1. Introduction
3D printing is a cutting-edge technology that has transformed almost every manufacturing sector, including construction, food engineering, pharmaceutical sciences, and regenerative medicines [1], [2], [3], [4]. It has the potential to produce objects with desirable complex geometrical architectures in an automated and reproducible manner [3,5,6].
Among various 3D printing technology variants, extrusion-based printing is the most widely used technology due to its simplicity, versatility, and user-friendliness [7]. It involves the extrusion of printable material formulations, so-called 'printing inks or bioinks (cell-laden formulations) or food-ink (intended for food manufacturing)', as a continuous strand (via the use of pneumatic/piston/screw-driven systems) onto the substrate to form 3D structures in a layer-by-layer format [7], [8], [9]. Rapid and controllable printing procedure, ability to tune the characteristics of the printed objects (by simply altering printing speed, applied pressure, and nozzle diameter), the possibility of creating structures of desirable (or clinically relevant) sizes, and multi-material/cellular printing ability, are some of the major advantages offered by this technology [8,9].
Despite significant advancements in this technology, developing suitable printing ink formulations for a specific application continues to be a challenge [10], [11], [12]. In this regard, pectin is a relatively new addition to the list of natural and synthetic materials that are compatible with extrusion printing technology [10,[13], [14], [15]]. Pectin is a naturally occurring polysaccharide derived primarily from the peel of citrus fruits and apple pomace, both of which are food industry byproducts [16,17]. Pectin has already witnessed wide applicability in food and pharmaceutical industries as well as in tissue engineering, due to its biocompatibility, biodegradability, excellent gelling properties, tunable physicochemical characteristics, and functional modification potential [16,18]. Inspired by these aspects, recent efforts have been directed towards optimizing pectin-based inks for additive manufacturing [13,19].
In this mini-review, we provide an overview of pectin-based printing inks for 3D printing applications. We discuss various strategies opted to date for developing pectin-based inks, in terms of their composition, preparation, and characteristics. Furthermore, we present the current state of the art application of printable pectin-based inks in tissue regeneration and food manufacturing.
2. Pectin-based printing inks: composition and crosslinking optimization
By definition, printing ink is an aqueous/organic solution containing a single or a mixture of biopolymers capable of rapid gelation or thickening upon extrusion. When living cells are used as a component in printing ink formulations, they are referred to as ‘bioink’ [14]. Formulating an adequate printable ink is often a daunting task as several chemical, physical, and biological parameters should be considered, including printability, printing fidelity, biocompatibility and cell-instructive degree, rheological behavior and gelation mechanism, mechanical stability and ease of manipulation [8].
Among the printing ink features, viscoelastic property is a major factor governing the printability and shape stability of the printed objects, particularly in the case of extrusion-based 3D printing strategies [20]. Fluid-like inks, like those usually obtained in the case of single component pectin-based ink, are often not suitable for extrusion-based printing, as they could not retain their shape post-nozzle extrusion, and tend to flow out over time [21,22]. Thus, formulation composition and associated sol-gel transition parameters must be considered adequately.
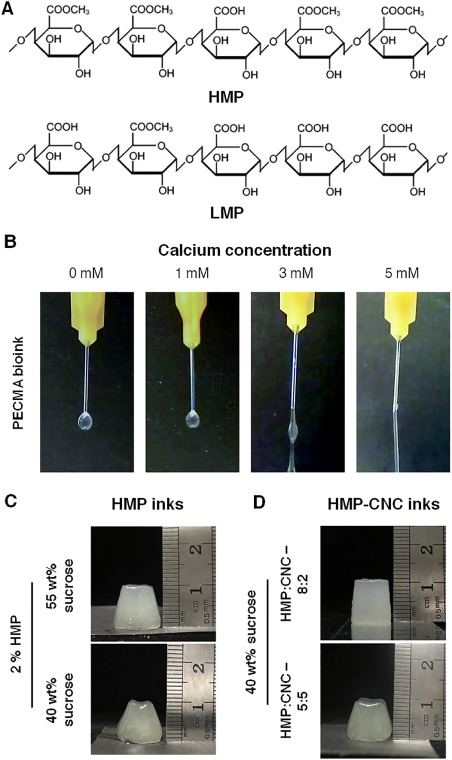
From a structural point of view, pectin is a polysaccharide composing of a linear chain of α-(1,4)-linked D-galacturonic acid units, which may be methylated [23]. Depending upon the degree of methylation (DM), it is usually classified as - high methoxyl pectin (HMP) and low methoxyl pectin (LMP) with DM values above or below 50%, respectively (Fig. 1A) [23]. This DM value has a profound impact on the functional properties of pectin or pectin-based formulations [23,24].
 Fig. 1
Fig. 1In the context of 3D printing pectin-based inks, some key parameters need to be carefully evaluated, including the DM, the overall ink formulation – blending with other polymers or with additive such as sucrose or nanocellulose – printing temperature, and the possibility to chemically modify pectin backbone to improve specific features of the printed object such as mechanical or cell-adhesion properties and/or ameliorate the crosslinking step (Table 1).
Table 1. Key parameters to be considered for the formulation of pectin-based inks for 3D printing applications.
| Parameter | How it influences 3D printing of pectin-based inks | How it can be generally controlled |
|---|---|---|
| DM value | Depending on DM value, various crosslinking strategies can be exploited |
|
| Crosslinking strategy | It affects the shape fidelity and stability of 3D printed objects. Depending on DM, pectin solutions can be solidified using divalent ions (most popular), positively charged biopolymers (polyelectrolyte complex), hydrogen bonding and hydrophobic interactions in combination with high sugar content. |
|
| Rheological behavior | The rheological behavior of the inks is fundamental to enable proper extrusion and shape retention upon it. |
|
| Chemical structure of the backbone |
By introducing selected moieties, it is possible to: - improve specific features of the printed object such as mechanical or cell-adhesion properties; - achieve new crosslinking strategies – e.g., UV polymerization. |
|
First, one should take into consideration the DM of the pectin to be used as this strongly influences the selection of crosslinking strategy. LMP, in fact, has numerous negatively charged carboxyl groups, which can electrostatically interact with divalent cations and crosslink pectin chains to form hydrogel via an egg-box model-based gelation mechanism [25]. The divalent cation used for ionic crosslinking of polymers is determined primarily by the following factors: cation-polymer or cation-water affinity, cation intrinsic toxicity, desired material properties and targeted application [26], [27], [28]. Amongst many, calcium (Ca2+) is the most exploited divalent cation used for pectin gelation [27]. As regard to printing inks, a recent study assessed the printability of the LMP (DM value – 21.7%)-based formulation in the presence of different calcium chloride concentrations (0 – 5 mM). The addition of low Ca2+ concentration, i.e., 0 or 1 mM, resulted in the formation of droplets at the nozzle and demonstrated fluidic nature. Ca2+ concentrations of 3 mM or higher, on the other hand, significantly increased the yield stress (3 mM: 1.18 Pa to 5 mM: 9.16 Pa) due to the ionic crosslinking of pectin chains. Noticeably, an irregular filament formed with 3 mM Ca2+ concentration, whereas a continuous filament was observed in the case of 5 mM Ca2+ (Fig. 1B) [29]. Alternatively, positively charged biomaterials such as chitosan can also cause the sol-gel transition of negatively charged LMP by forming polyelectrolyte complexes and have been successfully used in the formulation of 3D printable inks [30,31].
HMP, in contrast, gels via formation of hydrogen bonding and hydrophobic interactions between two pectin chains in acidic medium (pH < pKa) and in the presence of high sugar content (typically 55%) [23,24,27]. According to a study, single-component HMP ink with sucrose concentration of 40% or less exhibited fluidity and formed objects with irregular shapes, whereas those containing 55% sucrose content formed stable 3D printed structures (Fig. 1C) [24].
In addition, the formulation's composition also influences the printability and shape stability features. For instance, the incorporation of cellulose nanocrystals (CNCs) as additive in HMP-based ink with HMP:CNC ratio of 8:2 enabled the fabrication of stable 3D objects even with 40% sucrose content. The architectural features of the objects obtained with this composite ink were even better than those obtained with only HMP ink with 55% sucrose content, implying that sucrose requirements for gelation can be reduced in the presence of CNCs (Fig. 1D). However, changing this polymeric ratio to 5:5 or 2:8 severely harmed the object's appearance, emphasizing the importance of optimal HMP concentration [24]. In another study, using a temperature sweep test (ranging from 15 °C to 40 °C), the authors showed that the incorporation of different concentrations of LMP (0 - 2.5% w/v) in gelatin (5% w/v) increased the yield stress (from <5 Pa to >40 Pa in formulations containing 0 and 2.5% LMP, respectively). Furthermore, as LMP content increased, so did the sol-gel transition temperatures, which rose to around room temperature, i.e., 29.6 °C (2.5% LMP) from approximately 21.7 °C (0% LMP) [22].
Printing ink formulations based on pectin and Pluronic® F-127 have also been employed for printing 3D objects [32,33]. Due to the thermo-sensitive property of Pluronic® F-127, this blended ink remains liquid at a lower temperature (allowing for easy extrusion from printing nozzle) but gels on a printing bed maintained at 37 °C. However, structures printed with pectin/Pluronic® F-127 inks often exhibit low stability and may need further crosslinking with Ca2+ ions [32,33].
Other than pectin (or pectin-based blends), its semi-synthetic forms, such as pectin methacrylate (PECMA) [29] and pectin norbornene [34], have also been used for 3D printing applications. Due to their ability to undergo photocrosslinking, these materials offers several advantages, including higher resolution and better controllability over biochemical, mechanical, and biodegradability aspects of the printed substrates, which in turn offer tunability of cellular microenvironment [29,34]. Apart from that, these materials may also enable efficient printing with polymer concentrations as low as 1.5% w/v, which would otherwise be challenging [29].
Nonetheless, more extensive optimization and standardization studies are required to fully reveal the potential applicability of pectin in printing ink formulations, particularly those intended for bioprinting (either with mammalian or plant cells for tissue or food engineering, respectively).
3. 3D printed pectin-based constructs: application perspective
This section discusses the use of pectin-based inks for tissue engineering and food production. Table 2 contains a summary of all the relevant studies.
Table 2. Pectin-based, 3D printed constructs for tissue engineering and food manufacturing.
| Application | Printing method & system | Printing parameters | Printing ink formulation | Cells | Drug/protein loaded (if any) | Targeted tissue (if mentioned) | Remarks (if any) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Tissue Engineering | Extrusion (Regemat 3D V1) |
|
PECMA (varied degree of methacrylation – 21.7 – 56.6%), Cell adhesion peptide |
Human neonatal dermal fibroblasts |
– | Skin | Dual crosslinked network: ionic (CaCl2) and photo (Irgacure 2959) crosslinking. | [29] |
| Extrusion (BioBot1 Allevi 3D printer) |
|
LMP (DM – 18%), Alginate, Pluronic® F-127 | MIN6 cells | – | Pancreas | Structures with pectin maintained better viability of cells even under inflammatory conditions. | [40] | |
| Bioplotting (Ganbow Technology) |
|
Chitosan, Pectin | Mouse 7F2 osteoblasts | Pentoxifylline | Bone | Extrusion in NaOH bath for ink gelation. Osteogenic differentiation and maturation was evaluated. | [41] | |
| Extrusion (Regemat 3D V1) |
|
LMP norbornene (DM – 37%), Cell adhesion peptide, MMP-sensitive peptide |
Human neonatal dermal fibroblasts |
– | – | Dual crosslinked network: ionic (CaCl2 or BaCl2) and photo (VA-086) crosslinking. Choice of ionic crosslinker affected rate crosslinking, mechanical properties, and even cellular responses | [34] | |
| Extrusion (customized) |
|
LMP (DM – 36%), GPTMS | Human mesenchymal stem cells | – | – | GPTMS acted as crosslinker of pectin. | [21] | |
| Extrusion (Fab@home™ M3 bioprinter) |
|
LMP, Pluronic® F-127 | – | – | – | Dual crosslinked network: temperature induced Pluronic® F-127 gelation and ionic (CaCl2/oligochitosan) crosslinking. | [32] | |
| Extrusion (BioBot1 Allevi 3D printer) |
|
LMP, Pluronic® F-127 | – | – | – | Dual crosslinked network: temperature induced Pluronic® F-127 gelation and ionic (CaCl2/oligochitosan or chitosan) crosslinking. | [33] | |
| Extrusion (BioBot1 Allevi 3D printer) |
|
LMP, Pluronic® F-127, chitosan-coated gelatin/pectin microspheres | Mesenchymal stem cells | β-Estradiol (E2) loaded in microspheres | – | Dual crosslinked network: temperature induced Pluronic® F-127 gelation and ionic (CaCl2) crosslinking. | [52] | |
| Extrusion (BioBot1 Allevi 3D printer) |
|
Chitosan, Pectin | - |
Lidocaine hydrochloride |
Skin | Bioinks cooled to 25 °C to initiate sol-gel transition. Excellent flexibility was evident in printed structures post-lyophilization. Utility as a wound dressing. | [30] | |
| Extrusion (CELLINK® Inkredible) | – | HMP (DM ∼ 70–75%), Manuka honey | Human dermal fibroblasts | Propolis extract (incorporated as inclusion complex with β-cyclodextrin) | – | Potent antimicrobial effects observed. Honey improved the viscosity of inks. Utility as a wound dressing. | [45] | |
| Food Manufacturing | Extrusion (CELLINK® Inkredible) | – | HMP, Manuka honey | – | Cannabidiol (incorporated as inclusion complex with β-cyclodextrin) | – | Honey improved the viscosity of inks. Solid structured formed post-air drying. | [50] |
| Extrusion (customized) |
|
LMP (DM ∼ 12- 25%), Bovine serum albumin, Sugar | – | – | – | Pectin, Ca2+ crosslinker concentration and additives (like sugar and bovine serum albumin) governed structural characteristics | [48] | |
|
Extrusion (CNC Bench 3D 4046, GoCNC.de) |
|
LMP (DM ∼ 19%) | – | – | – | Printing method (simple/coaxial) governed structural characteristics | [49] | |
|
Extrusion (CNC Bench 3D 4046, GoCNC.de) |
|
LMP (DM ∼ 12%), Bovine serum albumin | Lamb's lettuce cell suspension | – | – | Inclusion of cells decreased mechanical and structural properties of printed constructs. Increased pectin concentrations negatively impacted cell viability. | [51] | |
| Not specified | Extrusion (FOODBOT-MF 3D printer, Shiyin Co. Ltd.) |
|
Buckwheat starch, HMP (DM ∼ 75%) | – | – | – | Optimization of bioink composition and printing parameters | [53] |
| Extrusion (SHINNOVE-S1, Shinnove Co. Ltd.) |
|
HMP (DM ∼ 65%) or LMP (DM ∼ 20%), Cellulose nanocrystals | – | – | – | Degree of esterification of pectin critically determines method of crosslinking, printability, and shape fidelity. | [24] | |
|
Extrusion (3D Discovery bioprinter, RegenHU) |
|
LMP (DM ∼ 12- 25%), TEMPO-oxidized cellulose nanofibrils | – | – | – | Optimization of bioink composition and printing parameters | [54] | |
| Extrusion (CELLINK® Inkredible) | – | Chitosan, HMP (DM ∼ 63- 66%) | – | – | – | Optimization of bioink composition and printing parameters | [31] |
3.1. Tissue regeneration
Tissue repair/ regeneration is a complex phenomenon and often needs multifactorial intervention to restore the injured/damaged tissues into their fully functional state [36,37]. In this regard, tissue engineering domain was introduced that combines the aspects of cellular biology, material sciences, and engineering sciences to generate tissue constructs for assistive repair/regeneration [36,38]. Among various strategies available for fabricating engineered tissue constructs, 3D printing, as current state-of-the-art technology, offers several advantages, including the ability to create complex and physiologically relevant geometries with good accuracy, high resolution, and reproducibility [9,10,39].
Recently, pectin-based bioinks have found their potential applicability for printing 3D constructs [13]. For instance, a single component RGD-conjugated PECMA (polymer concentration - 1.5% and degree of methacrylation - 21.7%) bioink laden with human neonatal dermal fibroblasts was used to bioprint 3D constructs for skin tissue engineering (Fig. 2A) [29]. The printed structures had a dual crosslinked network, which included ionic crosslinking with Ca2+ ions as well as UV light (7 mW cm−2) induced photocrosslinking using Irgacure 2959. This dual crosslinking allowed efficient printing of the 15-layered 3D structures of dimensions (17 (L) × 17 (W) × 2.4 (H) mm3. The bioprinted structures allowed the encapsulated fibroblasts to spread, proliferate, and secrete endogenous ECM matrix. Furthermore, the study showed that the swelling and mechanical properties of the printed constructs could be modulated simply by altering the polymeric concentration and photocrosslinking time, allowing us to tune fibroblast behavior.
 Fig. 2
Fig. 2Overcoming host immuno-rejection of constructs is one of the major challenges that limit the success of tissue engineering strategy. A potent strategy to address this limitation could be the use of semi-permeable immunologically isolated chambers and immunomodulatory biomaterials. Recently, alginate-Pluronic®-pectin bioink was used to print chambers for immuno-isolation of the pancreatic cells (Fig. 2B) [40]. Pluronic® improved ink printability and enabled the formation of micropores for efficient mass transfer while blocking the entry of host immune cells. Pectin, on the other hand, acted as an immunomodulatory material, inhibiting Toll-like receptor (TLR) 2/1 activation in THP1-reporter cell line while supporting the viability of MIN6 cells even under inflammatory stress conditions. When tested in C57BL/6 murine models, these acellular tri-component scaffolds exhibited good biocompatibility and supported neovascularization. However, since cell transplantation is often required to treat pancreatic diseases like diabetes, it would be interesting to further investigate the prospects of cell-laden pectin-based immunomodulatory 3D-printed constructs in animal models.
Incomplete and non-uniform crosslinking are common problem with bioprinted structures, including the pectin-based ones. Such issues limit the mechanical and biological performances of the bioprinted structures, necessitating the use of additional post-processing steps. Recently, for 3D printing, a newer single-pot procedure involving (3-glycidyloxypropyl)trimethoxysilane (GPTMS) crosslinker blended pectin inks was used [21]. When compared to a pectin-only formulation, the addition of GPTMS (0.984 g/g pectin) increased the viscosity and yield stress of the ink formulation by 40- and 6-folds, respectively. Shear-thinning behavior, combined with increased yield stress, ensured ready nozzle extrusion and shape stability of the printed structures. Post-printing, the structures were lyophilized and exposed to 37 °C temperature for complete crosslinking, resulting in interconnected micro- and macro-porous scaffold architecture. In vitro studies revealed that the scaffolds could support the growth of human mesenchymal stem cells for at least 10 days without compromising their viability. Furthermore, the authors also used this pectin-GPTMS printing ink formulation to create patient-specific complex anatomically shaped (nose and ear) constructs (Fig. 2C). Following a similar strategy, gelatin-pectin-GPTMS 3D printed woodpile-like constructs were fabricated and tested for biocompatibility with MG-63 cells [22]. This work adequately established pectin as a potent rheology modifier for gelatin-based bioinks for 3D printing of engineered scaffolds.
Pectin-based inks are also used in hard tissue engineering. 3D printed woodpile-like scaffolds were fabricated using a chitosan/pectin formulation directly in a sodium hydroxide coagulation bath [41]. Chitosan and pectin tend to form a polyelectrolyte complex, which allowed the researchers to achieve a higher wet compression modulus and a lower degradability of the scaffolds - highly desirable properties for bone regeneration. Moreover, chitosan/pectin printed scaffolds showed better compatibility with the 7F2 osteoblast cell line. When cells were subjected to the osteogenic culture medium for 21 days, they displayed higher alkaline phosphatase activity and mineralization than cells grown on only chitosan scaffolds.
Loading engineered tissue constructs with various bioactive molecules, such as growth factors, pharmaceutical compounds, phytochemicals, and others, is a potent strategy for improving their performance. When these bioactive molecules are released from constructs, they exert therapeutic effects such as antimicrobial, antioxidant, anti-inflammatory, angiogenesis-promoting, and others, thereby assisting in the tissue healing process [36,[42], [43], [44]]. A study used pectin-chitosan ink to create lidocaine hydrochloride-loaded wound dressing (Fig. 2D) [30]. The fabrication process involved grid patterning of the ink formulation, followed by lyophilization, while the drug remained entrapped in the pectin/chitosan polyelectrolyte network. The developed wound dressing was flexible and exhibited excellent skin tissue adhesive properties as well as a high potential to absorb wound exudates, both of which are necessary properties for effective wound coverage and keeping the wound moist. The wound dressing containing lidocaine hydrochloride demonstrated a burst release with approximately 60–70% cumulative release in the first hour. Such a release behavior would ensure immediate pain relief (via the action of lidocaine hydrochloride) at the wound sites. In another study, 3D printed bioactive films consisting of HMP, Manuka honey and cyclodextrin/propolis extract inclusion complex were fabricated [45]. These films had good mechanical, bioadhesive, and antimicrobial (against Escherichia coli and Staphylococcus aureus) properties. Moreover, they were biocompatible and promoted wound healing rates in vitro scratch assay conducted with human dermal fibroblasts. Notably, the aforementioned properties were highly dependent on the concentration of the inclusion complex in the ink formulation.
3.2. Food manufacturing
Similar to tissue engineering, 3D printing technology is also getting widely popular in food manufacturing for the creation of edible food products with complex geometries and in accordance with personalized nutrition requirements [2,46]. Several food-ink formulations have already been investigated for printing 3D food products [15,46,47]. In a similar vein, pectin, a natural plant material, has recently piqued the interest of researchers as a key component of food-grade inks.
In a recent study, LMP-based food-ink was formulated and optimized for the fabrication porous food products of different geometries like cube and teddy shapes (Fig. 3A) [48]. Partially crosslinked pectin inks (via the addition of 12.5 mM Ca2+ ions) exhibited viscoelastic properties and good printability; however, the printed objects had a low porosity (1.7%). However, when sugar syrup (50% v/v) was added to inks, the object's porosity increased to 3.1% and texture improved as compared to those obtained with 12.5 mM Ca2+ ion content. To further impart porous architecture to the objects, bovine serum albumin was added, allowing the air bubbles generated during the stirring process to be stabilized. These objects exhibited porosities as high as 21.9%.
 Fig. 3
Fig. 3Another study compared simple and coaxial extrusion printing strategies for LMP-based food product manufacturing [49]. In a former system, the pectin food-inks were first printed and then crosslinked with CaCl2 solution. The latter system, in contrast, involved coaxial extrusion of pectin inks (inner flow stream) and CaCl2 solution (outer flow stream). 3D cuboidal objects printed with either strategy were of high quality, with no internal defects. However, when compared to simple extrusion printing, coaxially printed objects had lower volumes and higher calcium content. Furthermore, despite having similar elastic properties, their failure behavior under high compression strain differed significantly. These differences in properties could be attributed to variation in gelation – structures printed via simple strategy, due to post-printing treatment, formed a continuous bulk material with homogenous crosslinking, while those printed coaxially exhibited weak inter-layer crosslinking due to partial ink gelation during the printing process.
To increase their nutritional/therapeutic values, 3D printed edible food products could also be loaded with various pharmaceuticals and/or different cells. For instance, food-inks containing pectin, Manuka honey, and cannabidiol/β-cyclodextrin inclusion complex were used to print 3D films [50]. The printed constructs demonstrated pH-responsive swelling release behavior, with a cumulative cannabidiol release of < 25% in simulated gastric fluid (pH 1.2) and simulated intestinal fluid (pH 6.8). However, in simulated colonic fluid (pH 7.4), a complete release of cannabidiol occurred via matrix dissolution mechanism, indicating that the developed constructs have colon-specific applicability. Such a system would ensure site-specific delivery of drugs like cannabidiol, preventing the risk of overdosing-associated side effects due to over-consumption of cannabis edibles. In another study, a cell suspension of Lamb's lettuce was loaded into pectin/Ca2+/BSA food-inks to print 3D cuboidal objects (Fig. 3B) [51]. As observed previously, the addition of BSA increased porosity and volume while decreasing mechanical properties of the printed objects. More importantly, the cells were uniformly distributed within the constructs and had a viability of 50–60%. The concentration of pectin used for printing had an effect on mechanical properties, porosity, and cell
 NHS chemistry.
NHS chemistry.