1. Introduction
In a chronic neurodegenerative disorder like Alzheimer's disease that accounts for millions of people worldwide having both early (4 to 5% in the age group of 30 to 60 years) and late onset, is believed to be primarily of genetic origin involving a number of genes [1]. In the early age group, different single gene mutation on chromosomes 21, 14 and 1 takes place resulting in abnormal amyloid precursor protein (APP), while at late onset, a single genetic risk factor, e.g., apolipoprotein E gene on chromosome 19 has the primary role to play [2], [3], [4]. Importantly, no drug treatments are available that can cure Alzheimer's disease, except for some medications that can alleviate symptoms or slow down its progression, e.g., cholinesterase inhibitors (e.g., donepezil, rivastigmine and galantamine) curb the breakdown of acetylcholine in the brain, and thereby aid in the treatment regimen of Alzheimer's disease [5], [6], [7], although all of them are associated with a number of side effects. Similar are the cases of deadly diseases like AIDS or cancer, where the ailing population is treated with a large number of drugs, leading to in situ toxicity and enveloping multiple drug resistance (MDR). A popular example is of human immunodeficiency virus (HIV) that develops MDR against antivirals, as it mutates rapidly under monotherapy. Cancer is another well known area where combination chemotherapy using neurotoxic drugs are the usual practice to avoid MDR [8].
In the above scenario, while relentless medical research is not only looking for possible treatment measures of some of the most widespread, deadly diseases of the current century, but also exerting a high level effort to eradicate the same by approaching and understanding its molecular mechanism. Interestingly, herewith we observe that in each of the above diseases the root cause is the genes and their expression [9], [10]. In this context, gene silencing is a procedure that refers to the ability of a cell for preventing the expression of specific genes as above at transcriptional or translational level [11], [12]. As it is evident, it is a molecular level strategy to selectively turn off specific genes in diseased tissues and hence, inhibit the progress of the same. It is the same as gene knock down, as, when silenced, its expression is not completely eliminated but reduced, by at least 70%. Hence, this approach provides a more complete view on the development of diseases, based on the genetic expression [13], [14], [15].
There are three major categories of gene silencing, a.) transcriptional b.) post transcriptional and c.) meiotic. Of these, transcriptional gene silencing (TGS) involves sequence-specific RNA degradation by post transcriptional gene silencing (PTGS) or decreased RNA synthesis by promoter methylation [16]. Meiosis refers to gene silencing by unpaired DNA, this in turn that DNA unpaired in meiosis causes silencing of all DNA homologues including genes that are themselves paired. Of the above, the post transcriptional gene silencing is the most widely adapted category of the process involving RNA interference (RNAi), which is a biological process encompassing the RNA molecules that inhibit gene expression, destroying the specific mRNA (messenger RNA) molecules in vitro [17], [18].
RNA interference therapy is primarily carried out using two types of small ribonucleic acid molecules, e.g., microRNA (miRNA) and siRNAs. In this, siRNAs and oligonucleotides are one the most powerful tools to combat against several diseases like cancer, acquired immune deficiency syndrome (AIDS), Alzheimer's etc. By binding with mRNA, the RNA fragments can suppress the expression of the protein sequence specifically [19], [20], [21], [22]. siRNAs produce RNA-induced silencing complex (RISC) after which the mRNA/siRNA duplex is degraded (Fig 1) [23], [24].
 Fig 1. Mechanism of action of siRNA conjugated nanoparticle
Fig 1. Mechanism of action of siRNA conjugated nanoparticlesiRNA forms tight complexes with the cationic nanoparticles via attractive electrostatic interactions [25]. During circulation and cellular internalization the siRNA must be dissociated from its cationic carrier before they are loaded into RNA-induced silencing complex (RISC) in the cytoplasm and initiate gene silencing. The physicochemical properties of the nanoparticle that dictates their molecular affinity to siRNA, can be altered by intracellular stimuli, such as change in pH in the endosome and cytosolic reducers, as a result the siRNA/polymer polyplex to disassemble. The specific changes which include the reduction of the cationic density and the molecular weight, and changes in the hydrophilicity/hydrophobicity of the nanoparticle siRNA complexes help in the dissociation of the polyplex. The protonation process and acid-responsive degradation within the endosome and glutathione (GSH)-mediated reduction in the cytoplasm, is the combination of the gradual stimuli-independent hydrolysis [25].
In last few years a number of research groups have focused on the utilization of this specific gene silencing mechanism [22], [26], [27] and in this regard, nanoparticles (NP) have further progressed largely. The half-life of the bare siRNAs in the systemic circulation is < 5 min but when it is modified, e.g., being encapsulated in liposome or tagged with cholesterol, its biological half-life enhances approximately by six times, e.g., to 30 min. However, once inserted in the cell, the intracellular half-life of the siRNA is 24 h, which is enough for gene silencing [28]. Among the other hurdles of siRNA therapy, the passage of the same through the blood brain barrier for treatment of Parkinson's or Alzheimer's diseases becomes the biggest challenge [29], [30], which can be readily taken care of by encapsulation of the siRNA within a nanocarrier. This serves a dual purpose of aiding a safe delivery of the encapsulated siRNAs to the target site in physiological condition, as, its bare counterpart readily degrades in the blood due to their fragile configuration [31], [32].
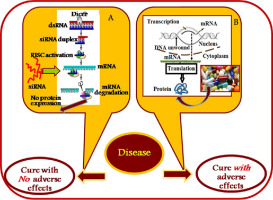
siRNA based gene silencing mechanism is a promising area of research as it aids in a novel treatment procedure by which it can selectively reduce the expression of the disease producing genes that lead to several incurable diseases including cancer, AIDS, Parkinson's, Alzheimer's etc., where the ordinary pharmaceutical formulations have normally failed (Fig. 2). Hence, herewith, the significance of the siRNAs is utmost, although, major challenges remain w.r.t siRNA stability and its delivery in vivo, including the need for their modification and their development as therapeutic agents [33]. In this regard, progress in nanotechnology has paved the way for leading to the development of efficient siRNA delivery systems, using a myriad of nanodelivery vehicles including polymeric, inorganic and organic nanoparticles [34]. In the present review, a detail discussion on the above category of nanoconjugate systems has been made, taking into account their possible application in the complete recovery and hence, eradication of the deadly diseases of the 21st century, e.g., AIDS, cancer etc. and thereby open up new directions in exploring the treatment procedure of the same.
 Fig. 2. Schematic representation of a comparative mechanism of action of A. siRNA and B. ordinary pharmaceutical formulations.
Fig. 2. Schematic representation of a comparative mechanism of action of A. siRNA and B. ordinary pharmaceutical formulations.2. Polymeric nanoparticles
Fig. 3 depicts the siRNA-nanoparticle (polymeric) conjugates related to chitosan, poly (ethyleneimine), poly(lactic-co-glycolic) acid, cyclodextrin, poly(ethylene glycol). Table 1 summarises the details of siRNA-polymer nanoconjugates.
 Fig. 3. Polymeric nanoparticles conjugated with siRNA, a.) chitosan, b.) PEI, c.) PLGA, d.) cyclodextrin, e.) PEG.
Fig. 3. Polymeric nanoparticles conjugated with siRNA, a.) chitosan, b.) PEI, c.) PLGA, d.) cyclodextrin, e.) PEG.Table 1. Details of siRNA-polymer nanoconjugates.
| Delivery vehicle | Disease | siRNA target site | References |
|---|---|---|---|
| Chitosan | Cancer | VEGF, RFP, EGFP, TNF-α, GAPDH, DPP-IV, Luciferase, TGFB1, TG2, RANK, EGFP, POSTN, FAK, PLXDC1 | [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48] |
| Chitosan | Antimalarial agent | AgCHS1, AgCHS2, Topoisomerase II | [49] |
| Chitosan | Brain diseases | P-glycoprotein | [51] |
| Polyethylenimine | Cancer | VEGF R2, BMI-1, luciferase, P-gp, WT1, TLR5, TLR7 | [54], [57], [58], [59], [60], [61], [62] |
| Polyethylenimine | Bowels disease | TNF-α | [56] |
| Poly(lactic-co-glycolic acid) | Cancer | STAT3, GFP, MBD1, luciferase, Cbfa-1, EGFP, BCL-w | [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [84] |
| Poly(lactic-co-glycolic acid) | HBV infection | HBsAg | [83] |
| Cyclodextrins | Cancer | RRM2, luciferase, GFP | [86], [87], [88] |
| Poly(ethylene glycol) | Cancer | VEGF, GFP, Luciferase, Bcl-2, EGFR, HIF-1α, MDM2, c-myc, CD13, KRas, MDR1, P-gp | [89], [90], [91], [92], [93], [96], [107] |
| Poly(ethylene glycol) | Nerve disease | NgR | [94] |
| Poly(ethylene glycol) | HBV infection | HBV | [95] |
| Dendrimer | Cancer | Bcl2, GFP, HSP27, TNF-α | [118], [119], [121], [122] |
| Dendrimer | HIV infection | Dicer substrate siRNAs | [110] |
| Other polymeric nanoparticles | Cancer | Plk1, Cy3, GFP, luciferase, Bfl1/A1, HIF-1R, EGFP, GAPDH, MDR-1, TNF-α, cyclin D1, BCL2L2, TRIB2 | [132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146], [148], [156], [158] |
| Other polymeric nanoparticles | Cardiac diseases | ERK | [136] |
| Other polymeric nanoparticles | Vascular dysfunction | Tie1, Tie2, VEcad, VEGFR-2, ICAM2 | [159] |
| Other polymeric nanoparticles | Plant | Cellulose synthases, NtCesA-1a, NtCesA-1b | [162] |
2.1. Chitosan
A polysaccharide based polymer, chitosan can be produced by deacetylation of chitin and from the structural element of the exoskeleton of crustaceans. Chitosan is found to be highly biocompatible and nontoxic for its biological existence [35], [36]. It is widely used as hemostatic agents in transdermal drug delivery on account of the versatility in properties. Further, the biopolymeric supramolecule can be derivatized as trimethyl chitosan, used as an efficient gene delivery.
In a report it has been found that poly-siRNA/glycol chitosan (psi-TGC) nanoparticle was used for silencing VEGF (vascular endothelial growth factor) and red fluorescent protein (RFP). They showed 95% suppression of VEGF in human prostate cancer cell line (Pc3) and 74% suppression of RFP in mouse melanoma cell line (B16F10) [37]. Another publication demonstrated knockdown of 70% of enhanced green fluorescent protein (EGFP) in H1299 human lung carcinoma cells by chitosan/siRNA containing sucrose as lyoprotectant [38]. They also silenced tumor necrosis factor (TNF-α) in RAW macrophage cell line by lyophilized chitosan/siRNA system [39] and GAPDH knock down was also found in H1299 cells. In another report 80% and 78% silencing was demonstrated of DPP-IV gene by siRNA/chitosan nanoparticle in HepG2 and Caco-2 cell lines [40], [41]. siRNA encapsulated nanoparticulate glycol chitosan polymer combined with polyethylenimine nanoparticles (siRNA–GC–PEI NPs) was also formed and treated in vitro using RFP expressing B16F10 tumor cell. Significant RFP gene inhibition was found in RFP/B16F10 bearing mice, due to high tumor targeting ability [42]. Luciferase gene and TGFB1 gene were also silenced by this nanoparticle [43], [44]. PEGylated chitosan was also used with luciferase siRNA that showed high stability in plasma [45]. Another study showed knock down of specific genes of proximal tubule epithelial cells of kidney and provided potential therapeutic strategy for several kidney diseases [46].
Among all the above work, the new area of siRNA delivery system is chitosan hydrogel (CH-HG) which can be directly injected in the tumor tissue. Han et al. showed the CH-HG comprising TG2 targeted siRNA significantly reduces the tumor growth in breast cancer (MDA-MB231) and melanoma (A375SM) [47]. Chitosan hydrogel/siRNA complex was synthesized with RANK siRNA and EGFP showed potential therapeutic approach [48]. Another novel tumor targeted delivery system is Arg-Gly-Asp-chitosan nanoparticle (RGD-CH-NP). Multiple growth-promoting genes (POSTN, FAK, and PLXDC1) were targeted by siRNA/RGD-CH-NP and found significant reduction of tumor growth [49].
Zhang et al. studied the siRNA/chitosan nanoparticles not only on human subjects and mice but also using Anopheles gambiae mosquito. They have silenced two genes by siRNAs responsible for chitin synthesis, e.g., AgCHS1 and AgCHS2 [50]. Another study was done on the antimalarial agent to cope with the drug resistance was a siRNA/chitosan nanoparticle targeting malarial topoisomerase II gene. There it was found that the parasitic growth of P. falciparum was inhibited significantly.
It is well known that there are several limitations of using bare siRNAs to hematopoietically relevant organs on account of their instability in blood serum, inability to cross cell membrane on account of their anionic nature, as well as, their rapid elimination via the renal system. Also, hydrodynamicdelivery of the same via high-pressure, high volume intravenous injection has very limited clinical use on account of severe side effects. Hence, to protect them against serum nucleases and to enhance their cellular uptake, a number of nanoparticles have been used as their vehicles/carriers, of which chitosan has been used by several researchers as a vehicle exhibiting significant stability of siRNAs in the blood [28] leading to protection of the same from degradation. Also, importantly, siRNA/chitosan nanocomplex can aid the passage of several drugs to the brain by suppressing p-glycoprotein expression in a blood-brain barrier model [51].
Despite chitosan nanoparticle has several advantages as a delivery vehicle, it exhibits a major disadvantage of allergic reactions when injected intravenously. Several researchers have shown that it can be nontoxic when used via oral route of administration [52]. The allergic response of this nanoparticle can be eliminated on conversion of this polymer into a monomer e.g., carbohydrate, amino acid etc. which does not lead to any hypersensitivity reaction when exposed to the circulatory system.
2.2. Polyethylenimine
Polyethylenimine is a type of polymer composed of amine group and two carbon aliphatic CH2  CH2
CH2 spacer. Due to its polycationic character it is used as a potent siRNA delivery vehicle [53]. Polyethyleneimine (PEI) can be PEGylated with Arg-Gly-Asp (RGD) peptide ligand which in turn can be used for delivering siRNA of vascular endothelial growth factor receptor-2 (VEGF R2).However due to successful delivery of this siRNA, it produced less angiogenesis and tumor growth, on account of a predominant role of VEGF R2 in the same [54]. In gastric carcinoma cells also CD44v6 was targeted by polyethylene glycol-polyethyleneimine (PEG-PEI) nanoparticle [55]. In bowels disease, TNF-α is the pro-inflammatory cytokine, the secretion of which can be controlled by siRNA/polyethyleneimine conjugate [56]. siRNA against BMI-1 was used in conjugation with polyethylenimine–laminarin complex and targeted to the breast cancer in vivo and in vitro [57]. Further modification of PEI was done on amines by ethyl acrylate, or introduction of negatively charged propionic acid and by acetylation of primary amines or succinic acid groups to the polymer structure. This modification showed higher level of gene silencing of luciferase gene and less cytotoxicity [58], compared to PEI before modification.
spacer. Due to its polycationic character it is used as a potent siRNA delivery vehicle [53]. Polyethyleneimine (PEI) can be PEGylated with Arg-Gly-Asp (RGD) peptide ligand which in turn can be used for delivering siRNA of vascular endothelial growth factor receptor-2 (VEGF R2).However due to successful delivery of this siRNA, it produced less angiogenesis and tumor growth, on account of a predominant role of VEGF R2 in the same [54]. In gastric carcinoma cells also CD44v6 was targeted by polyethylene glycol-polyethyleneimine (PEG-PEI) nanoparticle [55]. In bowels disease, TNF-α is the pro-inflammatory cytokine, the secretion of which can be controlled by siRNA/polyethyleneimine conjugate [56]. siRNA against BMI-1 was used in conjugation with polyethylenimine–laminarin complex and targeted to the breast cancer in vivo and in vitro [57]. Further modification of PEI was done on amines by ethyl acrylate, or introduction of negatively charged propionic acid and by acetylation of primary amines or succinic acid groups to the polymer structure. This modification showed higher level of gene silencing of luciferase gene and less cytotoxicity [58], compared to PEI before modification.
Navarro et al. have synthesized a novel conjugate with polyethylenimine (PEI) and phospholipid (dioleoylphosphatidylethanolamine,) DOPE for siRNA delivery. They have targeted P-glycoprotein (P-gp) of doxorubicin resistant MCF-7 human breast cancer cell [59]. The universal tumor antigen, Wilms' tumor gene 1 (WT1) was targeted by RNAi complexes formed by polyethylenimine (PEI-WT1) in lungs of mice with B16F10 lung metastasis. This study found that PEI-WT1 treatment is very effective for lung metastasis [60]. Cy3 labeled siRNA/tiRNA-PEI complex was used in targeted delivery in the liver of mice showed multi-targeted gene silencing [61]. Another research group showed that gene-specific silencing activity was done by TLR5 and TLR7 siRNA-PEI nanoparticles to transform tumor-infiltrating regulatory DCs (dendritic cell) into antitumor DCs [62].
An interesting comparative study was carried out on branched polyethylenimine (B-PEI) and linear polyethylenimine (L-PEI) that showed B-PEI to have a better cellular uptake and stability whereas L-PEI showed poor transfection efficiency [63]. Another comparative study was undertaken using monomer siRNA/L-PEI complexes and dimeric siRNA/L-PEI complexes, that exhibited a greatly enhanced cellular uptake and gene silencing in vitro in the case of the later, compared to the former [64].
The major limitation of this nanoparticle is its toxicity when used beyond a threshold concentration. Although, this limitation can be eradicated if a liposomal or any other biocompatible organic nanoparticle can be coated over this PEI nanoparticle.
2.3. Poly (lactic-co-glycolic acid)
Among the various types of nonviral vectors, the polymer, poly (lactic-co-glycolic acid) (PLGA), is found to be the most potent gene silencing vehicle [65], [66], [67], [68], [69]. For cancer immune therapy signal transducer and activator of transcription 3 (STAT3) by tumor-derived factors (TDFs) plays a vital role in dendritic cells (DCs). PLGA NPs containing siRNA polyplexes of PEI (PLGA-P) and PEI stearic acid derivative PEI-StA (PLGA-PS) was used for the knock down of STAT3. However PLGA-PS was found to release siRNA faster than PLGA-P. The silencing of STAT3 restored DCs by the upregulation of CD86 expression, large amount of secretion of TNF-R (tumor necrosis factor-receptor) and a substantial allogenic T cell proliferation. Further, PLGA encapsulation reduced the PEI associated toxicity as well [70]. Again STAT3 used with paclitaxel in conjugation with cationic polyethylenimine polymer (PLGA-PEI-TAX) showed promising result in cellular resistance in lung cancer cells [71]. EGFP-siRNA/PLGA was tested on H1299 cells which are stably expressing EGFP showed sustained release property of the siRNA [72]. GFP-siRNA–PLGA block copolymer was synthesized over which polyethylenimine was added and siRNA–PLGA/L-PEI micelles was formed that demonstrated superior gene silencing and cellular uptake [73]. GFP-siRNA was also conjugated with chitosan modified PLGA (CHT-PLGA) nanoparticles and tested on HEK 293 T cell line, that showed less toxicity [74]. MBD1 PLGA: Poloxamer nanoparticles (M-NPs) were used for potential therapy of pancreatic cancer [75]. Further, in prostate cancer, lipid coated PLGA nanoparticle was used with luciferase siRNA showed in vitro knock down of genes [76]. Another formulation was developed of PLGA-PEI nanoparticles using double emulsion-solvent evaporation technique, using luciferase as the oligonucleotide for silencing. Presence of PEI in PLGA nanoparticles led to approximately 2-fold higher cellular uptake of nanoparticles [77].
A formulation was developed as DEAPA-PVA-g-PLGA (diethylaminopropyl-amine-poly (vinylalcohol)-g-poly(lactide-co-glycolide) which was conjugated with GFP-siRNA and showed efficient delivery of siRNA within cell [78]. In another publication co-delivery of gene and siRNA was done using PLGA nanoparticle. coSOX9-pDNA/NPs and Cbfa-1-siRNA/NPs were used for co-delivery in vivo and in vitro [79]. Further modification in the formulation was done by using amine-modified-PVA–PLGA/siRNA nanoparticles, anti-luciferase siRNA was used in this system [80]. Specific formulation for inhalation was made by Jensen et al. [81]. The new spray dried anti-EGFP siRNA loaded nanocomposite microparticles have the potential use in inhalation therapy [81]. PLGA particles were incorporated with PEI produced another formulation where luciferase siRNA incorporation showed delivery of the siRNA [82] in a successful manner. In another publication, four formulations were developed and conjugated with siRNA. These are cationic PLGA–PEI, PLGA–chitosan and methoxy poly (ethylene glycol)–poly (lactide) (mPEG)–PLA/PEI, mPEG–PLA–chitosan nanoparticles. HBsAg (the surface antigen of the hepatitis B Virus (HBV) was studied and the mPEG–PLA–PEI nanoparticles mediated siRNA produced highest level of inhibition due to better characterization in terms of size and size distribution by laser scattering, surface charge by zeta potentialmeasurement, and surface chemistry by X-ray photoelectron spectroscopy (XPS) [83].
Andersen et al. have used polyethyleneimine (PEI) with a cetyl derivative to improve surface functionalization and siRNA delivery. The modified particle showed 2.6 times higher surface presentation of amines using the cetyl derivative compared to non-cetylated-PEI formulations and these particles successfully delivered siRNA in the murine macrophage cell line J774.1 and human osteosarcoma cell line U2OS. Anti-apoptotic oncogene BCL-w was silenced in these cell lines [84].
siRNA-PLGA nanoconjugate can be used for silencing the key mutated genes responsible for Alzheimer's disease, e.g., tau and amyloid precursor protein (APP), indicative of a great potential of the same for future prospects of treatment procedure of the above disease [22].
2.4. Cyclodextrin
Cyclodextrins are the group of compounds which are made up of cyclic polysaccharides. Due to its biocompatibility it is used widely with medicinal formulations. Cyclodextrin-containing polycations (CDP) are constructed with siRNA sequences targeting ribonucleotide reductase subunit M2 (RRM2) showed reduced tumor growth than non targeted nanoparticle given in the same dose [85]. Targeted delivery was achieved by β-cyclodextrin molecules enabling attachment with AD-PEG (adamantane containing polyethylene glycol). siRNA was loaded in this nanoparticle and luciferase reporter protein expression was knocked down in vitro [86]. A derivative of cyclodextrin, amino-β-cyclodextrin was used as siRNA delivery vehicle and the knockdown of green fluorescent protein (GFP) was achieved in vitro [87].
Being in use in medicinal formulations already, the above nanovehicle and its derivatives find huge prospects to be explored for delivery of siRNA in the treatment of some of the deadly diseases of the present times, under discussion.
2.5. Poly (ethylene glycol)
Poly (ethylene glycol) or PEG is a cationic polymer which can be used to prepare polyelectrolyte complex (PEC) micelles, with siRNA via a disulfide linkage (siRNA–PEG) by interacting with cationic polyethylenimine (PEI) which remains in the core. In prostate carcinoma cells (PC-3) vascular endothelial growth factor (VEGF) siRNA showed 96.5% silencing by VEGF siRNA–PEG/PEI PEC micelles [88]. PEGylated siRNA nanoparticle was tested in vitro and found to have controlled release siRNA GFP and luciferase [89]. GFP and VEGF were also conjugated with PEG and showed marked inhibition in MDA-MB-435 cells. These conjugates showed significant inhibition of IFN-α (Interferon-alpha) [90]. siRNA-PEG/NCs nanocomplex was transfected and showed cellular uptake and silencing of GFP and VEGF mRNAs [91]. Bcl-2 protein expression was also found to be down regulated in several cell lines by siRNA-PEG conjugate [92]. Polyethylene glycol-polyethyleneimine (PEG-PEI) complexed with nogo receptor (NgR) siRNA, a therapeutic target for CNS regeneration showed a potential use in neural regeneration [93]. Another PEGylated siRNA was synthesized and the anti-HBV siRNA showed its efficacy in vivo [94]. Another nanoparticle was obtained by mixing the carriers, e.g., DNA, protamine, siRNA, lipids, followed by further modification with polyethylene glycol and a ligand, anisamide. The targeted delivery showed ~ 70–80% of injected siRNA was accumulated in the tumor, ~ 10% was detected in the liver and ~ 20% recovered in the lung after the injection of the formulation in the xerograft model. siRNA targeted to epidermal growth factor receptor (EGFR) in the tumor which induced ~ 15% tumor cell apoptosis [95]. Efficient delivery of siRNA was done by monomethoxypoly (ethylene glycol)-poly(lactic-co-glycolic acid)-poly-l-lysine (mPEG-PLGA-PLL) triblock copolymers. Inhibition of GFP expression was found in human lung cancer SPC-A1-GFP cells [96]. Another triblock was synthesized which is poly (ethylene glycol)-block-poly(e-caprolactone)-block-poly(2-aminoethyl ethylene phosphate) (mPEG-b-PCL-b-PPEEA). This block polymer was complexed with acid ceramidase and luciferase siRNA which showed inhibition in acid ceramidase [97]. Another formulation was developed using PEGylated nanogel and quaternization of poly[2-(N,N-diethylaminoethyl) methacrylate] PEAMA moieties. It was complexed with siRNA of human surviving and luciferase gene showed subsequent endosomal escape in human hepatocarcinoma cell line [98]. Monomethoxy poly (ethylene glycol), poly (3-caprolactone) (PCL) and poly(2-aminoethyl ethylene phosphate) denoted as mPEG-b-PCL-b-PPEEA was formulated for safe delivery of siRNA. GFP-siRNA was used to study on HEK293 cells. Amphiphilic block copolymers poly(ε-caprolactone)-block-poly(2-aminoethylethylene phosphate) (PCL29-b-PPEEA21) and poly(ε-caprolactone)-block-poly(ethylene glycol) (PCL40-b-PEG45) was synthesized and conjugated with HIF-1α siRNA, that inhibited cell migration and angiogenesis in prostate cancer cell line (PC3 cell) [99]. Other nanoparticles (NP) were formulated using siRNA, a carrier DNA, a polycationic peptide, and cationic liposomes. The surface modification of the same was achieved using polyethylene glycol (PEG)-conjugated ligand, anisamide. The NP was presented to the target sigma receptor–expressing murine melanoma cells, B16F10. Moreover lung metastasis model was established by intravenous (IV) injection of the B16F10 cells and into C57BL/6 mice. A mixture of siRNA against MDM2, c-myc, and vascular endothelial growth factor (VEGF) co-formulated for targeting NP to the model for simultaneous suppression of these genes, resulting in lung metastasis to be reduced by 70–80%. When compared with the untreated mice, the survival time of the subject was prolonged by 30%, for the case of the treated subject. This targeted delivery showed less toxicity in the body [100]. Chen et al., produced LPD (liposome-polycation-DNA) nanoparticle, modified with NGR (aspargine–glycine–arginine) peptide, targeting aminopeptidase N (CD13) expressed in the tumor cells. c-myc siRNA formulation effectively suppressed the HT-1080 cells but not CD13-HT-29 cells and HT-1080 xenograft tumor. It showed partial tumor suppression and apoptosis [101]. Another targeted delivery was achieved by monodisperse ligand-PEG-siRNA conjugate. The folic acid targeting ligand was used and the NP showed specificity for folate receptor expressing cells [102]. In another report BCL-2 was silenced using triblock poly (amido amine)-poly(ethylene glycol)-poly-l-lysine (PAMAM-PEG-PLL) nanocarrier [103]. 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)] (DSPE-PEG) was synthesized and luciferase-targeted siRNA was complexed with it which showed enhanced cell binding and cellular uptake [104]. mPEG-DSPE-5000 conjugated with KRas siRNA tested in vivo toxicity and showed biocompatibility [100]. Targeted siRNA delivery was also done by folic acid-PEG-siRNA conjugate. Conjugate was shown to be internalised by folic acid receptor expressing cells [105].
Multidrug resistance is the major barrier for effective cancer therapy. Keeping this in view a nanoparticle was synthesized using P-glycoprotein (P-gp) targeted siRNA with poly (lactic acid) PLA–PEG-biotin conjugate. Efficient suppression of MDR1 gene e.g. P-gp gene was shown in this study in vivo in a mouse model [106]. PEG modified dendrimers have been used for protecting the siRNA molecule from degradation in one report and it has been seen that PEG modified dendrimer has high efficiency in in vivo delivery of GFP siRNA [107].
2.6. Dendrimer
Dendrimers are defined by three components: central core, interior dendritic structure and exterior surface with functional surface group. Polypropyleneimine (PPI) dendrimers were coated with poly (ethylene glycol) (PEG) and targeted with Bcl2 siRNA, and demonstrated cellular uptake and gene silencing in vitro on A549, A2780 and SKOV-3 cells and in vivo nude mice [108]. Cationic poly (amidoamine) (PAMAM) dendrimer was also used for siRNA delivery against humanized mouse model for HIV-1 infection. Dicer substrate siRNAs (dsiRNAs) were exhibited to suppress viral infection of HIV-1 [109]. In another publication PEG-conjugated PAMAM dendrimers (PEG-PAMAM) were synthesized and conjugated with GFP siRNA and the cellular uptake and gene silencing was studied in vitro and in vivo [107]. PAMAM dendrimers were demonstrated as an efficient siRNA delivery vehicle in PC3 cells by HSP27-siRNA. Further the same dendrimer has been used as a cocktail of dicer substrate siRNAs (dsiRNAs) which targeted HIV and cellular mRNAs which showed inhibition of HIV titer [110]. PAMAM dendrimers (G4 and G7) and dextran nanogels demonstrated TNF-α silencing in RAW 264.7 macrophages [111].
Despite the myriad of potential biomedical applications of dendrimers, previous research exploring the toxicity and biocompatibility of dendrimer based therapeutics suggests that some of them may display inherent toxicity, attributed to a combination of generation and charge [112], [113], [114], [115]. Although the mechanism explaining generational effects is still unclear, higher generation dendrimers have been observed to cause nanoscale holes in the cellular lipid bilayer resulting in higher toxicity. Some other researchers have found that higher generation attenuates higher toxicity [114], [115], [116], perhaps due to their conformational changes [112], [113], [114], [115], [116], [117], [118], [119]. Regardless of dendrimer generation, some cationic dendrimers have been shown to interact with negatively charged cell membranes [120]. An increasing number of studies using various cell lines have shown that exposure to cationic dendrimers results in cell membrane permeability, lysis and cytotoxicity [121], [122], [123], [124]. A comparative study on whole generation amine-terminated charged dendrimer and half generation carboxylic acid terminated dendrimer, undertaken using albino rats demonstrated more hemolytic toxicity in case of the former, obtained in the range of 15.3 to 17.3%. However, the PEGylated dendrimers showed decreased hemolysis of RBCs, to an extent of < 5% [125]. Moreover it was observed that galactose conjugated PPI (polypropylenimine) dendrimers were less hematotoxic compared to the parent PPI dendrimer and a significant reduction in cytotoxicity and hemolytic toxicity was observed for lactose and mannose conjugated PPI dendrimer [126]. In addition, various research on dendrimers exhibited significant decrease in RBC level after intravenous administration [127], [128]. However generation 5 PAMAM demonstrated compliment activation after 1 h of incubation at 37 °C, due to a net positive charge, exhibiting a reduction in hemolytic activity and hemagglutination has been seen after incubation of RBCs with dendriplexes for several hours [129]. Although dendriplex formulations are more suitable for intravenous injection, compared to cationic dendrimers alone, it is to be noted that dissociation of these complexes either in the pathway of the target or at the target site will discharge the individual constituents that themselves exhibit biological response [130].
2.7. Other polymeric nanoparticles
1,4-Butanediol (PbAE1) or 1,6-hexanediol (PbAE2) diacrylate-based polymers were formulated with siRNA conjugate and gene silencing of hepatoma cells and primary hepatocytes were studied and less cytotoxicity was demonstrated [131]. PEGylated anionic polymer targeted polo-like kinase 1 (PLK-1) oncogene and demonstrated the facilitated delivery of the siRNA [132]. In another publication Cy3-siRNA and GFP-siRNA were converted into polymeric nanoparticles and the stability of the siRNAs was studied [133]. Further, the cationic core and shell structure nanoparticles showed efficient delivery of luciferase siRNA [134]. Poly (acrylamidoethylamine) is another biocompatible material which is used for formulation of different types of cationic shell crosslinked knedel-like nanoparticles and linked with Cy3 siRNAs that showed good biocompatibility and cellular uptake in RAW 264.7 mouse macrophages [135]. The biologically active single walled carbon nanotubes (SWNTs) have been conjugated with poly (diallyldimethylammonium) chloride (PDDA) and hexamethylenediamine (HMDA), that demonstrated excellent gene silencing when it is tagged with signal-regulated kinase (ERK) siRNA on primary cardiomyocytes, although they exhibited negligible amount of cytotoxicity [136]. Poly (N,N-dimethylaminoethyl methacrylate) (PDMAEMA) is another type of polymer which was conjugated with GFP-siRNA and cellular cytotoxicity study was done [137]. Dimethylaminoethyl methacrylate copolymer is another efficient siRNA molecule (Cy3-siRNA, Bfl1/A1 siRNA) delivery vehicle which leads to silencing of specific genes on RAW 264.7 mouse macrophage cell line [138].
A comparative study was carried out using N-(1-aminoethyl)iminobis[N-(oleicyl-cysteinyl-histinyl-1-aminoethyl) propionamide] (EHCO) siRNA conjugate nanoparticle against two more conjugate systems comprising PEI/siRNA and TransFast/siRNA, that showed better biocompatibility of the EHCO/siRNA nanoparticle. PEGylation of this nanoparticle demonstrated reduced nonspecific cellular uptake in CHO-d1EGFP cells [139], The anti-HIF-1R siRNA tagging exhibited tumor growth inhibition in nude mice with human glioma U87 xenografts [140]. Cationic dextran nanogels (dex-HEMA-co-TMAEMA) are the other polymeric nanoparticles which is conjugated with luciferase and EGFP siRNAs and demonstrated gene silencing both in vivo and in vitro [141]. Hyaluronic acid (HA) based nanoformulations, conjugated with luciferase siRNA also showed their efficacy [142]. A study was conducted on multifunctional carrier (MFC), 1,4,7-triazanonylimino-bis[N-(oleicyl-cysteinyl-histinyl)-1aminoethyl) propionamide] (THCO) which was PEGylated and demonstrated higher transfection efficiency [143], compared to its non-PEGylated counterpart. Mono-cationic detergent (MCD) nanoparticles are produced in conjugation with siRNAs exhibited gene silencing in HeLa cells expressing luciferase gene [144]. Microsponges are another type of nanoparticle which consists of RNA interference (RNAi) polymers. Luciferase gene silencing was achieved in vivo using this polymer [145]. siRNA-encapsulated PCLEEP nanofibers are the nanocojugates that consists of the copolymers of caprolactone and ethyl ethylene phosphate. This complex silenced GAPDH (glyceraldehyde 3-phosphate dehydrogenase) by the encapsulated siRNAs [146]. Poly (amino ester glycol urethane)/siRNA polyplexes demonstrated luciferase silencing upto 92% in HT-1080 fibroblasts cells [147]. Poly(ethylene oxide)-modi Wed poly(beta-amino ester) (PEO-PbAE) and PEO-modiWed poly(epsilon-caprolactone) (PEO-PCL) nanoparticles are other two formulations that were developed and aided in efficiently silencing of multidrug resistance-1 (MDR-1) gene on SKOV3TR human ovarian adenocarcinoma cells [148]. Tamura and Yui had carried out a comparative study of different nanoparticles in delivery of siRNA, they formulated a polymer containing polyrotaxanes (PRXs), N,N-dimethylaminoethyl (DMAE) cyclodextrins (CDs) and poly(ethylene glycol) (PEG). They demonstrated that DMAE-PRXs render strong chemical binding with siRNA that leads to resistance in the exchange of anions, whereas 52CD-PRXs showed successful gene silencing of luciferase gene in HeLa cells [149]. In another study, an organic nanoparticle was synthesized where four helices in X motifs comprise siRNA, ribozyme, or aptamer with a central core of pRNA-X. An in vivo animal study proved that this nanoparticle with the ligand folate, after injection in the tail vein of mice can specifically deliver siRNA into the tumor and do not enter in the liver or any other organ [150]. Biodegradable polymerpoly(l-lactic acid) (l-PLA) and l-PLA-poly (ethylene glycol) (PEG) was used in a study and exhibited reduction in GFP signal in a transgenic mice model [151]. Silica-coated NaYF4 upconversion nanoparticles (UCNs) were synthesized which was tagged with folic acid and anti-HER2 (human epidermal growth factor receptor 2) antibody demonstrated cellular uptake and gene silencing of luciferase gene [152]. Biodegradable poly(amidoamine)s were used for synthesis of a nanoparticle which showed that 25% or 50% ethylene diamine (EDA) incorporation increases siRNA condensation and silencing of the EGFP gene [153]. Another monodispersed conjugated polymer nanoparticle PFNBr was synthesized which carried and efficiently transfected the siPlk1 into the PANC-1 cells [154]. Polo-like kinase 1(Plk1) siRNA was used in polymer which was produced by single-electron transfer living radical polymerization (SET-LRP) that showed no toxicity and aided in efficient delivery of siRNA [155]. Gelatin nanoparticles encapsulated tumor necrosis factor-α (TNF-α) and cyclin D1 (CCND1) siRNAs demonstrated high therapeutic potential in microspherebased oral systems (NiMOS) [156]. Silica coated upconversion nanoparticles (Si-UCNPs) demonstrated enhanced EGFP siRNA delivery in HeLa cells using NIR (near infrared) light [157]. Nanostructured poly-ε-caprolactone (PCL) scaffolds were used for encapsulation and delivery of siRNA for gene silencing of BCL2L2 and TRIB2 genes, in mesenchymal stem cells (MSCs) [158]. Low molecular weight polymeric nanoparticles were formulated by polyamines and lipids. These nanoparticles silenced five endothelial genes (Tie1, Tie2, VEcad, VEGFR-2, and ICAM2) in vivo which can be used for vascular dysfunction [159]. Human ovary cancer cell line SKOV-3luc expressing luciferase gene was transfected with siRNA conjugated polymeric nanoparticles comprising poly(lactide-co-2-methyl, 2-carboxytrimethylene carbonate)-g-polyethylene glycol-furan/azide, which demonstrated no associated cytotoxicity [160]. Polymeric nanoparticle was also conjugated with siRNA and targeted to intraperitoneal ovarian cancer cells in vitro and in vivo by HER2 antibodies [161].
Not only on human studies but also on plants the polymeric nanoparticles are used. Fluorescent conjugated polymer nanoparticles (CPNs) are tagged with siRNAs for cellulose syntheses, NtCesA-1a and NtCesA-1b documented that certain genes are responsible for cell wall regeneration in isolated protoplast [162].
Chitosan is a natural positively charged polymer which binds with the anionic siRNAs via electrostatic interaction to form siRNA-chitosan complex. PEI is a positively charged polymer which contain primary, secondary and tertiary amines, thus it produces complex with siRNAs which is characteristically known as “proton sponge effect”. Most of the polymeric nanoparticles are cationic in nature as a result they easily bind with the siRNAs which are anionic and produce a siRNA-nanoparticle complex.
The polymeric nanoparticles are highly biocompatible, biodegradable and mucoadhesive that enables these particles to be used in numerous pharmaceutical and biomedical applications, although, in general, stable suspension of these nanoparticles is difficult to be obtained on account of agglomeration of the same in aqueous medium.
Calando Pharmaceuticals, USA currently conducted a Phase I clinical trial for siRNA based therapeutic (CALAA-01) for the treatment of relapsed or refractory cancers. It is designed to target the M2 subunit of ribonucleotide reductase (RRM2), to inhibit tumor growth, and is encapsulated in cyclodextrin nanoparticle with the human transferrin (TF) protein where polyethylene glycol (PEG) has been used for enhancement of stability of the same [163].
3. Inorganic nanoparticles
Fig. 4 depicts the siRNA-nanoparticle (inorganic) conjugates, related to calcium phosphate, layered double hydroxide, gold, iron oxide and silica nanoparticles. Table 2 summarises the details of siRNA-inorganic nanoparticle conjugates.
 Fig. 4. Inorganic nanoparticles conjugated with siRNA, a.) LDH, b.) calcium phosphate, c.) Gold nanoparticle, d.) iron oxide nanoparticle, e.) silicon dioxide nanoparticle.
Fig. 4. Inorganic nanoparticles conjugated with siRNA, a.) LDH, b.) calcium phosphate, c.) Gold nanoparticle, d.) iron oxide nanoparticle, e.) silicon dioxide nanoparticle.Table 2. Details of siRNA-inorganic nanoparticle conjugates.
| Delivery vehicle | Disease | siRNA target site | References |
|---|---|---|---|
| Calcium carbonate | Cancer | VEGF-C | [164] |
| Layered double hydroxide | Brain disease | Htt | [165] |
| Calcium phosphate | Cancer | Luciferase, MDM2, c-myc, VEGF | [168], [172], [173] |
| Calcium phosphate | Bone disease | Osteopontin, osteocalcin | [169] |
| Gold nanoparticles | Cancer | GFP, luciferase, Plk1, EGFR, RRM2, cy5, GAPDH, VEGF, UBB, galectin-1, βgal | [178], [179], [180], [181], [182], [183], [184], [185], [186], [187], [189], [169], [192], [193] |
| Gold nanoparticles | HBV infection | HBsAg | [187] |
| Gold nanoparticles | Brain disease | DARPP-32 | [190] |
| Iron oxide nanoparticles | Cancer | BIRC5, GFP, Survivin | [195], [196], [197], [198], [199] |
| Silicon dioxide nanoparticle | Cancer | GFP, EGFR, P-gp, EphA2, luciferase, GAPDH, ATM, STAT3, GRP78 | [201], [202], [203], [204], [205], [206], [207], [208], [209] |
| Carbon nanotubes | Cardiac disease | ERK | [136] |
| Carbonate apatite | Cancer | ABC transporter, ABCG2, ABCB1 | [211] |
| Quantum dot | Neuro-degenerative disease | Cyclophilin B, MMP-9, BACE1, DARPP-32 | [212], [190], [215], [217] |
| Quantum dot | Cancer | HPV18 E6, HER2/neu | [214], [216] |
| Other inorganic nanoparticles | Cancer | GFP | [219], [220], [221] |
3.1. Calcium carbonate
This nanoparticle is an efficient delivery vehicle of siRNA, both in vivo and in vitro. Vascular endothelial growth factor-C (VEGF-C) siRNA conjugated with calcium carbonate nanoparticle demonstrated gene silencing in SGC-7901 cells and showed no obvious cytotoxicity [164].
3.2. Layered double hydroxide (LDH)
Ladewig et al. [166] have intercalated siRNA in the Mg-AlLDH nanoparticle, using siRNA-Htt sequence. NIH3T3 fibroblast cells and from the embryonic day 17.5 of (E17.5) C57Bl/6 mice embryos cortical neurons telencephalic lobes were dissected. Efficient delivery of the siRNA was studied by FACS, confocal and electron microscopy [165]. Double-stranded oligodeoxynucleotides (ODNs) was also loaded in Mg2Al(OH)6NO3–LDH and was transfected in human embryonic kidney cells (HEK293T) and the delivery was confirmed by western blotting [166]. The codelivery of drug and siRNA was achieved in MCF7, U2OS and HCT116 cell lines [167].
Although the area of LDH nanoparticles have been explored extensively as an efficient drug delivery vehicle, the prospect of using the same in molecular therapy is very less known, hence, there is a lot of opening in this area of research for treatment of various deadly diseases including cancer.
3.3. Calcium phosphate
Calcium phosphate (CaP) produces inorganic hybrid nanoparticle as it is a safe serum tolerable carrier system. Poly(ethylene glycol)-block-poly(aspartic acid) block copolymers, [PEG-P(Asp)] with CaP forms a potent transfection efficient nanoparticle. siRNA loaded in this nanoparticle targeting luciferase gene showed efficient gene silencing in HEK293 cell line [168]. Multi-shell (triple-shell) calcium phosphate-shRNA nanoparticles were prepared by a precipitation method was found to suppress the expression of osteopontin and osteocalcin proteins in the human osteoblast cells (HOB) [169]. Calcium phosphate nanoparticle with lipid coating (LCP) was synthesized for intravenous administration and used luciferase siRNA for silencing H460 cells [170]. Calcium phosphate/PLGA nanoparticle loaded with siRNA demonstrated no toxicity and excellent gene silencing in vitro in MODE-K cell line and in vivo, in intestinal inflammation of mice model [171].
However the targeted delivery of siRNA-calcium phosphate nanoconjugate remains a challenge till date. Yang et al. have developed lipid/calcium/phosphate (LCP) nanoparticles grafted with PEG and anisamide (AA) ligand for targeting sigma receptor expressed on B16F10 melanoma cells. This nanoparticle decreased luciferase activity in C57BL/6 mice in metastatic B16F10 tumor-loaded lungs by 78%. Moreover siRNA against MDM2, c-myc, and VEGF was coformulated in the targeted LCP-NP silenced the specific genes in tumor [172], [173].
The calcium phosphate nanoparticle with a bilayered coating of lipid (lipid calcium phosphate nanoparticle) can be extensively used as a nanocarrier for siRNA that might aid in inhibition/silencing of tyrosine kinase, a protein/gene constantly expressed in chronic myeloid leukemia (CML), which is one of the most dangerous type of blood cancer.