1. Introduction
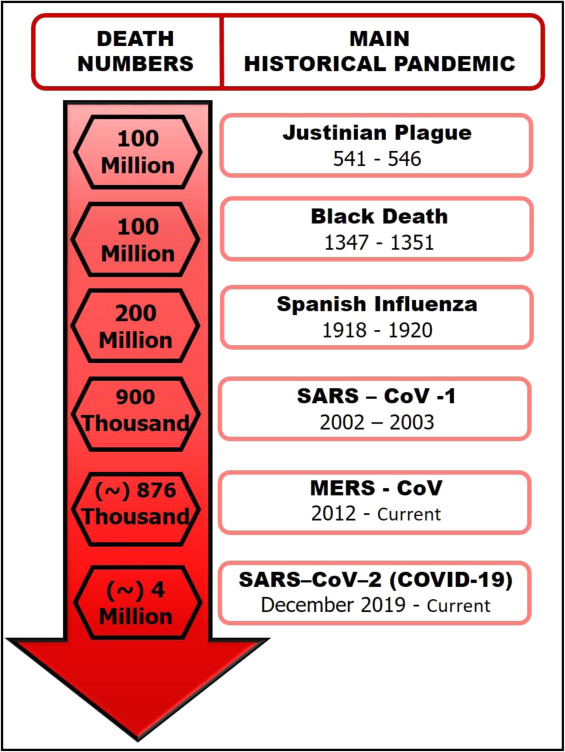
Since January 2020, the world has faced an infectious disease outbreak caused by a new zoonotic virus, SARS-CoV-2, responsible for the new coronavirus disease (COVID-19). Research has shown that emerging and re-emergence contagious diseases with pandemic potential have occurred regularly throughout human history (Fig. 1) since the Neolithic Era [1], [2], [3], [4]. A new infectious disease is often associated with the evolution of pathogens over time (genetic plasticity: mutation, re-assortment, and recombination) [5]. However, there are other conditions involved in the epidemic outbreaks [6], such as environmental (climate changes), ecological (deforestation), social (political and economic), and cultural factors (religion and eating habits) [7], [8].
 Fig. 1
Fig. 1Around 75% of epidemic diseases have a zoonotic origin (pathogens jump from a vertebrate animal to a human). Transmission can occur by direct contact with the animal, vectors (e.g., fleas, ticks, and mosquitoes), or indirect contact, such as food and water contamination [9]. Among the pathogens that managed to cross the species barrier (animal-human), about 44% have RNA viral origin [10]. Classic examples include H1N1, Hedra virus, Nipah virus, and human coronavirus (HCoV).
Although the response to epidemic outbreaks (restricted region) and natural disasters follow the same management cycle (mitigation, prevention, response, and recovery) [11], the equivalent does not apply in pandemic cases. Pandemic outbreaks present cascading effects, i.e., they do not occur as single events in a short time but in several waves over a long period until the entire population is immunized (vaccine or herd effect) [12]. During that time, there are constant changes; each new pandemic wave is different, as we can see in the current COVID-19 pandemic [13]. However, Health Agencies fight pandemics, taking into account each new stage change. The main ways used are information campaigns, social distancing, mask use, contact tracing, screening, quarantines, and rigorous mobility and travel restrictions [14].
The gradual return of each activity is significant and, probably, different for each region since the complexity and non-linearity (frequent waves) observed in a pandemic state can make it difficult to return to "normal" [12]. Therefore, the population must have a robust biosurveillance system with rapid identification of microorganisms and efficient diagnostic tools [7]. Thus, it would be possible to implement quick ways to measure biosafety and prevent/contain a possible epidemic outbreak. Some forms can be early detection of the disease, isolating and medicating the positive patient, tracking people who had contact with a positive patient, and promoting social distancing measures proportional to the risk of person to person transmission [14], [15].
The current SARS-CoV-2 pandemic has caused a “boom” in the diagnostic tools market. In 2020, regulatory agencies received hundreds of requests for clinical evaluation and formal approval of diagnostic tools for SARS-CoV-2. After a year of pandemic, such agencies have approved numerous molecular and immunological tests, but even so, many technical and operational problems are still reported [16], [17].
Success in controlling the COVID-19 pandemic is related both to the development of vaccines and mainly to the large-scale use of rapid and effective diagnostic tools. With the vaccines, it is possible to immunize the population, reducing cases of COVID-19. However, the satisfactory level of immunization is still unclear since further studies are needed to confirm the effect of protection against the COVID-19 variants [18], [19]. Thus, a rapid diagnostic remains the “key tool” for effective containment of the pandemic and has a high added value in reducing the damage caused by the future waves of COVID-19 [16].
This review describes the main features of the new HCoV, its origin, and possible factors related to the virus's emergence, known as severe acute respiratory coronavirus 2 (SARS-CoV-2), responsible for the current COVID-19 pandemic and millions of deaths around the world. It provides information about the standard tests used for diagnosis, recommended by the World Health Organization (WHO) and the Center for Disease Control and Prevention (CDC), as well as the efforts of the scientific community to improve the performance of those standard assays. In addition, it emphasizes the need to develop new diagnostic tools for fast viruses identification expanding the population's access to the early diagnosis, and avoiding virus dissemination. A promising candidate is electrochemical sensing platforms that offer benefits, including high sensitivity and selectivity, easy handling and interpretation (without specialized technicians), and easy execution in the field (without the need for laboratory structure).
1.1. Coronavirus
Coronaviridae, together with the Arteriviridae and Roniviridae families, belong to the order of Nidovirales. Coronaviridae family has two-virus genus, Coronavirus (CoV) and Torovirus. Four distinct genera are part of the CoV genus, known as alpha, beta, gamma, and delta; however, only alpha-CoVs and beta-CoVs infect mammalian hosts [20].
CoVs genome is composed of enveloped positive-sense RNA with approximately 30 kilobases (kb), carrying a leader sequence, ORF1ab, which encodes proteins responsible for viral replication and transcription and represents about two-thirds (67%) of the genome [21]. ORF1ab encodes, therefore, non-structural proteins (nsp) 1–16, including the RNA-dependent RNA polymerase (RdRp) complex [22]. The remaining third part of the genome encodes structural proteins: spike (S), envelope (E), membrane (M), nucleocapsid (N), and accessory proteins [23], [24]. S protein gives the spike-like projections on the surface of CoVs, conferring their distinctive aspect of a crown [21].
RdRp complex is a catalytic component composed of three non-structural proteins: nsp7, nsp8, and nsp12, responsible for cleaving ORF1ab. Nsp12 presents the active site, and the protein heterodimer nsp7-nsp8 activates nsp12. Therefore, the main component for RNA synthesis is such a protein set since RNA replication, and genome transcription occurs after cleavage of ORF1ab [25], [26].
Studies have shown that N and S structural proteins are closely related to CoVs pathogenicity. N protein plays an essential role both in viral genome packaging and viral transcription [27], [28]. In addition, researches have shown that N protein may also be associated with the host's immune response suppression, facilitating viral replication [29]. S proteins have two subunits (S1 and S2), with distinct functions. S1 binds to the cell surface receptor host through the receptor-binding domain (RBD) region present in this subunit. On the other hand, S2 mediates virus fusion with the host cell membrane [21].
Viruses with RNA genomes present high mutation rates due to peculiar features of RNA genomes not found in DNA genomes, such as high rates of replication, recombination, and segmentation in a short time. Thus, these features enable a rapid viral genome evolution, favoring variants emerging that can present new characteristics as virulence increases [30]. The CoVs, RNA viruses, have innumerable mutations, mainly in the S protein, which could not be different. These mutations can favor the adaptation of this protein to various host receptor types, causing changes both in the tropism and in the pathogenicity of CoVs [31].
The first report about CoV isolation was made in the 1930 s in samples from chicken infected with avian bronchitis virus (IBV); ever since, many other zoonotic hosts were a source for virus isolation (birds, pigs, cows, dogs, cats, bats, and others) [32], [33]. The animal-human species barrier was crossed in the mid-1960 s, when the first human coronavirus, HCoV-229E, was isolated from patients with common cold symptoms [34]. Those viruses predominantly cause respiratory and intestinal infections and induce a wide range of clinical manifestations. Initially, pathogenicity classification categorized HCoVs as low pathogenic in humans (HCoV-229E, HCoV-NL63, HCoV-OC43 and HKU1) and symptoms were associated primarily with mild and self-limiting upper respiratory tract infections, such as common cold [35]. However, in the 2000 s, more specifically in 2002 and 2012, two new HCoV were isolated from patients with severe respiratory symptoms. These new viruses were officially named severe respiratory syndrome (SARS-CoV) and the Middle East respiratory syndrome (MERS-CoV). Phylogenetic studies showed that both belonged to the beta-CoV genera and originated from bats. Although CoVs have bats as their original host, they are not directly transmitted to humans, needing an intermediate host: civet for SARS-CoV and dromedary camels for MERS- CoV [36].
SARS was considered the first Pandemic of the 21st Century [37]. In 2002, cases of SARS-CoV-1 were first reported from Guangdong province (China) and quickly spread to the entire continent. This pandemic resulted in over 8422 cases in 26 countries and about 900 deaths [38]. In 2012, MERS-CoV infection spread from Saudi Arabia to more than 27 countries across the Middle East, Europe, North Africa, and Asia [36]. According to the last update (May 5, 2020) published by WHO, MERS-CoV number of cases reported globally was 2553 with 876 associated deaths [39].
1.2. COVID-19: origen, spread, and pathogenesis
In December 2019, many patients were diagnosed with unexplainable pneumonia. All patients, initially, were epidemiologically associated with the Huanan market from Wuhan province, China. WHO was alerted for the possibility of an unknown viral outbreak. In January 2020, these cases of pneumonia and a new HCoV type were associated [40].
The phylogenetic analysis showed that this new HCoV presented more than 95% homology with bat coronavirus and more than 80% similarity with SARS-CoV [41]. Given this homology, the new HCoV was named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses (ICTV), causing COVID-19 [40].
On August 20, 2021, WHO reported a total of 209,876,613 confirmed cases of SARS-CoV-2 and 4,400,284 deaths worldwide. Until August 19, vaccine doses administered were 4,562,256,778. On March 27, 2020, China had the highest number of confirmed cases, with 82,078 cases, followed by Italy, with 80,539 cases. Nowadays, (July 19, 2021), the USA presents the most confirmed cases (33,723,155), followed by India (31,144,229) and Brazil (19,342,448). The countries most affected in the first quarter of the pandemic, China and Italy, apparently managed to contain the outbreak and reduce the virus spread, currently presenting 119,698 and 4,287,458 of the confirmed cases (cumulative total), respectively. About the death numbers (cumulative total) caused by COVID-19, the country that is currently in the first position is the USA (603,790), followed by Brazil (541,266) and India (414,108). Italy is in eleventh (127,867) and China is in ninety-eighth position (5.616) [42].
The viral infection starts with the entrance of the virus into host cells. In the case of HCoVs, the RBD domain present in the S1 subunit of S protein recognizes the receptor located on the host's cell surface, initiating virus entrance into the cytosol. In both SARS-CoVs, the RBD domain specifically recognizes angiotensin-converting enzyme-2 (ACE2) receptor in host surface cell, whereas in the MERS-CoV, the RBD domain specifically recognizes dipeptidyl-peptidase-4 (DPP4) receptor, also called CD26 [43].
Although the entry of the SARS-CoV-2 into the host's cell occurs through the attachment between ACE2 receptor and S protein, new researches have shown that S protein can also bind to other receptors, such as NRP1 (neuropilin-1) receptor [44]; however its mechanism is not yet fully understood.
Native S protein is present on the viral envelope surface in its closed form (inactive S protein), in which RBDs domains are inaccessible to the cell receptor. After protein cleavage, the S1 subunit opens (active S protein), exposing the RBD domain [45]. Depending on the type of protease present in the host's tissue, SARS-CoV-2 can enter cells by two distinct pathways, the early (non-endocytic) and the late (endocytic) pathways. Proteases expressed in the cellular membrane, such as trypsin, furin, and TMPRSS2, lead SARS-CoV-2 to the non-endocytic pathway. These host's enzymes cleave the S protein, causing conformational changes that expose the S2 subunit and, consequently, virus fusion immediately with the host's plasma membrane [43]. In the absence of those enzymes, the virus enters by the late pathway, within vesicles (endosomes) after S protein cleavage by endosomal proteases (clathrin and non-clathrin types) [44]. The non-endocytic pathway shows to be much more efficient, reaching an infection rate of 100–1000 times higher [46].
ACE2 and NRP1 receptors are expressed in multiple tissues from the human body, as oral mucosa, lungs, kidneys, liver, heart, intestines, brain, blood vessels, and others [47]. That is why SARS-CoV-2 also can invade these tissues and induce systemic inflammation, leading to multiple organ dysfunction syndromes [48]. Much research has shown that SARS-CoV and SARS-CoV-2 recognize the same host-receptor. However, SARS-CoV-2 presents greater pathogenicity and is more virulent than SARS-CoV. These features are due to some alterations found in the SARS-CoV-2 (Fig. 2). Such as (i) attachment to more than one receptor (ACE2 and NRP1) [44]; (ii) entrance by two different pathways [46]; (iii) cleavage site for furin protease in the S protein [49]; and structural differences in RBD domain that provides more binding motif to ACE2 receptor, favoring more vital interaction [50].
 Fig. 2
Fig. 2According to CDC [51], the primary SARS-CoV-2 infection route exposes to respiratory droplets carrying the infectious virus. Virus-containing respiratory droplets can spread when an infected person (symptomatic or asymptomatic) exhales droplets (breathing, speaking, singing, coughing, sneezing) close to another person. However, less common but possible is the transmission by contact with a surface that has been contaminated [51].
WHO and CDC suggest that the SARS-CoV-2 RNA shedding period (time between exposure to the pathogen and first symptoms appearance) varies from 2 to 14 days with an average of approximately five days, similar to SARS-CoV [52]. However, SARS-CoV-2 duration in the human body is not yet fully understood since it can vary from person to person. Some studies have reported that time may be more prolonged. Zhou et al. [53] did a retrospective study with 191 patients from Wuhan hospitals (China) and observed that SARS-CoV-2 RNA could be detectable for an average period of 20 days, with the shortest time eight days and the longest, 37 days (more than one month). Similar results were observed for Mancuso et al. [54] in patients of various hospitals from Italy. Li et al. analyzed the genomes of the firsts patients from Wuhan hospital. They founded different results from those published by Zhou et al. Li and collaborated showed that such genome samples presented a mean viral RNA time detected of 53.5 days, with the longest time of 83 days (approximately three months) [55]. Other researchers also analyzed samples from Wuhan hospital, but these studies took samples from infected patients at different periods. They reported that the average of the RNA shedding was 92 days, and the longest was 118 days (approximately four months) [56]. Thus, the presence of SARS-CoV-2 RNA in the human body can be longer than initially suspected. However, there is still insufficient data to affirm whether this prolonged time of the virus in the body may be related to a more extended transmission period [57].
SARS-CoV-2 infection induces the production of three antibodies (IgA, IgM, and IgG). The seroconversion process can begin simultaneously or sequentially from the 7º to 14º day (on average 5º days) after the appearance of the first symptoms [58]. IgA and IgM antibodies provide early defense, while IgG is responsible for long-term immunity and immunological memory.
SARS-CoV-2 IgM e IgG can be detectable from 4º and 7º day of the initial symptoms, respectively [59], unlike SARS-CoV-2 IgA, which can be detectable from 2º day [60]. Studies show that the IgA levels were significantly higher than those of IgM in both severe and non-severe patients, making it a pivotal biomarker to COVID-19 pathogenesis [60], [61].
It is not yet clear how long the organism can provide enough IgG titers to keep immunization. Research shows that antibody titers decrease 2–6 months after acute infection, indicating that reinfection may occur [62]. Reinfection is occurring in diverse countries. However, there is not yet a consensus about this process, since it may be associated with: (i) non-durable protective immunity [58]; (ii) different strains of the same virus [63] or; (iii) both [62].
Older age, pre-existing comorbidities (chronic hypertension, diabetes, obesity, chronic lung disease, cardiovascular disease), and pregnancy are considered risk factors for developing severe COVID-19 [64]. So far, little information about vertical transmission is available, but there are some report-cases about the SARS-CoV-2 RNA presence in the fetal side of the placenta. Most children do not present symptoms, but they are considered one of the main COVID-19 transmitting agents for close family members [65].
Infection and death rates have been distinctive both within the same country and among countries [66], [67]. Moreover, it is essential to highlight that the behavior of COVID-19 in different continents is unclear [40]. That reinforces how much we have yet to learn about COVID-19. Aspects of SARS-CoV-2 infection lika pathogenicity, host's immune response, ethnicity, viral mutation rate, rapid variant appearance and vaccine efficiency are not fully understood, motivating even more scientific community to elucidating all questions involved in SARS-CoV-2 infection.
1.2.1. Impact of SARS-CoV-2 variants in the world
Studies carried out by WHO and CDC reported the presence of multiple SARS-CoV-2 variants circulating the world. Analysis of 10,022 SARS-CoV-2 genomes revealed more than 67,700 variants [68]. The term "variant" or "strain" designates the viral particle with one or more mutations, leading to changes in some phenotypic features, antigenicity, transmission, or virulence [69]. One of the most worrying mutations in the SARS-CoV-2 was point mutation in the S gene (from ASP614 to GLY614), giving rise to a new strain called D614G. This amino acid change allowed the virus to increase its transduction and infection capacity up to 8X [70].
According to CDC, the D614G strain has caused great concern, since from this variant descend the most other variants are circulating globally. Based on the SARS-CoV-2 control effect, CDC classified the variants into three categories: decrease immune function (antibody neutralization capacity), decrease diagnostic accuracy, and decrease efficiency of treatments and vaccines. The sorting order starts from the lowest to the highest level: (1) Variant of Interest (VOI); (2) Variant of Concern (VOC); and (3) Variant of High Consequence (VOHC) [71]. However, the variant status might escalate or deescalate based on emerging scientific evidence; therefore, CDC constantly updates the ranking of each variant [72].
WHO classifies variants according to CDC; however, it differs from the United States classification. Due to these differences, WHO proposed using Greek letters (label), such as Alpha, Beta, Gamma, as a practical way of discussing variants by non-scientific audiences. The variants and their classifications are described in Table 1 [72].
Table 1. SARS-CoV-2 Variant Classifications by CDC.
| VOI | WHO label | 1º Identified place | Spike protein substitutions |
|---|---|---|---|
| B.1.427** | Epsilon | U.S. (California) | L452R; D614G |
| B.1.429** | Epsilon | U.S. (California) | S13I; W152C; L452R; D614G |
| B.1.525 | Eta | U.K./Nigeria (December 2020) | A67V; 69del; 70del; 144del; E484K; Q677H; F888L; D614G |
| B.1.526 | Lota | U.S. (New York) (November 2020) | L5F, (D80G*), T95I, (Y144-*), (F157S*), D253G, (L452R*), (S477N*), E484K, D614G, A701V, (T859N*), (D950H*), (Q957R*) |
| B.1.617.1 | Kappa | India (December 2020) | (T95I), G142D, E154K, L452R, E484Q, D614G, P681R, Q1071H |
| B.1.617.3 | – | India (October 2020) | T19R, G142D, L452R, E484Q, D614G, P681R, D950N |
| P.2 | Zeta | Brazil (April 2020) | E484K, (F565L*), D614G, V1176F |
| VOC | WHO label | 1º Identified place | Spike protein substitutions |
| B.1.1.7 | Alpha | The U.K. | 69del, 70del, 144del, (E484K*), (S494P*), N501Y, A570D, D614G, P681H, T716I, S982A, D1118H (K1191N*) |
| B.1.351 | Beta | South Africa | D80A, D215G, 241del, 242del, 243del, K417N, E484K, N501Y, D614G, A701V |
| B.1.617.2 | Delta | India (October 2020) | T19R, (V70F*), T95I, G142D, E156-, F157-, R158G, (A222V*), (W258L*), (K417N*), L452R, T478K, D614G, P681R, D950N |
| P.1 | Gamma | Japan/Brazil | L18F, T20N, P26S, D138Y, R190S, K417T, E484K, N501Y, D614G, H655Y, T1027I |
(**) Deescalated from VOC on June 29, 2021 (Efficient response to therapy and vaccine)
(*) detected in some sequences but not all
VOIs present genetic modifications that change to receptor binding and decrease antibodies functions, reducing the efficacy of the diagnostic, therapy, and vaccination and increasing the transmissibility and disease severity. VOCs present an improvement of the attributes found in VOIs. Such variants reduce diagnostic capability; they also increase the virus’s resistance to one or more classes of therapies, and significantly decrease the host immune function and the protective effect of the vaccines, raising the transmissibility and disease severity (e.g., increased hospitalizations or deaths). These variant types might require one or more appropriate public health actions, such as notification to WHO; efforts to spread control (testing and quarantine implementation); research to determine mutation rate and update in the diagnostics, treatments, and vaccines; and closely monitoring by federal agencies. VOHC has all attributes found in the VOCs; furthermore several attributes increase the concern level by health agencies. Fortunately, to date, no variant has been found to belong to this classification. Variants of this classification would have high impacts on medical countermeasures, such as a high increase in the diagnosis failure; significant reduction in vaccine effectiveness; reduced susceptibility to the various approved therapies; increased hospitalizations, and high severe clinical disease, with increased mortality [72].
As an RNA virus, SARS-CoV-2 presents a propensity for rapid evolution, which results in a great diversity of variants with different characteristics from their prior strain. The first dominant variant identified was D614G, and since then, scientists have described several others, some of which are considered VOCS.
One of the main concerns among SARS-CoV-2 variants is the B.1.617.2 (Delta) variant. According to WHO, this variant was first identified in India in late 2020 and quickly spread to more than 80 countries; however, it is more prevalent in India and the UK [73], [74].
B.1.617.2 presents several point mutations, including ASP614 to GLY614 in the S protein (D614G), as shown in Table 1. Besides the D614G mutation, other mutations, L452R, P681R, and T478K, cause significant public-health concerns [75]. The L452R mutation is more transmissible and has greater efficiency for immune evasion in vaccine sera; P681R increases transmission, and T478K generates greater infectivity [76].
Reseachs shows that the B.1.617.2 is increasing exponentially worldwide, and probably soon, it could become the dominant variant. This Delta strain appears to be spreading 60% faster than the Alpha strain (B.1.1.7), which already had a transmissibility rate of about 50% compared to the original strain of SARS-CoV-2 [77].
On June 6, 2021, the Health Ministry of India announced the discovery of a new variant originating from the Delta strain (B.1.617.2), called Delta Plus (B.1.617.2.1). In 3 months, Delta Plus is already present in nine countries: the USA, UK, Portugal, Switzerland, Japan, Poland, Nepal, Russia, and China [78]. The most prevalent version of this Delta plus variant worldwide is designated AY.1. However, other versions are AY.2 e AY.3, mainly restricted to the United States [79].
Delta plus variant has the addition of a point mutation, K417N, that is associated with immune escape, although its impact on transmissibility remains unclear [80]. In the original strain, RBD K417 residue is linked to ACE2 D30 residue by a salt bridge, considered one of the strongest non-covalent bonds. Thus, these residues contribute to the high binding affinity of SARS-CoV-2 to the ACE2 receptor [81]. Although K417N mutation loses three atomic contacts, reducing the contact of this residue to D30 of ACE2 and destabilizing the salt bridge, it increases by six atomic contacts the binding of the neighboring L455 to D30 of ACE2. Thus, K417N mutation slightly increases the binding affinity of S protein to ACE2 [82], [83].
Many aspects of the Delta plus variant still need to be better studied. It remains unclear whether this additional mutation will make the Delta plus variant more transmissible, infectious, or resistant to antibodies than the original Delta variant. Another point to be clarified is how effective the current vaccines will be against this new variant.
2. COVID-19 diagnostics: main approaches
Rapid diagnostic, social isolation and masks are critical for control outbreaks and prevent viral spread. COVID-19 has non-specific manifestations, which can range from no symptoms to severe symptoms and death. Such a non-specificity aspect can be a problem for the health system because it can hamper correct diagnosis and epidemiological mapping (Fig. 3A).
 Fig. 3
Fig. 32.1. Reference standard assays
Health organizations recommend reverse transcription-polymerase chain reaction (RT-PCR) as the “gold standard” for the SARS-CoV-2 RNA detection [84] and serologic assay to identified SARS-CoV-2 antibodies from clinical specimens [85], [86], [87]. Nucleic acid assay identifies viral gene presence, measures active infection and guides public authority to contain the viral spread. Antibodies detection tests identify previous infections, helping to understand the behavior and progression of the disease, contributing to epidemiological data survey.
On June 11, 2020, WHO added the chest-image tools to the test list for COVID-19 diagnostic workup [88]. Among various imaging tools, the chest computed tomographic (CCT) showed greater sensitivity [89]. CCT is a routine technique for lung lesions evaluation in patients with some pulmonary dysfunction. Consequently, CCT can help in COVID-19 diagnostic and progression (from symptom presentation to home discharge). However, CCT is an option cases like RT-PCR unavailable; RT-PCR result delayed; symptomatic patient with negative RT-PCR; patients with moderate to severe symptoms, which present some risk-factor; and patients with worsening of the respiratory condition [88], [90]. In this way, CCT can be an integrated part of the medical screening system, particularly for patients who need emergency care.
2.2. Main features about reference standard assays
2.2.1. SARS-CoV-2 RNA detection
The start point for COVID-19 diagnostic is SARS-CoV-2 RNA detection by nucleic acid amplification tests (NAATs). NAATs assays can amplify low numbers of DNA copies in millions of orders of magnitude with high sensitivity and specificity, being excellent tools for the early diagnosis of infectious diseases. WHO recommends using RT-PCR, a reverse transcription reaction followed by PCR, for SARS-CoV-2 RNA detection utilizing samples collected from the upper respiratory tract for spontaneously breathing patients (oropharynx and nasopharynx regions) and lower respiratory tract for mechanically ventilated patients [91].
The targets for SARS-CoV-2 RNA detections, approved by CDC and WHO, have some distinctions. N1 and N2 genes are recommended by CDC [92], while RdRp, E, and N genes are recommended by WHO [91]. E gene is the first line-screening assay, and RdRp and N genes are used for confirmatory tests. The gene human-RNase P (RP) is a positive control [93].
Although the RT-PCR technique is well known as an accurate method for detecting viral genetic material, many studies show that this technique can present false-negative results for SARS-CoV-2 RNA [94], [95], [96]. Many factors may generate false-negative results. Two critical points are sample quality (stage of the disease, viral load, virus replication, RNA extraction, location, and collection way) and the PCR assay (reagents, melting temperature, primers, and probes sets) [91], [97].
Additional limiting factors for extensive usage of NAATs for infection disease diagnosis are infrastructure, high-cost reagents and equipment, refrigeration for reagents and samples, and trained professionals. Due to the need for this infrastructure, these assays are inappropriate for use in resource-poor settings [98].
2.2.1.1. Other molecular techniques for SARS-CoV-2 RNA detection
Reverse transcription loop-mediated isothermal amplification (RT-LAMP) is an alternative approach to the traditional RT-PCR method. Compared with the other amplification methods, the LAMP methodology is faster and the whole process takes place at a constant temperature. Such an innovative technique uses two important features to decrease reaction time: a polymerase with displacement activity and a set of 4–6 primers designed to create loop structures in the template containing primer annealing sites for amplification. The effect is an amplification product (amplicon) identified by color, turbidity or fluorescence detection in a few minutes. Besides shorter reaction time, the assay can occur in simple devices, some of them developed to be used in no power condition, as battery-coupled machines or fast colorimetric tests, revealing a sensitive and easy-to-use potential for point-of-care diagnosis [98]. These features make the LAMP assay an appropriate detection tool for outbreaks of emerging and reemerging diseases [99].
However, although RT-LAMP presents advantages compared to conventional RT-PCR, it has some intrinsic limitations, such as a complex pre-study to select the better-conserved target gene region and the design of more efficient primer sets. In addition, the RT-LAMP can present false-positive results during its post-amplification process, characterized by the PCR inhibition internal control, which can lead to non-specific amplification products [100], [101], [102].
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR/Cas-based assay) is a sophisticated technique of gene-editing that has revolutionized the molecular biology field. CRISPR is an important bacterial defense mechanism against an invading genome, characterized by small nucleic acid sequences repeated throughout the bacterium genome. There is a "nucleic acid spacer," called Protospacer Adjacent Motif (PAM), between each short palindromic sequence. PAM is a short non-coding sequence, which indicates the cleavage site for an enzyme known as Cas. Then, the CRISPR sequence is transcribed in small RNA fragments (crRNA or guide-RNA), which have the ability to annealing with foreign DNA. The guide-RNA (gRNA) orients the Cas enzyme until cleavage site, and next, the invading genome is eliminated [103].
Although numerous Cas enzyme types exist, only those with nuclease activity, such as Cas-12 and Cas-13, are used for diagnosis. Furthermore, for the CRISPR/Cas system to be used as a detection tool, the gRNA must be genetically modified to bind a specific target sequence. After, the cleaved fragments link to a fluorescent probe to be quantified by fluorescence spectroscopy [104]. Some reports show that when this system is associated with a pre-amplification method, there is a sensitivity improvement of the assay, allowing the detection of deficient levels of concentration of target [105]. Therefore, the recent viral spread of SARS-CoV-2 has led to numerous adaptations of CRISPR/Cas systems for COVID-19 diagnosis.
Broughton et al. [106] reported developing a CRISPR–Cas12-based assay, called SARS-CoV-2 DNA Endonuclease-Targeted CRISPR Trans Reporter (DETECTR). This platform uses RT-LAMP simultaneously. Researchers designed primer sets for E and N genes (both recommended by WHO and by CDC). They used two sgRNAs projected (i) to bind in sequences of the E gene found in three SARS-like coronaviruses (SARS-CoV-2, bat-SL-CoVZC45, and SARS-CoV) and (ii) to bind in sequences of N gene found only in SARS-CoV-2. DETECTR considers positive results for SARS-CoV-2 detection of two genes (E and N). Seventy-eight samples from American patients were analyzed (36 positives for COVID-19 and 42 samples with other viral respiratory infections). The sample-to-result time was around 45 min, with a detection range between 70 and 300 copies/µl of input, and the positive and negative predictive values were 95% and 100%, respectively.
Zhang et al. published a protocol modification in the SHERLOCK platform for SARS-CoV-2 detection. Methodology evolved S and Orf1ab genes as targets and used synthetic samples of COVID-19 RNA fragments. Detection ranged between 20 and 200 attomolar (aM) (10–100 copies/µl of input), and assay time (from RNA purified) was less than an hour [107].
Metsky et al. [108] provided developing algorithms and machine learning models for rapidly designing nucleic acid detection assays and multiplex panels connected to a system called ADAPT. They analyzed 67 viral genomes responsible for several viral infections, including SARS-CoV-2, phylogenetically close to SARS-CoV-2 and other respiratory viruses. The ADAPT showed that only one sgRNA sequence is required for targeting SARS-CoV-2 RNA, unlike protocols described by Broughton et al. and Zang et al., which used two gRNA. Metsky et al. tested sgRNA obtained by ADAPT in an experimental assay based on the SHERLOCK platform. They observed that only one sgRNA, unlike SHERLOCK original protocol, is enough for high sensitive detection from synthetic SARS-CoV-2 RNA (10 copies/µl of input),
In summary, the CRISPR/Cas system has shown be promise technology for diagnosing various diseases [109], [110], [111]. However, there are still many caveats related to this technique. Among them, it is possible to highlight: (i) the need for a professional with experience in protein purification and in molecular biology techniques for that all reaction components are made and used correctly; (ii) difficulties in obtaining pre-designed kits (reaction components); (iii) decreased sensitivity and specificity of the assay when using the pre-made kits; and (iv) all the disadvantages (pre-mentioned) related to the amplification assays [103], [105].
2.2.2. SARS-CoV-2 antibody detection
Immunoassays are also approaches used in the frontline testing for specific diagnoses of COVID-19. These assays can provide information on whether a person was infected recently (active viral infection) or in the past [112].
After one year and a half of SARS-CoV-2 discovery, there are still several gaps to be understood about the immune response against the viruses. Some studies related S and N viral proteins as primarily responsible for inducing immune response [113]. N protein has shown to be more immunogenic among HCoV proteins, but it induces antibodies with a shorter duration than ones against S protein. Studies have reported that S protein antibodies can be viable 11 years after patient exposition to some HCoV type [114]. Such findings make S protein the target of choice for studies related to the production of vaccines [29].
SAR-CoV-2 immune memory is not yet fully understood. Some cases reported have suggested a short timeframe, around four months, between recovery and possible reinfection in the same person [62]. Therefore, WHO and CDC have emphasized that immunodiagnostic tests should be used in cases of evaluating the infection progression, epidemiological screening, and in the research environment [115].
The main immunological tests used for diagnosing SARS-CoV-2 infection include neutralization antibody (NA), rapid diagnostic test (RDT), chemiluminescence immunoassay (CLIA), and enzyme-linked immunosorbent assay (ELISA) [116].
In NA assay, the patient's sample is added to a cell culture medium and incubated for 3–5 days. The functional ability of antibodies against SARS-CoV-2 is then determined. Although more specific and accurate, NA assay requests a long period and biosafety certificates laboratories (BSL2 or BSL3), making them impracticable for rapid tests [116].
RDT is a simple, fast, and inexpensive test based on the lateral flow immunoassay (LFIA) method to detect specific antibodies (IgG, IgM, or IgA) of SARS-CoV-2. In this technique, a drop of a patient's sample (whole blood, serum, or plasma) is spilled on a disc strip, where the reaction occurs. The result is released on average 20 min later, presenting the potential to be used as point-of-care tests. However, this method has shown poor accuracy, less than 20% compared to ELISA, causing a high rate of false-negative results [117].
CLIA is a method that combines chemiluminescence with immunoreaction (antibody-antigen interaction). When the target recognizes and interacts with a receptor linked to a luminescent molecule (luminol, acridine, and their derivatives), such a molecule is released. It returns to its fundamental state, emitting light [118]. The intensity of light emitted has a linear relationship with the concentration of the measured substance [119], with an average time-to-result of 1–2 h [116].
ELISA is the most common immunodiagnostic method. This assay has a similar concept to the CLIA. Immunoreaction is also associated with light emission from a chromogenic agent released in the reaction, and the intensity of emitted light corresponds to the target concentration in the sample [120].
ELISA is robust and easy to perform regarding immunoassays. Nevertheless, for SARS-CoV-2, these tests have low sensitivity and specificity when compared with antibody detection of other infection agents. These caveats are related to the unclear SARS-CoV-2 antigens definition for antibody detection; or usage of different targets for antibodies, as S protein, N protein, or both; or cross-reaction with other HCoVs antigens (high antigenic homology), and dependence on antibody production kinetics [112], [121], [122], [123].
2.2.3. Routine assays and CCT images
Blood routine tests can complement diagnosis. Some reports identify alterations in blood components suggestive of early SARS-CoV-2 infection, as lymphocyte count reduction and an increase of serum amyloid A (SAA), C-reactive protein (CRP), and erythrocyte sedimentation rate.In severe cases, an increase in the rates of creatine kinase (CK), lactate dehydrogenase (LDH), and aspartate transaminase (AST) can be detected [124]. Because these alterations can be associated with other viral infections, specialists should interpret results with caution. In cases of doubt, a more specific test, as RT-PCR, is recommended.
SARS-CoV-2 causes typical pulmonary alterations in most COVID-19 patients, such as ground-glass opacities, multifocal patchy consolidation, or interstitial changes with a peripheral distribution. These abnormalities are not specific to COVID-19 since they exist in other pathologies [125]. Nevertheless, CCT has played a crucial role in symptomatic patients with negative RT-PCR, helping diagnosis by images alterations, clinical and laboratory evidence, and information if the patient had contact with a patient diagnosed with COVID-19 [97].
3. Challenges to control the COVID-19 pandemic
The global scientific community has come together to improve existing assays and develop new ones that can help understand the pathogenesis and epidemiology of SARS-CoV-2, and expand the diagnostic assays for this virus and thus contribute to the pandemic end. However, despite all the worldwide aid, there are still several challenges to achieving an excellent diagnosis performance (effective and accurate SARS-CoV-2 detection) (Fig. 3A/B). Among them, can be a highlight: (i) non-specific symptoms, which may be confused with other diseases; (ii) high correlation phylogenetic and immunologic with other coronavirus species, which may cause false-positive results and (iii) overload of diagnostic capacity, causing delays in the patient samples analysis [16], [40], [65], [97].
Proper sample collection is essential for an effective diagnosis of infectious diseases. According to WHO [126], the ideal samples for genetic detection of SARS-CoV-2 are those collected in the patient's airways. From patients who present infections at an early stage, mild disease, or asymptomatic, samples should be collected by swabs, in the upper airways region, both in the nasopharyngeal and oropharyngeal regions. However, when the RT-PCR result is negative, but there is a strong clinical suspicion that the result is false-negative, the patient should do a new test with a sputum sample. Nevertheless, if the patient has a severe respiratory problem, the medical team may resort to endotracheal aspirate or bronchoalveolar lavage.
According to the global health agencies, only in the 21st century were recorded unprecedented outbreaks caused by infectious diseases, e.g., Dengue, SARS, MERV, Ebola, Zika, and recently SARS-CoV-2. In 2004, WHO organized a meeting to discuss prevention strategies for the emergence of new infectious outbreaks with the potential pandemic. The meeting showed world leaders the damages that a long pandemic could have on both a country's health and the economy. Moreover, how a fast viral containment could help mitigate the social and economic impact. The main strategies suggested were to be improved in all approach types, including rapid communication between agencies and countries to avoid, control, and reduce the impact caused to society [127].
As highlighted at the meeting, it is imperative to emphasize that a quick and effective diagnosis is crucial to mitigate the disease outbreaks effect. Recent outbreaks reinforce the need to use a point-of-care (POC) approach to control the disease epidemic. A study made by Kost et al. [128] and Perkins and co-workers [129] point out the need to implement in the school curriculum the learning of essential themes related to public health, more specifically about the point-of-care test (POCT).
The biggest Ebola outbreak took almost two years (2014–2015) and could have been avoided if the lessons about infectious disease outbreaks had been strictly followed. The ability to perform effective diagnosis and control of the outbreak took about a year and cost about $2–3 million. According to Perkins, world authorities must consider that “diagnostic development and validation are time-consuming; they should be carried out in anticipation of epidemics rather than in response to them” [129]. Unfortunately, despite the efforts made by the health agencies and scientific community, the world authorities still do not manage an efficient response. In the SARS-CoV case, the first outbreak occurred eighteen years ago. It was impossible to implement preventive safety measures to contain the spread of the disease, such as rapid and effective diagnosis, drug development, and rapid deployment of medical infrastructure.
COVID-19 showed to the world the importance of good resource management in cases of a pandemic. SARS-CoV-2 made us reflect on the importance of constant investments and incentives in the science and technology. Such incentives could improve to the basic principles of science, increasing technological support for developing new tools capable of predicting, diagnosing, and containing new pandemic outbreaks.
After implementing collaborative data support between academia and industry was possible to observe the rapid development of new diagnostic tools, drugs, and vaccines against COVID-19 [16]. This connection takes us to the definition of translational medicine (MT), an area that is currently expanding. MT presents an interconnected approach, covering each stage of the research to the commercialization of the final product, which streamlines the entire process, benefiting the population [130].
Thereby, diagnostic technology investments could provide the health system with new approaches, as high-performance tools, which present fast response, high specificity and sensitivity in detection, ease of use, low cost, portability, and PCR-free (Fig. 3B). With such improvement approaches, we would probably be able to achieve the ideal scenario for pandemic control.
3.1. Biomarkers: the importance of specific target for diagnosis
A crucial step for SARS-CoV-2 diagnosis was the fast sequencing of the entire genome and its availability in public databases by Chinese. Genome analysis is considered the main form to find specific biomarkers for infectious disease detection. Biomarkers are molecular signatures associated with the presence, severity, or disease type necessary for diagnostic, prognosis, monitoring, and therapy.
Multi-omics analysis (genomics, transcriptomics, proteomics, and metabolomics) has proved to be a robust approach to identify several molecular signatures associated with COVID-19[131]. Such approaches provide a holistic molecular perspective of biological systems compared to traditional analysis. For SARS-CoV-2 (Fig. 4), omics studies have revealed deregulations of some inflammatory factors, metabolites, and other molecules found in blood, including amino acids, carbohydrates, fatty acids, and glycerophospholipids [132].
 Fig. 4
Fig. 4Zhang et al. [133] analyzed 140 samples from positive RT-PCR patients for SARS-CoV-2 and observed a positive correlation between decreasing in blood eosinophil and lymphocyte with the severity of the disease. Messner et al. [134] analyzed other 188 serum samples. They found 27 potential biomarkers (complement factors, the coagulation system, inflammation modulators, and pro-inflammatory factors) differentially expressed depending on the COVID-19 severity grade. Among these classes of biomarkers, the interleukin 6 (IL-6) was more elevated in severe disease presence. The IL-6 is associated with inflammatory cascade and, consequently, presents an essential role in the host immune modulation. So, IL-6 can be a relevant predictive biomarker of the SARS-CoV infection progression [135].
Ellinghaus et al. [136] analyzed 3815 samples from Italy and Spain patients (1610 positive samples and 2205 controls). They performed a genome-wide association study (GWAS), which associates specific genetic variations with particular diseases [137]. In total, they analyzed 8,582,968 single-nucleotide polymorphisms (SNP) common to both countries. The results show two independent gene variants associated with the severe form of COVID-19, rs657152 (A or C SNP) at locus 9q34.2 and rs11385942 (insertion-deletion GA or G variant) at locus 3p21.31. The 9q34.2 locus is responsible for encoding ABO antigen erythrocyte proteins. According to the analysis, patients with blood group A present a higher risk of developing severe COVID-19 than any other blood group. However, the 3p21.31 locus contains genes that encode chemokines, whose function is to attract immune cells to infection sites. Modification in these genes can lead to an increase in the immune response. Furthermore, the SLC6A20 gene, also present in the locus, encodes neuronal norepinephrine transporter (NET) that interacts with ACE2, the SARS-CoV-2 cell-surface receptor [138], [139]. Polymorphisms in this gene have been associated with neurological and blood pressure disorders [125], leading to higher susceptibility to severe forms of Covid-19 [140], [141].
Kong et al. [142] have found in 24 positive patients for COVID-19 that vascular endothelial growth factor D (VEGF-D) is the most critical indicator related to the severity of COVID-19. VEGF-D is a glycoprotein responsible for (i) mitogenic process in endothelial cells; (ii) angiogenic process; (iii) vascular permeability induction; and (iv) linfangiogênese (lymphatic vessels growth). Because VEGF-D is present in various tissues (lung, heart, small intestine, fetal lung, and at lower levels in the pancreas, colon, and skeletal muscle), its overexpression increases the probability of a systemic pathology [143]. High levels of VEGF-D active coagulation cascade and increase D-dimer levels in plasma. That is why VEGF-D and D-dimer are the biomarkers that can predict COVID-19 progression to severe form [144].
Overmyer et al. [132] analyzed 128 blood samples from COVID-19 patients with various severity grades and outcomes by multi-omic analysis. All information is added in a web tool (covid-omics.app), enabling the current global efforts to understand the behavior of infection by the SARS-CoV-2. They found 219 biomarkers correlated with COVID-19 severity, belonging to different sets of clusters (proteins, transcripts, and lipids). Such biomarkers are involved in the deregulation of platelet function, blood coagulation, acute phase response, and endotheliopathy. The Large-multi-omic analysis made by Overmyer and collaborators confirmed the data published by many reteaches realized at the beginning of the COVID-19 pandemic. According to Overmyer et al., the main predictive biomarkers for the severe development of COVID-19 are HDL-cholesterol reduction, increased neutrophil counts, increased VEGF, von Willebrand Factor (VWF), thrombin and D-dimer levels; and elevated concentrations of the IL-6 and IL-10. IL-6 detection indicates worsening of the patient's condition [132].
In summary, multi-omics approaches can allow a complete view of COVID-19 pathophysiology [145]. Additionally, such analysis can aid in the discovery of the molecular signature, which may be present or altered by the SARS-CoV-2 infection [146]. These assays have great importance for the advance of both precision and personalized medicine [131], [147]. Most biomarkers discovered for COVID-19 are not specific for only SARS-CoV-2 infections but can be helpful in the prognostic, severity, and monitoring of COVID-19 [132], [146]. In addition, biomarkers can be deposited in a network database and connected to epidemic mathematical models, generating algorithms capable of predicting, controlling, and preventing infectious disease outbreaks and a pandemic [132], [148].