption capacity of the MPSS up to 11.5 times of their own dry weight. The submicroparticles preserved their hydrophobic properties after 20 recycling cycles and proved to be a promising adsorbent for the oil spill recovery. Another study, focusing on environmentally safe and low cost nanoparticle production conditions, was carried out by Mirshahghassemi and Lead (2015). The nanoparticles were synthesized by a hydrothermal method and coated with polyvinylpyrrolidone (PVP). A crude oil sample in synthetic seawater with and without Suwannee River fulvic acid (SRFA) was prepared by sonication to ensure uniform mixing of the components typically occurring in the top 5 cm of water following an oil spill. It was shown that the average median particle size increased from 11.2 to 16.1 nm as a result of oil adsorption. The PVP-coated nanoparticles adsorbed about 4 times their own weight of oil, but showed lower removal efficiency for long chain alkanesespecially at the highest SRFA concentration in the synthetic seawater solution.
Some studies have shown promising adsorption properties of nanocompositesor other materials prepared with incorporated magnetic nanoparticles. Magnetic nanobiocomposite material based on industrial waste collagen and superparamagnetic iron oxide nanoparticles was prepared and tested for selective oil absorption and magnetic tracking ability by Thanikaivelan et al. (Thanikaivelan et al., 2012). The fiber-like nanobiocomposite magnetic material absorbed oil up to twice its own weight. The oil containing material was later converted into magnetic graphitic carbon, which can be used in various applications. Surface modified floating macroporous materials were prepared and tested for adsorption of different oils by a group of researchers from Brasil (Carvalho et al., 2016). Silica-encapsulated iron oxide nanoparticles were thermally treated to produce macroporous material, which was further modified by octadecyltrimethoxysilane in order to introduce hydrophobicity. The porous structure, as well as hydrophobic properties of the material, contributed to increased oil adsorption.
Modified components from the crude oil itself were used by Abdullah et al. (2016) as an oil spill collector. Asphaltenes extracted from a heavy crude oil were sulfonated and applied as coating agents to create hydrophobic nanoparticles. Stable hydrophobic properties of the material as well as good dispersibility in non-polar solvents and the crude oil itself were confirmed. The nanoparticles were further tested for heavy crude oil collection and showed 90% oil removal at 1 to 25 magnetite to crude oil ratio.
2.3. Mitigation of undesired phase separation
A variety of compounds present in crude oils can contribute to deposit formation during production and processing operations. It can be desirable to prevent the formation of these deposits and thereby avoid flow assurance problems.
The injection of self-heating nickel-ferrite Ni nanoparticles has been proposed as a possible solution for gas hydrate formation problems (Bhatia and Chacko, 2011). The nanoparticles would be injected into a well causing a temperature increase and subsequently lead to disturbance of the hydrate thermodynamic equilibrium. The laboratory experiments for the hydrate dissociation and methane release were performed on artificially synthesized gas hydrates. It was shown that the self-heating temperature of the Ninanoparticles was proportional to the frequency and the square of the magnetic field strength.
Another troublesome deposit that can be encountered in the production tubing is paraffin wax. Cobalt-nickel nanoparticles in combination with specifically developed polymers can be used for melting the wax deposits (Haindade et al., 2012). The experimental setup allowed for injection of the nanoparticles inside the annulus between the production tubing and casing. An external alternating magnetic field was then applied, causing the nanoparticles to vibrate and generate heat. The heat was transferred to the tubing where the wax deposits melted. The melted material was then produced together with the oil stream.
Naphthenic acids present in the crude oils can cause deposit formation, corrosion, emulsion stabilization, and pollution of the produced water. Existing processes for their removal have varying degrees of success and there is a need for more efficient and environmentally friendly methods to decrease the acidic content and improve the crude oil quality. Recently, we have designed and compared two types of amino-functionalized magnetic nanoparticles capable of selective extraction of naphthenic acids from both model and crude oil systems(Simonsen et al., 2017). The amino-functionalized samples showed about 2.7 times higher adsorption capacity compared to the silica-coated nanoparticles.
Removal of asphaltenes from heavy crude oils may improve their processing leading to reduced costs and increased energy efficiency. Six different types of nanoparticles (including magnetic oxides, , MgO, and NiO) were studied for adsorption and subsequent catalytic oxidation of asphaltenes (Nassar et al., 2011). Although and NiO nanoparticles exhibited the highest surface areas, the highest adsorption capacity was registered for. Asphaltene adsorption from model solutions was shown to be metal-oxide-specific and to depend on the type and strength of the interactions between asphaltenes and surface.
3. Magnetically driven nanoparticle recovery
Development of efficient recovery techniques for magnetic nanoparticles is central for their practical applications. The majority of techniques are based on the use of magnetic flux or field gradients. A variety of the methods that can be used for magnetic separation of nanoparticles from a liquid medium are discussed in the following section.
3.1. High-gradient magnetic separation
High-gradient magnetic separation (HGMS) is an analytical technique used to isolate magnetic species from a nonmagnetic environment utilizing column flow. It was originally proposed for use in mineral beneficiation, water and waste treatment, chemical processing, and separation of micron-size magnetic particles (Oberteuffer, 1973, 1974). Fig. 3 shows the schematic principle of HGMS. The setup generally consists of a column packed with magnetically susceptible wires or spheres, which create large field gradients by dehomogenizing the magnetic field sent through the column, and subsequently trap the magnetic material (Gerber and Birss, 1983).
 Fig. 3. Schematic illustration of the HGMS column.
Fig. 3. Schematic illustration of the HGMS column.A variety of applications of the HGMS for separation of magnetic nanoparticles have emerged in the recent years. Parameters, such as magnetic field gradients, flow rates, nanoparticle sizes and concentrations, and pH of the carrying fluids, have been evaluated in detail through experimental work and computer modeling.
One of the first attempts was reported by Kelland (1998). The generation of 5–20 nm magnetic nanoparticles was separated by both matrix and continuous flow through an axial HGMS device at magnetic field strength of 0.01 T. The results confirmed the applicability of both methods in ferrofluid separation and in narrowing down the size distributions of the nanoparticles. Ravnik and Hriberšek (2013) studied the performance of a HGMS device in removal of polystyrene magnetic spheres from a laminar steady flow. Magnetisable wires in the separation unit were placed outside the separation channel, and an external nonuniform magnetic field was applied. A numerical algorithm was used for simulation of a dilute suspension flow. The particle collection efficiency increased with the magnetic field strength up to a saturation point and decreased linearly with the increasing flow rate. It was concluded that the designed HGMS device was not applicable for the high flow rates, but the number of parallel separation channels could be increased to reach optimum separation efficiency. Moeser et al. (2004) carried out an evaluation of the nanoparticle capture in a bench-scale HGMS column and developed a model describing the process. Polymer- and phospholipid-coated magnetite nanoparticles were dispersed in water (0.25 wt % Fe3O4), and the suspension was flushed through the column. The individual polymer-coated nanoparticles could not be permanently captured by the HGMS, in contrast to the phospholipid-nanoparticle aggregates where 87% capture was achieved. It was theoretically estimated that the individual nanoparticles with a magnetic core smaller than 20 nm could not be permanently trapped by the wires. The difference in the separation efficiency of the two nanoparticle types was attributed to the volume fraction of the magnetite. Capture of polymer-coated iron oxide clusters with diameters larger than 50 nm at high flow rates was studied by Ditsch et al. (2005). The recovery rates were higher than 99.9% and most of the losses were attributed to single nanoparticles with diameters smaller than 30 nm. A model was developed to describe the nanocluster flow through the column consisting of multiple wires. Fletcher et al. (Fletcher, 1991) also suggested that isolation of particles in magnetic field gradients <100 T/m could be limited to particles with diameters of 50 nm or larger. The predictions showed that movements of the particles with smaller diameters are dominated by Brownian motion, and that the drag force opposing particle motion cannot be overcome by the magnetic forces.
Most of the studies on the recovery of magnetic nanoparticles with the HGMS technique suggest that the process must be optimized for every specific application. The fine particles, or nanoparticles, must be passed through in large enough volumes and should also be initiated to form aggregates or clusters in order to be efficiently captured (Moeser et al., 2004; Ditsch et al., 2005).
3.2. Field flow fractionation
Field flow fractionation techniques have been used for decades for separation of macromolecules, solid particles, and even microorganisms (Schimpf et al., 2000; Katasonova and Fedotov, 2009). In combination with applied magnetic fields, they can be successfully applied for separation of both magnetic micro- and nanoparticles. Magnetic field flow fractionation (MFFF) has been reported as one of the most promising separation methods based on the application of external magnetic field to initiate the nanoparticle magnetization. Fractionation, in contrast to magnetic filters and centrifugation, is capable of separating the treated medium into several fractions. Fig. 4 shows an example of a MFFF separation principle. The sample is typically passed through a channel and separated as the magnetic field is applied perpendicular to the channel. Nanoparticles are separated depending on their size and composition.
 Fig. 4. Example of the MFFF separation principle.
Fig. 4. Example of the MFFF separation principle.Latham et al. (2005) studied the purification of maghemite γ- and cobalt ferrite Co nanoparticles with a capillary MFFF setup. The method allowed for successful separation of the nanoparticle mixture into two monodisperse fractions. It was suggested that the nanoparticles that interact strongly with the field are restricted to slower flow streams near the wall of the channel, while those with weaker interaction reside toward the center of the channel. A differential magnetic catch-and-release (DMCR) method was developed by the same group in order to separate polydisperse flow of magnetic nanoparticles according to their size (Beveridge et al., 2009). The DMCR combined elements of both chromatographic and magnetic fractionation techniques and resulted in efficient nanoparticle capture depending on their size, viscosity and velocity of the carrying phase as well as the magnetic field. Quadrupole magnetic field-flow fractionation is another method that was tested for purification of dextran-coated magnetic nanoparticles (Carpino et al., 2005). The system consisted of a channel placed inside a quadrupole magnet. It was concluded that the method can be used for successful separation of polydisperse systems.
3.3. Magnetic separation in electric fields
Electrical Field-Flow Fractionation is another promising technique that can be used for separation of mainly water/nanoparticle dispersions in narrow channels by application of an electric field. In this method, the magnetic nanoparticles are separated based on their electrophoretic mobilities and sizes.
Cyclical Electrical Field Flow Fractionation (CyEFFF) was used by Tasci et al. (2013) to separate gold and iron oxide surface-coated magnetic nanoparticles in alternating electric fields. In this setup, the oscillating voltages were applied to the electrodes resulting in a cyclical electric field and the particles moving back and forth inside the channel. It was shown that the CyEFFF was capable of separating 50 nm iron oxide nanoparticles with both lipid and polystyrene sulfonate coatings based on their electrophoretic mobilities. Dragiciu et al. (Draghici et al., 2010) manipulated the flow of aqueous solutions with bare and functionalized magnetite nanoparticles by an array of Cr/Au electrodes. The electrodes were used to apply 1.5–5 V voltage across the solution. It was observed that the nanoparticle movement from the inlet to the outlet reservoir could be controlled under direct, or in reversed polarization.
4. Potential environmental and health IMPACTS
The application of nanoparticles on industrial scale entails substantial material release into the air, water or soil, exposing them to people working on sites, occupants of the territory as well as animal life (Nowack and Bucheli, 2007). The currently available toxicology reports and studies evaluating the potential short and long-term adverse effects of nanoparticles on the human health and environment are rather few and do not always provide the complete outlook due to lack of reliable information about particular nanomaterials. One of the major problems with the use of nanoparticles is their lack of stability, which can lead to oxidation, reduction or dissolution of the material, and potential release of undesired components (Tejamaya et al., 2012; Thio et al., 2011). Strict safety measures and evaluation routines for working with synthetically synthesized nanoparticles during large-scale processes have to be developed to prevent their release and destabilization to avoid potential harm.
According to the current review, magnetic iron oxide nanoparticles in particular are some of the most relevant candidates for applications in the oil and gas industry. Therefore, it is interesting to review the toxicological aspects and potential risks related to their properties. As it was mentioned in the introduction, the iron oxide nanoparticles exhibit low degree of cytotoxicity compared to other materials, and can be considered biocompatible. Most of the literature on the topic assesses the safety of iron oxide nanoparticles with respect to cellular/bacterial response and biomedical applications (Schlorf et al., 2011; Soenen et al., 2011; Calero et al., 2014). Calero et al. (2014) assessed safety of the nanoparticle-cell interactions based on iron oxide nanoparticles functionalized with three types of surface coatings introducing different surface charges: (3-aminopropyl-triethoxysilane (APS), aminodextran (AD), and dimercaptosuccinic acid (DMSA). The results confirmed low toxicity of the nanoparticles and also suggested that the amount of nanoparticles penetrating into cells depended on the type of coating. This effect can potentially be used to control the amount of substances accumulated by the cells. The effect of uncoated and polyvinyl alcohol (PVA) coated superparamagnetic iron oxide nanoparticles to cause arrest in cell life-cycles was studied by Mahmoudi and co-workers (Mahmoudi et al., 2009). Both uncoated and PVA-coated nanoparticles showed significant cell death and cell cycle arrest at the highest concentrations due protein attachment on the nanoparticle surfaces and subsequent formation of protein “corona”. However, the PVA-coated samples showed better biocompatibility due to a different protein-surface affinity. Baumann et al. (2014) related the toxicity and immobilization effects of iron oxide nanoparticle functionalized with four types of coatings to the nanoparticle colloidal stability and the release of ions from the core material.
A safety assessment of chronic oral exposure to low doses of iron oxide γ-nanoparticles in growing chickens was performed by Chamorro et al. (2015). The rather short 2 week-long study revealed that the ingestion of nanoparticles showed no toxicological symptoms on bird growth parameters, intestinal or hematological alterations. No nanoparticle accumulation in liver, spleen, or duodenum was registered. While a variety of studies report effects of the iron oxide nanoparticles on various types of human cells, no scientific data is currently available on the potential health effects of the short/long-term exposure in humans.
5. Summary
The synthesis of high-quality magnetic nanoparticles, covering a wide range of compositions and tailored properties, has grown in importance over the past decade as a result of high interest in the field of fundamental research, and potential technological applications.
An overview of separation applications where magnetic nanoparticles potentially can be used has been presented. Emulsion separation, oil spill clean-up, produced water purification, and removal of other unwanted compounds can all be accomplished by the use of functionalized nanoparticles. A majority of the sources in the literature holds iron oxide nanoparticles as the most promising material based on its low production costs, low toxicity, and convenient synthesis and modification routes. The reported synthesis and functionalization processes for such nanoparticles typically include 2 to 4 steps and can be carried out within a matter of hours. The separation/adsorption capacities for functionalized nanoparticles are around 90% in case of water separation from emulsions, 2–11-fold sorption by dry material weight in case of oil spill collection, and up to 99% for removal of oil droplets from produced water. The typical number of nanoparticle recycling cycles without change in the original properties and adsorption capacities varied between 5 and 20 depending on the surface coating and washing procedure. In most of the applications, nanoparticle interfacial properties as well as strong magnetic response played an important role in the efficient adsorption and rapid separation, respectively. The review of the magnetically driven separation technologies suggested that effective separation of the nanoparticles depends on their size or agglomeration degree, composition, and flow rates. Nanoparticle recovery as high as 99% could be achieved by optimizing the process and material characteristics.
It is important to prevent the irreversible consequences that exposure to nanoparticles can cause to human health and environment by studying all the potential risks prior to the applications of nano-scale materials. Moreover, establishing laws for regulation of nanoparticles release and potential exposure should be mandatory for every specific process. A general conclusion is that the risks related to nanoparticle exposure, or amount of accumulated material, are highly dependent on the type of the nanoparticle coating and other multiple nanoparticle properties.
Acknowledgment
The authors acknowledge the financial support from VISTA – a basic research program in collaboration between The Norwegian Academy of Science and Letters, and Statoil.