1. Introduction
Tissue repair and regeneration is highly dynamic and complex, yet relatively slower process [1]. Over the years, an enormous amount of research has been directed towards devising effective strategies to accelerate this healing process, establishing a separate domain of science – “Tissue Engineering and Regenerative Medicines (TERM)” [2], [3], [4]. TERM combines various biomaterials, cells, and engineering principles to fabricate tissue constructs that could assist in the healing process by providing an adequate microenvironment for cell growth and restoration of their functional performance [2], [3], [4].
One of the major considerations for developing an engineered tissue construct is to impart adequate bioactive properties that could potentially stimulate the regenerative process and that too in a tissue-specific manner. Among many such approaches, incorporation of growth factors, pharmaceutical compounds (antioxidant, anti-inflammatory, and anti-microbial), and various nanomaterials (such as bioactive glass, bioceramics, and metal/non-metal materials) have been employed widely [2,[5], [6], [7], [8]]. Despite several proof-of-concept studies, these approaches have their shortcomings, often limiting their clinical translation. The growth factor-based approaches are often linked with low stability and high costs [9,10], while pharmaceutical compounds and nanomaterials may raise cytotoxicity and even drug-resistance issues [11,12].
In this regard, an alternative strategy is to fully harness the therapeutic properties of crude herbal plant extracts. Plants, in fact, are employed for thousands of years to treat various diseases, such as osteoarthritis, ulcers, cancer, heart disorders, bone fracture, diabetes, and others [13]. In the past decade, researchers have made a conscious effort to incorporate herbal extracts into engineered tissue constructs owing to their bioactivities, environmental-friendly nature, potential non-toxicity, cost-effectiveness, and easy isolation procedures [14]. In particular, extracts with antioxidative, antimicrobial, and anti-inflammatory activities could prevent complications and delays in the tissue repair/ regeneration process. At the same time, those with pro-angiogenic potency and the ability to induce stem cell differentiation may promote regenerative outcomes [14], [15], [16].
With this perspective, this review summarizes the current state-of-the-art herbal scaffolds for tissue engineering applications. The review gears off with the introduction of tissue regeneration specific properties of the herbal extracts. Then, a glimpse of various biomaterials and fabrication strategies for preparing herbal scaffolds is provided. Later, the application perspectives, challenges, and prospects are discussed. We believe this review would contribute to a better understanding of current advances and available opportunities on the role of plant extract-loaded constructs in regenerative medicine.
2. Herbal medicine extracts: properties relevant to tissue regeneration
Plants/herbs have long been a part of traditional medicines all around the world. They contain a variety of phytochemicals such as phenolic compounds (e.g. phenolic acids, flavonoids and tannins), alkaloids, terpenes [17] and even functional phytohormones [18], which are known to exert, directly or indirectly, a multitude of healing effects. However, in the following sub-sections, we would limit our discussion to effects that have precise relevance to tissue engineering applications, such as antimicrobial, antioxidant, anti-inflammatory, pro-angiogenic, and stem cell differentiation.
2.1. Antimicrobial, antioxidant, and anti-inflammatory properties
As already stated, tissue healing is a highly complex phenomenon and factors such as oxidative stress, bacterial infections, and severe inflammation may critically delay the process [1,19]. Excessive generation of free radicals such as reactive oxygen species (ROS) causes detrimental effects on cellular viabilities [20]. Colonization by microorganisms, a major issue in tissue engineering, could lead to sepsis if left unattended [21,22]. In the presence of these free radicals and microbial infection, severe inflammatory reactions are generally observed at the surgical site, posing a big challenge in this field of research.
In view of that, pharmacological agents and/or nanomaterials have been incorporated into tissue-engineered constructs to confer antioxidative, antimicrobial and anti-inflammatory properties and facilitate tissue regeneration [5,6,[23], [24], [25], [26]]. Nevertheless, the usage of these components has some limitations, particularly associated with their complex isolation procedures, relatively low standardization of nanomaterial synthesis protocols, toxicological and immunological concerns, and increased risk of antibiotic resistance (with increasing use of antibiotics). Moreover, the long-term impact of nanomaterials, particularly on the environment and human health, is not thoroughly assessed [11,12].
In this regard, using herbal extracts could be a potentially safer alternative, mainly due to their natural origin and striking potencies to confer antioxidant, antibacterial, and anti-inflammatory activities. To exert potent antioxidant effects, the phytocompounds interact directly or indirectly with the free radicals [23], whereas anti-inflammatory effects of plant extracts are mediated by blocking the production of cytokines/chemokines and inhibiting the nitric oxide pathway [27]. As for the antibacterial activities of plants, phytocompounds work by disrupting the synthesis of important bacterial proteins, cell walls, and cell membranes. Besides, these molecules could also inhibit bacterial DNA replication and other vital metabolic pathways [28].
Here, it must be noted that these antioxidant, anti-inflammatory, and antimicrobial properties are not explicitly limited to a specific phytocompound; a synergistic effect is often exerted. For instance, the ethyl acetate fraction of Cissus quadrangularis L. was found to possess strong antioxidant potency and an effective antimicrobial activity towards Gram-positive bacteria such as Bacillus cereus and Staphylococcus aureus [29]. Similarly, Carica papaya leaves and pulps extracted using methanol and ethanol demonstrated high antioxidant and broad-spectrum inhibition of Gram-positive and Gram-negative bacteria [30]. Hydroethanolic extract of Nasturtium officinale conferred protection against Gentamicin-induced nephrotoxicity in Wistar rats due to its antioxidative and anti-inflammatory (via a decreased expression of Tumor necrosis factor α and nitric oxide) properties [31]. Antioxidant-rich extracts (methanolic and aqueous) obtained from fruits of Terminalia bellirica Roxb. were active against multiple drug resistant Acinetobacter spp., Pseudomonas aeruginosa, and Methicillin-resistant S. aureus [32]. Few other herbal extracts reported to exhibit such properties, include Moringa oleifera (aqueous leaf extract) [33,34], Tinospora cordifolia (aqueous extract) [35], Calendula officinalis (methanolic petal extract) [36]. Aloe barbadensis Miller (methanolic extract of gel powder) [37], Lawsonia inermis (n-butanolic leaf extract) [38], Camellia sinensis [39], and Zataria multiflora Boiss [40].
2.2. Pro-angiogenic properties
Angiogenesis is another factor that critically determines the quality and speed of reparative or regenerative processes in a tissue post-injury [10,41,42]. An early and rapid vascularization is desired to ensure adequate nutrient supply and oxygenation to the injured tissues, thereby preventing hypoxia development and subsequent induction of tissue necrosis [41,43]. Moreover, angiogenesis also plays a crucial role in the recruitment of stem cells and immune cells as well as various growth factors at the injured tissue site to synergistically promote the reparative process [10,44].
One of the most potent strategies to improve tissue angiogenesis is the administration of pro-angiogenic growth factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), and others [9,10]. However, despite being a widely explored strategy, its clinical translation is limited by the labile nature and short half-life of the growth factors, uncontrolled pharmacokinetics, risk of delivering supra-optimal doses, and high production cost [10].
In this regard, the use of bioactive plant extracts with pro-angiogenic properties could alternatively be used [15]. To date, various plant extracts have been reported to possess pro-angiogenic properties. For instance, hydroethanolic extracts of Dalbergia odorifera promoted the induction of angiogenesis in a dose-dependent manner (3–10 µg/mL) in transgenic zebrafish embryo models. Moreover, extract treatment was also able to restore the VEGF receptor kinase inhibitor II-induced blood vessel loss in vivo [45]. Oral administration of Angelica dahurica hydroethanolic root extract (1.1 mL/0.2 kg body weight) promoted wound healing in diabetic rat models with an evident increase in vascularization at the wound sites compared to the untreated group [46]. Aqueous root extracts of Rehmannia glutinosa (1.85 g/kg oral dose) improved the healing rates of foot ulcers in diabetic rat models with a significant increase in the number of capillaries at the granulation site in the treated groups [47]. Drynaria fortunei aqueous extracts treatment was also reported to promote angiogenesis both in vitro (human umbilical vein-derived endothelial cell (HUVEC) tube formation assay) and in vivo (Cultrex assay and CAM assay) [48].
In addition, extracts of other medicinal herbs, including Angelica sinensis (aqueous root extracts) [49,50], Carthamus tinctorius (aqueous flower extracts) [51], Pueraria thunbergiana (hydroethanolic flower extracts) [52], Achyranthese bidentata Bl. (whole root extracts) [53], Cyathula officinalis Kuan (whole root extracts) [53], Rubia cordifolia (whole root extracts) [54], Scutellaria baicalensis (aqueous root extracts) [55], and Astragalus membranaceus (aqueous root extracts) [56] have also been reported to exert potent pro-angiogenic effects in one or the other in vitro or in vivo models.
2.3. Stem/progenitor cell proliferation and differentiation promoting properties
Stem cell technology has already revolutionized the field of TERM due to their considerable self-renewal potency and ability to differentiate into multiple cell types, offering viable prospects in the repair of the injured tissues/organs [57,58]. Moreover, the use of stem cells also offers an opportunity to develop patient-specific regenerative therapies. Amongst many, mesenchymal stem cells (MSCs – derived either from bone, bone marrow, adipose tissue, or umbilical cord), and hematopoietic stem cells are the most widely evaluated stem cell types in vitro, in vivo, and even in the clinical phase trials [58]. However, (i) maintenance of the stemness and (ii) efficient guiding towards definite lineages, particularly under in vitro conditions, are the major challenges associated with this domain [59].
To address these issues, various recombinant cytokines and growth factors are used extensively, which faces limitations similar to those discussed in the previous section [59]. As an alternative strategy, crude plant extracts have been used for treating stem cells [59]. For instance, leaf (10 µg/mL) and root (5 µg/mL) extracts of Tinospora cordifolia and Withania somnifera, respectively, were reported to promote proliferation and significantly delay cellular senescence in the Wharton's jelly-derived MSCs [60]. Similarly, Alpinate oxyphyllae aqueous extract (100 µg/mL) enriched the MSC population via restoration of their stemness. Moreover, MSCs pre-conditioned with these extracts were able to rescue H9C2 cells from doxorubicin-induced cellular aging, more effectively compared to unconditioned ones [61]. Ginkgo biloba extracts (25 mg/L) also significantly reduced the senescence in endothelial progenitor cells [62]. Herbal extracts of Ginkgo biloba [63], Agastache rugosa [64], Symphytum officinale [65], Cissus quadrangularis [66], Cassia occidentalis L. [67], Drynaria fortunei J. Sm [68], Fructus Ligustri Lucidi [69], Dipsacus asper [70], and Morinda citrifolia [71,72], were shown to enhance osteogenesis. An augmented chondrogenesis in MSCs was linked to the treatment of crude extracts from plants like Arctium lappa L. [73], Cladophora glomerata [74], and Dioscorea opposita Thunb [75]. On the other hand, Angelicae Sinensis [76], Polygala tenuifolia [77], Zingiber purpureum Rosc. [78], Bacopa monnieri [79], and a combination of Panax ginseng - Polygonum multiflorum Thunb. [80], promoted neurogenesis.
These aspects, taken together, have immensely motivated researchers to incorporate plant extracts with such bioactive properties in engineered tissue constructs to enhance their therapeutic performances.
3. Herbal constructs: from the biomaterial perspective
Biomaterials are a major component of the tissue engineering domain [81,82]. These materials, on one hand, provide external support for cell attachment, proliferation, migration, and differentiation. On the other hand, they act as a carrier matrix wherein different bioactive compounds, including herbal extracts, could be loaded and subsequently delivered to the target site. The choice of biomaterial to design the scaffold and the selection of loaded bioactive molecules are two crucial parameters that determine the successful use of developed scaffolds for tissue engineering [81,83].
A wide range of natural and synthetic polymers have been explored in the literature to prepare herbal scaffolds [14]. Natural polymers are derived from natural resources and act as an attractive alternative to design scaffolds owing to their inherent biocompatibility, biodegradability, and bioactivity. Natural polymers include polysaccharides (e.g. chitosan, dextran, cellulose, starch, alginate, pectin, hyaluronic acid), proteins (e.g. collagen, gelatin, fibrin, keratin), and even decellularized extracellular matrices. However, low mechanical properties, undesirable contaminants and immunogenicity risk (associated with few of them), limited tunability aspects, and batch to batch variation in properties depending on the source used, are few disadvantages of these materials [83], [84], [85]. In contrast, synthetic polymers are developed in the laboratory and show various attractive features like low immunogenicity, defined composition, suitability for different chemical functionalization, tunable mechanical properties, and adjustable degradability. Examples of synthetic polymers include polycaprolactone (PCL), polyvinyl alcohol (PVA), polyethylene glycol (PEG), polylactic acid (PLA), nylon, and others. However, in the case of synthetic polymers, special attention needs to be paid during synthesis to control their chemical properties and avoid the formation of toxic byproducts upon degradation [83,84,86]. Nonetheless, different strategies such as blending of natural and synthetic polymers and/or incorporation of various additive materials (e.g. metal/non-metal micro- or nanoparticles) can be opted to achieve scaffolds with desirable biodegradability, bioactivity, and mechanical properties [87], [88], [89].
As a bioactive component, herbal extracts have received significant interest in designing engineered tissue constructs due to their attractive properties (as discussed in the previous section) [14,90]. These extracts could be incorporated either via direct blending in the polymeric/biomaterial-based formulation or post-loading in nano-delivery vehicles or immersion/layer-by-layer coating procedures [91], [92], [93]. The phytochemicals present in these herbal extracts could interact with the biomaterials via their polar and non-polar groups, establishing hydrophobic interactions, hydrogen bonding, and van der Waals interactions [90,94,95]. These interactions critically alter the physicochemical and mechanical properties of constructs as well as modulate the drug release profiles of loaded extracts. For instance, Biswas et al. fabricated PLA based constructs via the blending of Panchavalkala, a positively charged herbal nanodrug with particle size ∼40 nm. Homogeneous distribution of the nanodrug in the polymer matrix (observed via transmission electron microscopy (TEM)) and shifting of FTIR peaks associated with nanodrug as well as PLA clearly indicated a good interaction between the components. These interactions were accountable for a delayed drug diffusion from the constructs, thus leading to a sustained and controlled release [96]. In a similar manner, the interaction between the amine functional groups of chitosan with the components of Cassia fistula leaf extract, as confirmed through FTIR analysis, played an important role in determining the swelling behavior and release profiles of chitosan beads [97]. In another study, interactions between collagen, silica, and Cynodon dactylon extract were shown to be responsible for improved mechanical property and lower degradability of the scaffolds [98]. In addition, non-covalent interactions between fish scale collagen and Macrotyloma uniflorum extract components resulted in surface smoothening, reduction in porosity, and increase in the tensile strength and percentage elongation of the fabricated sponges [99].
4. Herbal constructs: different fabrication strategies
The design and processing of herbal constructs with optimal properties for their applications are essential for their success. In fact, depending on the application, different fabrication technologies can be selected based on the desired construct's performance in terms of morphology, mechanical properties, surface physico-chemistry and eventual controlled degradation and release rate of the herbs embedded within. To date, fabrication technologies, including solvent casting, lyophilization, hydrogel formation, electrospinning, and 3D printing, have been used for developing herbal constructs.
4.1. Solvent casting
Solvent casting is a method employed to create scaffolds based on the evaporative properties of some solvents. The polymer is first dissolved in a solvent having high volatility and then cast in a mold. A layer of material membrane adhering to the mold is formed once the solvent gets evaporated. This method is straightforward, simple, and inexpensive and does not need specialized equipment. However, toxic solvents are often used in this process, which may cause denaturation of proteins or other active pharmaceutical agents incorporated within. To avoid the retention of toxic solvent within the scaffold, they have to be fully dried using a vacuum desiccator.
Using this method, Daisy et al. fabricated graphene oxide (GO)/polyhydroxybutyrate/alginate nanocomposite films loaded with curcumin and Gymnema sylvestre extract. All materials were first dissolved separately in chloroform and then mixed. The solvent was evaporated, depositing the mixture into a Petri dish. Further, for extract loading, nanocomposite was dispersed in an aqueous solution containing extracts and then dried at 50 °C [100].
4.2. Lyophilization/freeze-drying
Lyophilization or freeze-drying is a low-temperature dehydration process [101]. At first, the solution is frozen at a low temperature (−70 °C to −80 °C). It follows a primary drying process, in which pressure is lowered (to a few millibars) through a partial vacuum, and the ice within the material is removed by direct sublimation. Finally, during a secondary drying process, most of the unfrozen water in the material is removed by desorption [101]. Porosity, pore sizes, and structures of the fabricated scaffolds largely depend on parameters of the starting polymer solution, such as the ratio of water, viscosity, and applied processing parameters [102,103]. Apart from being a relatively simple procedure, another advantage of this process is the elimination of several rinsing steps and stability of bioactive components (due to low-temperature processing) as dispersed water, and polymer solvents are removed directly. However, long processing time and risk of heterogeneous freezing, which affects scaffold homogeneity, are the major limitations of the process.
Using this strategy, pectin/sodium alginate biopolymeric dressing were fabricated by Sutar et al. Solution containing both the polymers and glycerol was crosslinked with calcium chloride, then froze and lyophilized. The scaffold was then coated with Croton oblongifolius extract by dipping method and vacuum drying. A porosity of ∼90% was achieved in the scaffolds [104]. In another study, Veerasubramanian et al. added Avena sativa extract within konjac glucomannan and keratin polymeric formulation. This solution was further crosslinked with sodium trimetaphosphate, dispensed onto Teflon molds, and lyophilized. Avena sativa extract incorporation significantly affected porosity (74% in respect to 88% without extract) due to the intercalation of pores by the β-glucan present in the extract [105].
Thongtham et al. opted for a slightly different strategy to incorporate Cissus quadrangularis extract into the lyophilized scaffolds. First, extract loaded nanoparticles were prepared using double emulsion technique with PCL and PVA as oil and aqueous phases, respectively. These nanoparticles were further added to the polymeric formulation containing chitosan, collagen, and hydroxyapatite, which was then cross-linked with glutaraldehyde and lyophilized. The use of this strategy significantly prevented initial burst release and prolonged the extract release rate. Scaffolds exhibited an interconnected porous architecture with pores of 90–100 µm [106].
4.3. Hydrogel formation
Hydrogels are crosslinked 3D networks made of hydrophilic polymer chains linked by hydrogen or covalent bonds, van der Waals interactions, or other physical or chemical bonds [107,108]. Thanks to their hydrophilic structure, they are capable of holding large amounts of water and can swell in aqueous media. Moreover, tunable biodegradability and mechanical properties, injectability, self-healing, and shear thinning properties could be added advantages of many hydrogels applied in biomedical research [109], [110], [111].
These hydrogel matrices can also be used for encapsulating synthetic drugs and/or herbal extracts. For instance, Theobroma cacao extracts were dispersed directly into the bulk Carbopol 940 hydrogels and applied for wound healing [112]. Alternatively, Pterocarpus marsupium heartwood extract was first loaded into chitosan nanoparticles (prepared by ionic gelation method using tripolyphosphate) and then blended in Carbopol 940 hydrogel. Encapsulation of chitosan nanoparticles in hydrogels ensured a sustained release of herbal extracts [93]. Similarly, in another study, Achyrocline satureioides herbal extract was loaded into the oil-in-water nanoemulsion. Further, the hydrogel containing loaded nanoemulsions were prepared using Carbopol® Ultrez 20, as a gelation agent. This hydrogel-nanoemulsion system exhibited relatively slower and sustained release kinetics as compared to the bare nanoemulsions [113]. A hydrogel formulation consisting of silk fibroin, chitosan, and β-glycerophosphate was also employed to load and deliver Dimocarpus longan seed extracts. Chitosan/β-glycerophosphate combination imparted thermosensitivity to the hydrogel system, allowing sol-gel transition at 37 °C while maintaining fluidity at a lower temperature for easy injectability at the tissue sites [114].
Despite the fact that hydrogels show great potential, they are still affected by many limitations [115]. Due to their low mechanical properties and their fragile nature, the feasibility of applying hydrogels in many biomedical areas is still limited. To overcome low mechanical properties of hydrogels, functional nanomaterials (like gold, silver, graphene, and titanium nanoparticles) have been incorporated to form composites [116]. In addition, novel hydrogel materials with more stable and stronger characteristics are needed and remain an important direction for future research.
4.4. Electrospinning
Electrospinning is a widely used fabrication method to produce fibers with varying diameters from nanometers to micrometers [117,118]. The main components of the electrospinning set-up are a collector, a syringe pump, and a high voltage power supply. When the high voltage (of the kV order) is applied, a potential difference is created between the needle of a syringe filled with a polymer solution and the collecting target. As the surface tension of the polymer at the needle tip is counter-balanced by localized charges generated by the electrostatic force, the droplet elongates and stretches, forming a Taylor cone. When the surface tension is overcome, a continuous polymer jet is ejected, which travels towards the collecting target [117,118]. Working on both, the solution properties like surface tension, conductivity and viscosity and the electrospinning parameters, such as tip-collector distance, applied voltage, solution flow rate, temperature, humidity, the type of collector (i.e., static, rotating drum), fibers with varying diameters can be fabricated [117], [118], [119].
Electrospinning is probably the most used fabrication method found in the literature to fabricate scaffolds containing medicinal herbs. To date, different methods for loading pharmaceutical agents, including blending, coaxial- and emulsion-based, into electrospun fibers have been opted [120]. In blend electrospinning, the bioactive agents are directly dissolved in the polymer solution. For example, Naeimi et al. blended Nepeta dschuparensis extract and honey into PVA and chitosan solution to prepare nanofibrous mats under 17 kV applied voltage. As compared to PVA/chitosan fibers (90–150 nm size), honey and herbal extract containing formulation resulted in larger fiber size due to increased viscosity [121]. In another work, Kalachaveedu et al. added different ratios and concentrations (10% or 20%) of extracts derived from 4 plants, namely Aristolochia bracteolate, Acalypha indica, Thespesia populnea and Lawsonia inermis in Guar gum-PVA solution and electrospun at 20 kV [122]. Here also, the addition of herbal extracts increased the size of the fiber. Besides, the fibers with higher extract concentration exhibited higher non-uniformity and breaks. Different from the simple blending strategy, the coaxial strategy uses a coaxial needle to obtain core-shell fibers. Recently, core-shell nanofibers were produced by Ramalingam et al. The shell was composed of PCL/gelatin/Minocycline hydrochloride antibiotic whereas the core consisted of gelatin/Gymnema sylvestre extracts [123]. The prepared fibers were smooth, continuous, bead-free, and had sizes around 300–450 nm. Thanks to the external protective shell, direct contact of biological agents in the core with the environment can be prevented, which could be beneficial when dealing with unstable pharmaceutical molecules. On the other hand, in emulsion electrospinning, either water-in-oil (W/O) or oil-in-water (O/W) emulsions are created to be electrospun encapsulating hydrophilic or hydrophobic agents in the polymer solution. With this strategy, the requirement of a special coaxial needle can be omitted. As regard to herbal scaffolds, emulsion electrospinning is yet not applied and provide avenues for future studies.
Electrospinning has been demonstrated as a simple, widely used, quick approach to fabricate many types of nanofibrous scaffolds. Nevertheless, it is challenging to construct scaffolds with complex structures utilizing this technique. Moreover, scaffolds containing a homogeneous distribution of pores are also hard to fabricate. Furthermore, the high voltage applied in this process may also affect the bioactivity of pharmaceutical agents, reducing their therapeutic efficiency [117,118].
4.5. 3D printing/additive manufacturing
In the last 20 years, additive manufacturing (AM) has imposed itself as one of the most appropriate methods for producing 3D scaffolds for tissue engineering applications [124], [125], [126]. AM consists of a variety of technologies to build 3D objects by adding layer-upon-layer of material, generating objects with specific geometries. 3D geometries are first designed using computer-aided design software, then the 3D model is read by the 3D printer as a build file of 2D layers to produce the final product [127,128]. AM can be classified into three different main approaches: (i) laser-based, (ii) nozzle-based, and (iii) printer-based. Owing to the computer-controlled nature of fabrication, scaffolds with customized shape and physicochemical and mechanobiological features can be printed with a high degree of automation, reproducibility, and good accuracy [129,130]. Moreover, it is possible to achieve variable features on the same object.
Recently, few of the studies have fabricated 3D printed herbal scaffolds. For instance, Satureja cuneifolia extract was blended with sodium alginate/PEG to develop scaffolds using extrusion 3D printing. After printing, scaffolds were crosslinked by spraying calcium chloride solution on them and further applied for diabetic wound healing [131]. A different AM technology was used by Robertson et al. to fabricate porous β-tricalcium phosphate (β-TCP). β-TCP powder was loaded into a binder jet 3D printer, printed, cured (at 175 °C), and sintered (at 1250 °C). Scaffolds were further coated with polydopamine and Cissus Quadrangularis extract and used for bone tissue engineering applications [132]. Baniasadi et al. developed 3D geometries by direct-ink-writing of TEMPO-oxidized cellulose nanofibrils (TOCNF) reinforced Aloe barbadensis Miller bio-hydrogels. Hydrogels showed good viscoelastic properties, which allowed extrusion of thin filaments through a 630 µm diameter nozzle. Samples were then soaked in calcium chloride solution for crosslinking. The 3D structures exhibited high wet stability and a porosity higher than 80% [133].
Despite the progress made with 3D printing technologies, few major challenges still exist in this domain. In the case of a multi-scale hierarchical structure, most extrusion-based 3D printing nozzles have limited printing resolution and could only mimic the tissue at a relatively low level. Therefore, advanced nozzles should be designed to print structures having a significantly smaller diameter without causing nozzle clogging. Alternatively, light-based printing technologies could allow us to achieve better resolution. However, in particular reference to herbal scaffolds, the application of 3D printing is still in its infancy and needs further in-depth investigation.
5. Tissue engineering applications of herbal scaffolds
With a clear understanding of the therapeutic activities of herbal extracts, biomaterials opportunities, and fabrication methodologies, we would now focus on the application perspectives of the herbal constructs. In particular, the combination of herbal extracts and biomaterials unites the advantages of both components to create bioactive scaffolds with better regenerative potential. Besides, biomaterials enable the control over the release of the loaded herbal extracts. Controlled release is crucial to ensure the presence of the herbal extracts for a prolonged period instead of rapid clearance from the target sites. Rapid clearance of the herbal extracts may lead to short-term improvement, and hence, a repeated administration of extracts might be needed to achieve adequate healing. However, frequent administration might cause discomfort and inconvenience to the patients as well as increase medical costs and reduce patient compliance. Last but not least, controlled release of extracts could also reduce the associated side effects [134,135].
In most of the studies, the herbal extracts are incorporated into the biomaterials to modulate the cell behavior and immune response as well as to prevent microbial infections. Apart from that, the addition of herbal extracts to the biomaterials might also alter the physicochemical properties, i.e., microstructure, mechanical strength, wettability, swelling ratio, biodegradation, porosity, morphology, thermal characteristic, etc., of the resulting constructs. Tuning the construct's physicochemical properties is of prime importance as they critically affect cytocompatibility and cell responses (including functionality) [136,137].
As the old saying (by Paracelsus) goes - “All things are poison, and nothing is without poison; only the dose makes a thing not a poison”, thus, the dose of herbal extracts incorporated into scaffolds must be taken into consideration. In general, the crude herbal extracts are added in a small amount (< 20% w/w). A higher concentration is often associated with undesirable cellular responses [138].
The key findings reported in various studies that incorporated herbal extracts to the biomaterials for tissue engineering applications have been summarized in Table 1. We have only listed papers published between 2016 and 2021.
Table 1. Summary of studies employing crude herbal extract-loaded constructs for tissue engineering applications.
| Application | Biomaterials and additives | Plant extract (solvent used) | Extract loading strategy | Additional drug loaded (if any) | Construct fabrication strategy | Cells | Therapeutic activity (evaluated in vivo) | Remarks | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Wound healing | PVA | Cordia myxa (L) (hydroethanolic) | Direct blending | Electrospinning | Human foreskin fibroblasts | Full thickness skin defect mice models | Potent antioxidant activity | [147] | |
| PCL, chitosan | Melilotus officinalis | Direct blending | Electrospinning | SNL76/7 fibroblasts | Potent antibacterial activity | [163] | |||
| Alginate, pectin | Croton oblongifolius (ethanol) | Coating | Lyophilization | Human dermal fibroblasts | Potent hemostatic and antibacterial activity | [104] | |||
| Carbopol gel, chitosan nanoparticles | Pterocarpus marsupium (water) | Loaded in chitosan nanoparticles | Hydrogel formation | Full thickness skin defect diabetic rat models | Potent antibacterial activity | [93] | |||
| PCL | Terminalia bellerica | Direct blending | Electrospinning | NIH/3T3 fibroblasts | Potent antibacterial activity | [164] | |||
| Chitosan | Aloe barbadensis Miller | Direct blending | Tetrasodium EDTA | Hydrogel formation | NIH/3T3 fibroblasts | Potent antibacterial activity | [165] | ||
| Alginate (top and bottom layers), chitosan (middle layers) | Aloe barbadensis Miller (middle layer), Dracaena cinnabari resin particles (in top layer) | Direct blending | Lyophilization (iterative) | Human foreskin fibroblasts | Full thickness skin defect rat models | Multilayer wound dressings developed. | [148] | ||
| PVA, chitosan | Nepeta dschuparensis (hydroethanolic) | Direct blending | Electrospinning | Burn wound rat models | Honey was also blended in the formulation | [121] | |||
| PCL, gelatin | Althea officinalis (water) | Direct blending | Electrospinning | L929 fibroblasts | [92] | ||||
| Alginate, PEG | Satureja cuneifolia (methanol) | Direct blending | 3D printing | L929 fibroblasts | Potent antibacterial activity | [131] | |||
| PLA, collagen | Aspalathus linearis (water) | Direct blending | Silver sulphadiazine | Electrospinning | Human keratinocytes | Potent antioxidant and antibacterial activity | [166] | ||
| Alginate, poly(hydroxybutyrate), GO | Gymnema sylvestre (hexane and methanol) | Immersion | Curcumin | Solvent casting | WS1 human dermal fibroblast | Potent antioxidant activity | [100] | ||
| Guar gum, PVA | Acalypha indica, Aristolochia bracteolata, Lawsonia inermis, Thespesia populnea (ethanol) | Direct blending | Solvent casting | Human MSCs | Full thickness skin defect rat models | Extracts from different plants were added in different ratios | [122] | ||
| PCL (bottom layer), PVA-carboxyethyl chitosan (top layers), simultaneously spun both formulation (middle layer) | Matricaria recutita L. | Direct blending (in PVA-carboxyethyl chitosan) | Electrospinning | Adipose-derived stem cells | Multilayer wound dressings developed. Potent antioxidant and antibacterial activity | [138] | |||
| Dimethylaminoethyl methacrylate, hyaluronic acid | Didymocarpus pedicellatus (hydroethanolic) | Direct blending | Hydrogel formation | Full thickness skin defect diabetic rat models | [152] | ||||
| PVA, gelatin | Carica papaya (acidified water) | Direct blending | Electrospinning | NIH/3T3 fibroblasts | Potent antibacterial activity | [167] | |||
| PVA, chitosan | Azadirachta indica (methanol) | Direct blending (in chitosan) | Electrospinning | Bi-layered scaffold. Potent antibacterial activity | [168] | ||||
| PCL | Gymnema sylvestre (methanol) | Direct blending | Electrospinning | Human dermal fibroblasts | Potent antibacterial activity | [169] | |||
| PCL, gum arabic | Calendula officinalis | Direct blending | Electrospinning | L929 fibroblasts | Potent antibacterial activity | [170] | |||
| PCL, Zein, gum arabic | Calendula officinalis | Direct blending | Electrospinning | L929 fibroblasts | Potent antibacterial activity | [171] | |||
| Carrageenan, chitosan capped sulfur nanoparticles | Grapefruit seed | Direct blending | Solvent casting | L929 fibroblasts | Full thickness skin defect rat models | Potent antibacterial activities | [149] | ||
| Chitosan (base), gelatin-PVA (top) | Lithospermi radix (hydromethanolic) | Direct blending | Electrospinning | L929 fibroblasts | Full thickness skin defect rat models | Bilayer wound dressing. Potent antibacterial activity | [172] | ||
| Alginate, PEGMA | Aloe barbadensis Miller, Moringa oleifera (water) | Direct blending | Lyophilization | Human dermal fibroblasts | Potent antioxidant, anti-inflammatory and antimicrobial activities | [143] | |||
| PCL-chitosan-PEO-keratin (shell), PEO (core) | Aloe barbadensis Miller | Encapsulation (PEO core) | Electrospinning | L929 fibroblasts | [142] | ||||
| Konjac glucomannan, human hair keratin | Avena sativa (ethanol) | Direct blending | Lyophilization | NIH/3T3 fibroblasts | Full thickness skin defect diabetic rat models | Potent antioxidant, antibacterial activity | [105] | ||
| Nylon 66 | Beta vulgaris (acidified hydroethanolic) | Direct blending | Electrospinning | Normal human epidermal keratinocytes | [173] | ||||
| Collagen, silica | Cynodon dactylon (hydroethanolic) | Direct blending | Lyophilization | NIH/3T3 fibroblasts | Full thickness skin defect rat models | [98] | |||
| Chitosan, PEO | Lawsonia inermis (hydroethanolic) | Direct blending | Electrospinning | Human foreskin fibroblasts | Full thickness skin defect rat models | Potential antibacterial activity. | [174] | ||
| Gelatin, PVA | Centella asiatica (hydromethanolic) | Direct blending | Electrospinning | L929 fibroblasts | Full thickness skin defect rat models | Potential antibacterial activity. | [175] | ||
| PVA, pectin | Hippophae rhamnoides (water) | Direct blending | Lyophilization | L929 fibroblasts | Full thickness skin defect rat models | [176] | |||
| Collagen | Coccinia grandis (water) | Direct blending | Lyophilization | NIH 3T3 fibroblasts, HaCaT cells | Full thickness skin defect rat models | Potent antibacterial activity. | [177] | ||
| Collagen containing gelatin-collagen microparticles | Calendula officinalis (hydroglycolic) | Dip coating | Lyophilization | Full thickness skin defect lagomorph models | Scaffold without extract performed better in wound healing | [150] | |||
| Nanoemulsion (oil phase: Egg lecithin, medium chain triglycerides; aqueous phase: polysorbate 80), carbopol® Ultrez 20 gelling agent | Achyrocline satureioides (hydroethanolic; added in oil phase) | Direct blending into nanoemulsion | Vitamin E (added in oil phase) | Hydrogel formation | Protection against oxidative stress generated by UVA/UVB light. | [113] | |||
| PVA, chitosan | Centella asiatica, Portulaca oleracea and Houttuynia cordata (water) | Direct blending | Electrospinning | Human patients with facial acne vulgaris | Potent antioxidant and antibacterial activity | [144] | |||
| Bone regeneration | PCL | Cissus quadrangularis (water) | Dip coating | Electrospinning | Human MSCs | Calvarial bone defect rat model | GO was added to dip coating solution | [91] | |
| Collagen, Hydroxyapatite | Punica granatum (hydroethanolic) or Myrciaria sp. (acidified hydroethanolic) or Vitis sp. | Direct blending | Hydrogel formation | NIH/3T3 fibroblasts | Potent antibacterial activity | [153] | |||
| Alginate, carboxymethyl cellulose | Spinacia oleracea (hydroethanolic) | Direct blending | Lyophilization | MG-63 cells | [154] | ||||
| Collagen-chitosan-Hydroxyapatite formulation, PCL nanoparticles | Cissus quadrangularis (hydroethanolic) | Loaded in PCL nanoparticles | Lyophilization | MC3T3-E1 murine pre-osteoblasts | [106] | ||||
| β-tricalcium phosphate | Cissus Quadrangularis (methanol) | Coating | 3D printing | Human fetal osteoblast cells | Distal femur bone defect rat model | Fabricated scaffolds were coated with polydopamine prior to extract loading | [132] | ||
| Chitosan, silk fibroin | Dimocarpus longan seed (water) | Direct blending | Hydrogel formation | MC3T3-E1 murine pre-osteoblasts | Thermo-sensitive gelation. Potent antibacterial activity | [114] | |||
| PCL | Cissus Quadrangularis (ethanol) | Coating | Electrospinning | Human MSCs | Fabricated scaffolds were coated with GO prior to extract loading | [178] | |||
| PCL-PEG-PCL | Elaeagnus angustifolia (water) | Direct blending | Electrospinning | Human dental pulp stem cells | [179] | ||||
| PDX | Aloe Barbadensis Miller | Direct blending | Electrospinning | SaOS-2 cells | [155] | ||||
| Chitosan, carboxymethyl cellulose | Cissus quadrangularis | Direct blending | Lyophilization | SaOS-2 cells | Potent stem cell differentiation | [180] | |||
| PLA, Hydroxyapatite | Equisetum arvense (hydroethanolic) | Direct blending | Electrospinning | Human Adipose-derived stem cells | [156] | ||||
| Chitosan, alginate | Pterocarpus angolensis (water) or Eucomis autumnalis (water) | Direct blending | Lyophilization | Porcine Adipose-derived stem cells | Potent cell differentiation and anti-inflammatory activity | [157] | |||
| Not specified | Cotton fabric base, micro-emulsion (oil phase: almond oil; aqueous phase: tragacanth gum; surfactant: Triton X-100) | Chamomile (hydroethanolic; added in aqueous phase) | Direct blending into micro-emulsion | Hydrogel formation | Potent antimicrobial activity | [159] | |||
| Chitosan, gelatin | Cinnamomum zeylanicum (ethanol) | Direct blending | Electrospinning | NIH/3T3 fibroblasts | Potent antibacterial activity | [160] | |||
| TOCNF | Aloe barbadensis Miller | Direct blending | 3D bioprinting | Efficient printability of bioinks | [133] | ||||
| PCL, PEG | Nasturtium officinale (ethanol) | Direct blending | Electrospinning | Human Adipose-derived stem cells | Potential antioxidant activity. | [162] | |||
| Alginate, cellulose nanofibers | Oryza sativa (water), Tinospora cordifolia (water) | Lyophilization | Potent antibacterial activity | [161] |
5.1. Wound healing
Wound healing is a complex process that can be divided into 4 phases, i.e., hemostasis, inflammation, proliferation, and remodeling, that are overlapping in space and time [139]. It involves the crosstalk and interplay of multiple types of cells and mediators. Hemostasis is important to stop the bleeding by forming a fibrin clot that also serves as a provisional matrix to support cell migration. The activated platelets release growth factors and cytokines, which recruit the neutrophils and macrophages to initiate the inflammation phase. These phagocytic cells engulf and remove the cellular debris and foreign particles. At the same time, the cytokines secreted by these immune cells intensify the inflammation, and the growth factors released induce the migration of keratinocytes, fibroblasts, and endothelial cells to begin wound re-epithelialization, granulation tissue formation, and angiogenesis, respectively, which are the hallmarks of the proliferation phase. Afterwards, the wound undergoes remodeling to remove excessive collagen and increase strength. However, the healed tissue never regains the strength of the original skin [140,141].
To date, many herbal extracts are known to be involved in one or more wound healing events, such as promoting blood clotting, fighting against infection, expediting wound epithelialization, correct redox imbalance, reducing inflammation, and enhancing angiogenesis without causing any cytotoxicity [[14], [15], [16],59]. Thus, herbal extracts are suitable to be incorporated into the scaffolds to fasten wound healing. Besides, synergistic effects of herbal extracts and scaffolds could prove vital to further expedite the wound healing process along with reduction in the associated risk of chronic wound formation [14].
From the literature search, herbal extracts were found to be more commonly used for skin tissue engineering compared to the engineering of other tissues. This might be driven by – (i) higher number of herbal extracts that have been found to promote wound healing traditionally and in the previous scientific studies, (ii) it is easier to prepare the engineered skin substitutes and test its safety and efficacy in vivo, (iii) high prevalence of skin injuries and the huge and urgent need for better therapy to treat acute and chronic wounds.
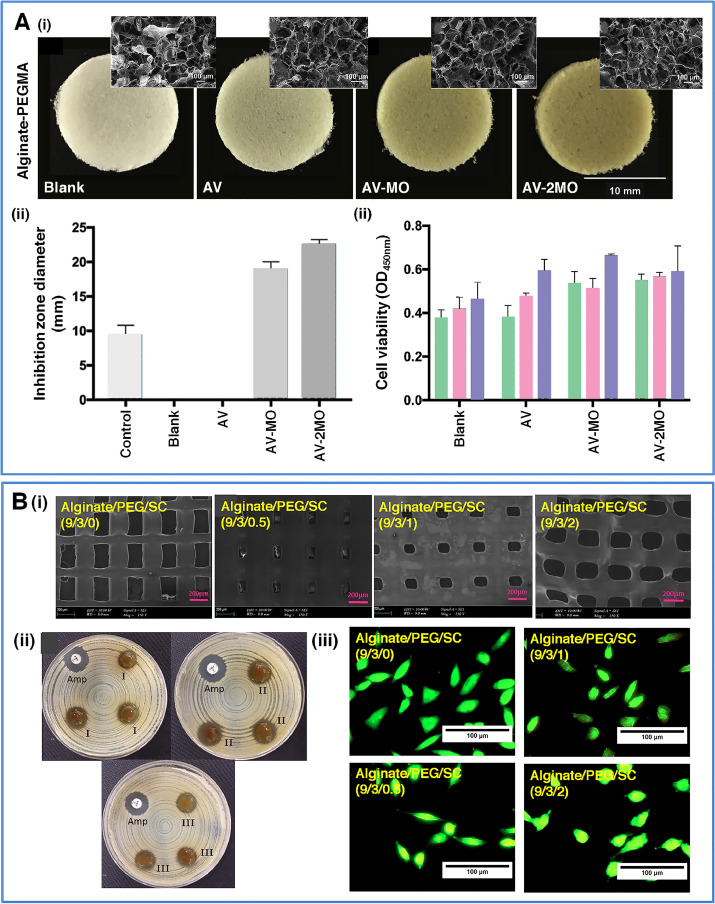
In a recent study, Zahedi et al. prepared electrospun PCL/chitosan/polyethylene oxide (PEO)/keratin/Aloe barbadensis Miller fibrous mats and coaxially electrospun mats with core (PEO/Aloe barbadensis Miller) and shell (PCL/chitosan/keratin) [142]. The core-shell fibrous mats exhibited better mechanical properties and supported higher adhesion of L929 murine fibroblasts compared to simple mats. Rubio-Elizalde et al. incorporated Aloe barbadensis Miller (to increase the wettability) and Moringa oleifera extracts (for antioxidative and antibacterial activities) to the lyophilized alginate/PEG-methyl ether methacrylate (PEGMA) scaffolds (Fig. 1A) [143]. Results showed that only the scaffolds with Moringa oleifera showed significantly higher ROS and nitric oxide scavenging capacity, as well as a potent antibacterial activity against S. aureus as compared to the blank scaffold. Importantly, all the scaffolds were not cytotoxic to human skin fibroblasts. In another study, the authors applied a 3D printing approach to prepare alginate/PEG scaffold supplemented with Satureja cuneifolia extract (Fig. 1B) [131]. Physicochemical characterizations showed that the 3D printed scaffolds with 2% extract (w/v) concentration had higher tensile strength, lower swelling ratio, and slower degradation compared to those with 0.5% and 1% extract. The printed scaffolds exhibited selective antibacterial activity against Gram-positive S. aureus but not towards the Gram-negative E. coli. Notably, extract-loaded scaffolds were biocompatible in nature; however, they supported a lower L929 murine fibroblast proliferation compared to the extract-free scaffolds. An electrospun PVA/chitosan fibrous patch containing extracts from Centella asiatica, Portulaca oleracea and Houttuynia cordata was shown to possess a strong antibacterial activity, especially against Propionibacterium acnes, while exhibiting only a mild vascular irritation in vitro [144].
 Fig. 1
Fig. 1