1. Introduction
Unconventional resources are classified as the reservoirs with too low permeability such as tight oil or gas, gas shales, and coalbed methane reservoirsor with too high viscosity such as oil shales or heavy oil (Holditch, 2013). In Canada, there are about 1.7 × 10 12 barrels of heavy oil and bitumen in place (Kantzas and Brook, 2004). Heavy oil reservoirs in Alberta and Saskatchewan, with viscosity in the range of 50 to 50,000 mPa s (cp) and densities similar to that of water at reservoir temperature and pressure, contain some solution gas leading to produce around 5% of the original oil in place through solution gas drive mechanism (Bryan and Kantzas, 2008). The remaining oil in place is a remarkable target for improved oil recovery techniques. Many of these reservoirs are unconsolidated sand deposits with high porosity and permeability. Therefore, the main reason for the low primary recovery has been attributed to the oil high viscosity. Accordingly, thermal enhanced oil recoverytechniques have been applied in numerous projects to lower oil viscosity. However, since many heavy oil reservoirs in Alberta and Saskatchewan are too thin or small there is a tremendous heat loss to the overburden and underburden formations making thermal techniques less efficient (Farouq Ali, 2006, Luo et al., 2007). In addition, a lot of Canadian shallow bitumen reservoirs such as Surmont leases, Kearl Lake, and Wabiskaw-McMurray possess top water zones (Zhou et al., 2016), where thermal recovery techniques are not good candidates either. In these cases, the low recovery of thermal methods has been attributed to the heat loss and steam quality reduction due to the water influx from top zone into the heated chamber (Zhou et al., 2016).
As an alternative to the thermal techniques, solvent-based processes have been suggested to exploit heavy oil reservoirs having thin pay zones (Denney, 2012). These processes are based on pressurizing the reservoir to dissolve solvent into the heavy oil. This solvent dissolution lowers oil viscosity and enhances mobility (Diedro et al., 2015, Qazvini Firouz and Torabi, 2012). However, these processes may not be applicable in many of Western Canadian reservoirs that are in the post cold heavy oil production with sands (CHOPS) stage. These post CHOPS reservoirs contain very high permeable networks of wormholes, created after a sand production during primary production (Rangriz Shokri and Babadagli, 2016), through which a solvent can finger. Therefore, a very significant volume of solvent is required to sufficiently pressurize the system leading to a remarkable reduction in oil viscosity (Bryan et al., 2013). Furthermore, the rate of solvent mass transfer into the oil and preventing formation damage through precipitation of insoluble asphaltenes are the other crucial challenges with which should be dealt to have a successful solvent-based EOR processes (Diedro et al., 2015).
Chemical enhanced oil recovery techniques have been suggested to improve heavy oil mobility in thin post CHOPS reservoirs in Alberta and Saskatchewan. Polymer flooding is one of the conventional chemical EOR techniques to improve oil mobility by increasing the viscosity of the injected fluid. This method seems to be more challenging in heavy oil systems, since to match or exceed the viscosity of very viscous heavy oil too viscous polymer solution should be injected into the reservoir resulting in injectivity problems (Kumar et al., 2012). Thus, alkaline-surfactant flooding has been recognized as a more cost-effective and feasible chemical EOR technique in thin post CHOPS heavy oil systems (Bryan, 2008, Bryan and Kantzas, 2007, Bryan et al., 2008, Bryan et al., 2013, Denney, 2009, Kumar et al., 2012, Liu et al., 2007, Mai et al., 2009, Naderi et al., 2015). In these processes, an alkaline-surfactant cocktail is injected into the reservoir to create an emulsion in the reservoir. Injected alkali reacts with acidic crude oil to create in-situ soap/surfactant (Ashrafizadeh et al., 2012, Farouq Ali, 2006, Kumar et al., 2012). Surfactants are amphiphilic compoundscontaining the hydrophilic head and hydrophobic tail. They are expected to settle at the oil-water interface, reduce interfacial tension and create oil in water emulsion. The in-situ created emulsions act as a flow barrier to divert flow from high permeable channels/wormholes into the un-swept regions of the reservoir (Bryan et al., 2013, Chen et al., 2013, Pei et al., 2013, Reisberg and Doscher, 1956). The main advantage of alkaline-surfactant flood compared to polymer flood is that the flow conformance can be achieved by injection of an alkaline-surfactant cocktail with viscosity similar to that of water rather than injection of too viscous polymer into heavy oil reservoirs. Furthermore, due to the reaction of appropriately designed alkaline-surfactant cocktail with heavy oil low viscosity oil in water emulsion is created, which can flow more easily compared to very viscous heavy oil. However, it seems that the mentioned emulsion-assisted mobility control is not sustained and the emulsion droplets are not stable enough to tolerate the driving force during the flooding and as a result, the improvement in oil recovery would be only short term (Bryan et al., 2013). After this early time improvement in oil recovery, the main mechanism switches to entrainment of oil droplets as oil in water emulsion, which is a less effective mechanism to improve oil recovery compared to the plugging mechanism.
Furthermore, since surfactants are susceptible to thermal degradation and/or adsorption and precipitation on the pore surface (ShamsiJazeyi et al., 2014) they cannot be applied in harsh reservoir environments of high temperature or high salinity conditions (Kim et al., 2016a). Stabilization of oil in water emulsions by solid particles such as clays, quartz, feldspar, gypsum, coal dust, asphaltenes, and polymers (Bragg, 1999, Pickering, 1907), as opposed to conventional surfactant-assisted emulsion stabilization techniques, can be used to enhance the efficiency of an alkaline-surfactant flooding. This enhancement can be obtained by either boosting the strength of the in-situ flow barrier or working as a drive fluid to push the oil towards the producing well (Bragg, 1999). Nanoparticles have great chemical and physical stability in harsh reservoir conditions (Metin et al., 2013, Xu et al., 2016, Yu and Xie, 2012) and their application as emulsifiers are reported to be more cost-effective compared to surfactants, with reduced environmental impact (Griffith et al., 2016).
There are many articles addressing the remedial effect of nanomaterials to stabilize oil in water emulsions applied in different fields of study including foods, cosmetics, pharmaceuticals, oil and gas, etc. There is not a comprehensive review on the topic for enhanced oil recovery applications. It is worth emphasizing that since the viscosity of an emulsion is larger than that of the continuous phase (Kumar et al., 2012), so the viscosity of water in oil emulsion is even larger than that of the oil. Therefore, in heavy oil systems, it is preferred to have oil in water emulsion and avoid water in oil emulsion which requires a very large pressure gradient for typical flow rates. Accordingly, this work is aimed to critically review the capability of NPs to stabilize oil in water emulsion. It is worth emphasizing that in this review we focused on macro-emulsions as opposed to micro-emulsions. Formation and characteristics of these two types of emulsions are completely different from each other. The droplet diameter of macro-emulsions is greater than 0.1 μm (Sharma and Shah, 1985) which in turn, leads to effectively scatter light and make them appear milky/turbid (Totten et al., 2003). Furthermore, macro-emulsions are thermodynamically unstable and can be produced by applying a mechanical energy (Sharma and Shah, 1985). On the contrary, micro-emulsions are thermodynamically stable (Qi et al., 2017), transparent/clear (De Gennes and Taupin, 1982), and can form spontaneously (Sharma and Shah, 1985) upon gentle mixing of the components with no high shear requirement. For further details about micro-emulsions the reader is referred to Gelbart and Ben-Shaul, 1996, Langevin, 1988, Sharma and Shah, 1985, Totten et al., 2003 among others. This review builds the argument that nanoparticles can be used to stabilize oil in water macro-emulsions and to fortify alkali surfactant injections so that they become an effective technology for enhanced recovery of heavy oil. For simplification we used the word “emulsion” instead of “macro-emulsion” all throughout the article.
2. Nanoparticle-assisted stabilization of oil in water emulsions
Nanoparticles have been suggested as modifiers or alternatives to surfactants as emulsion stabilizers (Binks et al., 2007a, Pilapil et al., 2016). In this regard, although the nanoparticles’ function is very similar to that of surfactants (Xu et al., 2015) there are some differences between the two surface-active agents. The main difference is attributed to the stronger tendency of nanoparticles to adsorb at the interfaces (Binks, 2002) compared to surfactants which can be dynamically adsorbed or desorbed at the interface (Worthen et al., 2013). Therefore, nanoparticle-stabilized emulsions are reported to be very stable and as a result, they can survive harsh reservoir environments. Thus, water pathways fingered through a water-flooded reservoir can be more efficiently plugged by emulsion droplets stabilized by NPs and the resultant flow divergence would be more sustained. Besides channel plugging, oil entrainment in the form of oil in water emulsions has been put forward as the other emulsification-assisted EOR mechanism. As mentioned, oil in water emulsions possess a considerably lower viscosity compared to oil. Therefore, they can flow more easily than the oil towards the production well. If the size of NP-stabilized oil in water emulsions is not comparable to that of pore throats they cannot plug the water pathways. In this case, since the emulsion stability has been fortified by NPs they can survive and remain stable to reach a producing well (Zhang et al., 2010) and increase oil recovery through oil entrainment. Therefore, NP-assisted enhancement in either capability of emulsion droplets to plug the preformed water pathways or oil entrainment has attracted the attention of many researchers, and is reviewed in the following sections.
The standard model of nanoparticle stabilization assumes attachment of the nanoparticles to droplet surfaces. Though Pilapil et al., showed that nanoparticle stabilization is also possible when nanoparticles do not attach to droplet surfaces (Pilapil et al., 2016), this review focuses on the standard model. The stability of solid-stabilized emulsions is increased with an increase in the adhesion of nanoparticles at the oil-water interface (Yoon et al., 2016). Nanoparticle tendency to like water or oil can be quantified in terms of its wettability (measured by contact angle), just like that of a surfactant which is described by hydrophile-lipophile balance (HLB) number. Three-phase contact angle has been recognized as a critical parameter to design a nanoparticle-stabilized emulsion determining particles position relative to the interface between aqueous and oleic phases (Melle et al., 2005). Particle wettability is also a decisive factor to determine the type of emulsion (oil in water versus water in oil emulsion).
As a rule of thumb, the phase in which the surfactant is more soluble would be the continuous phase. Therefore, hydrophilic surfactants (high HLB) tend to form oil in water emulsion, while hydrophobic surfactants tend to form water in oil emulsions (Bryan and Kantzas, 2007, Worthen et al., 2013). By analogy to surfactants, hydrophilic nanoparticles (with three-phase contact angle of smaller than 90°) tend to create oil in water emulsion, while hydrophobic nanoparticles (with three-phase contact angle of larger than 90°) tend to create water in oil emulsions (Melle et al., 2005). Furthermore, it is believed that the stability of a solid-stabilized emulsion is increased when the three-phase contact angle is in the vicinity of 90° (Melle et al., 2005). Cui et al., also reported that the surface activity of particles with contact angle slightly above or below 9° is critically increased which in turn, leads to an increase in the stability of water in oil or oil in water emulsions, respectively (Cui et al., 2010a, Cui et al., 2010b). In addition, the drop size of emulsions (prepared at any volume fractionof water and toluene) stabilized by either very hydrophilic or very hydrophobic particles is large (larger than 100 μm) and as a result, the emulsion is unstable to coalescence. However, emulsions stabilized by particles with intermediate hydrophobicity are of submicron size and stable to coalescence (Binks and Lumsdon, 2000a).
Silica has been widely used as the most common and cost-effective NP to enhance emulsion stability, so the main focus of this review is on silica NPs. Silica NPs are generally too hydrophilic with very high tendency to remain in aqueous phase and tend not to settle at the oil-water interface. Therefore, different approaches have been applied to increase their hydrophobicity and surface activity. These techniques can be broadly categorized into in-situ and ex-situ activation methods. Silica NPs can be in-situ activated due to the effect of different types of surfactants including anionic, cationic, non-ionic, and zwitterionic, which are all discussed in more detail in different sections. On the other hand, ex-situ activation techniques such as silanisation (Balard et al., 2012, Binks and Whitby, 2005, Binks and Lumsdon, 2000a, Jiang et al., 2016, Aveyard et al., 2003, Dickson et al., 2004, Safouane et al., 2007, Tyowua et al., 2017, Grate et al., 2013, Sun et al., 2014, Sun et al., 2015) and surface modification by formation of covalent bonding or polymer grafting (Espinosa et al., 2010, Hunter et al., 2009a, Hunter et al., 2009b, Saleh et al., 2005, Saigal et al., 2010, Worthen et al., 2013) have been applied to lower silica NPs hydrophilicity. As mentioned, applying an external mechanical energy is required to create a macro-emulsion. This energy can be provided through different ways to produce different levels of energy input. In this regard, manual shaking can represent providing a low level of energy, while using homogenizers or fluid flow through porous medium may provide medium energy level. At low and medium energy input levels, NPs need to be already surface-activated by either surfactant or particle coating to reach the interface and stabilize emulsions. Depending on the balance of hydrophilicity and hydrophobicity on the NP surface, more vigorous mixing techniques like using ultrasonicators may be needed to bring the NPs to the interface and enable attachment.
2.1. In-situ activation of NPs by different surfactants
Considerable work has been done to investigate the effect of different types of surfactant to facilitate NP adsorption onto the oil-water interface. In this technique besides the effect of surfactants adsorption on NPs surface, the free surfactant molecules may also have the opportunity to adsorb by themselves at the interface to decrease IFT (Worthen et al., 2013). In this regard, the interaction between NP and surfactant, which is a function of the charge and amphiphilic feature of both surfactant and NP, was recognized as the most important factor. Therefore, this section is subdivided by the type of surfactant and their interaction with NPs having different charges.
2.1.1. Cationic surfactant
There are some articles addressing the enhancement in emulsion stability due to addition of negatively-charged NPs to cationic surfactant solutions (Binks et al., 2007a, Lan et al., 2007a, Schmitt-Rozieres et al., 2009, Zhu et al., 2015). Binks et al., investigated the effect of a pure cationic surfactant, hexadecyltrimethylammonium bromide (CTAB) with critical micelleconcentration (CMC) in water at 30 °C equal to 0.9 mM, and hydrophilic silicaNP (with an average diameter of 15 nm) to stabilize n-dodecane in water emulsion (Binks et al., 2007a). It is worth mentioning that they tried to investigate the surfactant and NPs interaction at diverse pH values; one at pH = 3 close to the NPs' isoelectric point of zero charge which occurs at pH between 2 and 3, and the other at pH = 9.5 corresponding to the NPs’ very high negative surface charge. They used a homogenizer operating at 11000 rpm for 2 min to mix equal volume of n-dodecane and water (total 10 cm3). Based on their results, at pH = 9.5 and in the absence of surfactant there is no emulsion due to the effect of different concentrations of silica NPs (0.1, 0.5, 1.0, 2.0, and 31.6 wt. %). At this high pH, NPs are very hydrophilic with very high negative charge (zeta potential of – 40 mV). Thus, they do not tend to sit at the oil-water interface. However, at pH = 3 NPs have lower negative charge and so they are less hydrophilic which in turn the emulsion prepared with only NPs (0.5 wt. %) is oil in water and stable to coalescence. Binks and Whitby also reported an improvement in emulsion stability by controlling the pH of silica NP dispersion (Binks and Whitby, 2005). Based on their work, lowering of pH, or adding an electrolyte, changes particle charge and flocculation which in turn temporarily enhances emulsion stability.
At both acidic (pH = 3) and basic (pH = 9.5) conditions, the stability of an n-dodecane in water emulsion is enhanced due to the effect of CTAB alone as an emulsifier and the emulsion stability increases with an increase in CTAB concentration (Binks et al., 2007a). Binks et al., reported that at 10−4 M CTAB there is no emulsion but with an increases in CTAB concentration to 10−3 M (around CMC) a stable emulsion is generated (Binks et al., 2007a). With a further increase in CTAB concentration to 10−2 and 10−1 M the emulsion stability is further enhanced. In CTAB-silica NP mixture, they used 10−4 M CTAB, which cannot produce emulsion alone, along with different concentrations of NPs (0.1, 0.5, 1, 5, 10, 30 wt. %) which are neither emulsifier alone. Based on their results, addition of only 0.1 wt. % NP to 10−4 M CTAB solution can produce a considerable amount of emulsion which although creams with time but is kind of stable after 24 h (they did not mention how long the emulsion is stable and just presented photographs of vials taken after 24 h, which depicts the emulsion stability). They reported 0.504 wt. % particles required to mix with 10−4 M CTAB to produce an emulsion which is stable to coalescence. In another set of experiments, they kept NP concentration constant as 2 wt. % and increased CTAB concentration from 10−4 M to 10−1 M. At low CTAB concentrations (10−3 or 10−4 M) there is low surfactant adsorption onto NPs. Therefore, NPs still have enough negative charges leading to enough repulsion in between. As a result, NPs dispersion is stable without any sedimentation. Upon addition of higher concentrations of CTAB, i.e., 10−3 M (around CMC) and up to 10−2 M, NPs increasingly begin to settle down. At these CTAB concentrations, monolayer adsorption of surfactant molecules onto the surface of negatively charged silica NPs results in Van der Waals attraction between alkyl chains on the surface of neighboring particles leading to NPs sedimentation. The degree of this NPs sedimentation is the highest when the system contains 10−2 M CTAB. The n-dodecane in water emulsion stability is the highest at this condition (i.e., 10−2 M CTAB along with 2 wt. % NP) which corresponds to the least NPs charge. At higher CTAB concentrations, the sedimentation of NPs due to addition of CTAB is decreased demonstrating that a fraction of particles are re-stabilizing. At these conditions, surfactant molecules are attached onto the NPs surface through bilayer adsorption leading to positively increase in NPs charge and the resultant charge repulsion. The emulsion stability is again reducing at these conditions demonstrating that the system should be tuned to an optimum condition at which NPs are the least stable and have the lowest surface charge to produce the most stable emulsion. To exclude the effect of energy input into the system by the homogenizer, they repeated their experiments and used gentle manual shaking (20 times in 8 s) to make an emulsion. They reported the exact similar trend but with larger oil drops; therefore, they concluded that at low values of shear surfactants can adsorb to the NPs surface to stabilize the emulsion.
Kim et al., also addressed a remarkable improvement in decane in brine (8% NaCl and 2% CaCl2) emulsion stability due to the combined effect of cationic surfactant (dodecyl trimethyl ammonium bromide (DTAB)) and hydrophilic silica NP (Kim et al., 2016a). They performed a comparative study using different kinds of surfactants with different charge type including cationic (DTAB), anionic (alkydiphenyloxide disulfonate), non-ionic (polyoxyethylene 100 monostearate), and zwitterionic (lauryl hydroxysultaine) surfactants along with silica NP to emulsify create decane in brine (8% NaCl and 2% CaCl2) emulsions (Kim et al., 2016a). To make an emulsion, decane (as the oil phase) was co-injected along with the aqueous phase containing nanoparticle and different surfactants through a sand pack column. This study was aimed to investigate the combined effect of NP and surfactants as emulsifiers using minimum amounts of nanoparticles and surfactant to create oil in brine emulsion. Some preliminary experiments were done to obtain the minimum concentration of NP or surfactant to create an emulsion when only either nanoparticle or surfactants were used. Based on this study, the lowest NP concentration to generate a decane in brine emulsion is 0.01 wt. %, which was claimed to be one to two orders of magnitude lower than those used in literature. In addition, when only surfactant was used the lowest surfactant concentration to make an emulsion is as follows: 0.005% for cationic, 0.0005% for zwitterionic, 0.0005% for non-ionic, and 0.1% for anionic surfactants. Afterwards, the potential interaction between NP and different surfactants at these minimum concentrations were investigated. Kim et al. reported an enhancement in emulsion stability due to the combined effect of NP and all the surfactants except for the anionic one (alkydiphenyloxide disulfonate) and the order of enhancement is as follows: cationic > non-ionic > zwitterionic > anionic. For the cases of cationic and non-ionic surfactants, the emulsion stability is remarkably enhanced for over three months. It is worth mentioning that beside the highest degree of emulsification, charges of the reservoir rock can play an important role in choosing the best surfactant. For example, non-ionic surfactants would be the better choice compared to cationic surfactants to apply in negatively-charged sand stone reservoirs, since the surfactant adsorption/loss can be prevented.
In another study, Pei et al., also reported that the stability of oil (biodiesel) in brine (0.5 wt. % sodium chloride) emulsion enhances due to the combined effect of hydrophilic silica NP and hexadecyltrimethylammoniumbromide (CTAB) (Pei et al., 2015). To prepare an emulsion, they used a homogenizer for 2 min operating at 10000 rpm. In static emulsion stability tests, they used 0.01, 0.03, 0.1, 0.2, 0.3, 0.4, and 0.5 wt. % silica NP along with 0.1 wt. % CTAB as the aqueous phase. They reported that the emulsion is stable even after 60 days due to the effect of nanoparticle added to the surfactant-stabilized emulsion and with increasing NP concentration the emulsion stability is increased. Furthermore, Yoon et al., also reported an enhancement in n-decane in water emulsion stability due to the combined effect of silica NP, cationic surfactant (DTAB), and anionic polymer to form a complex colloidal layer adhered to the oil-water interface (Yoon et al., 2016).
2.1.1.1. Stabilization mechanism
2.1.1.1.1. Cationic surfactant effect to alter NPs wettability
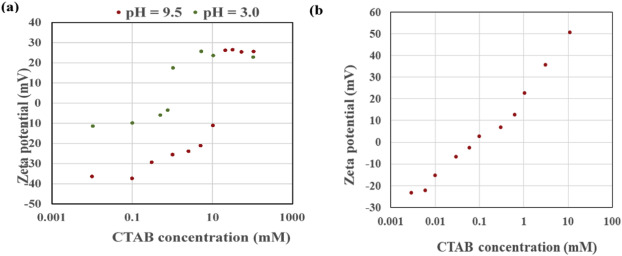
Addition of a cationic surfactant to a hydrophilic NPs dispersion has been reported to alter NPs' wettability (Binks et al., 2007a, Binks and Rodrigues, 2009). In one study, Binks et al., reported wettability alteration of very hydrophilic silica NPs due to adsorption of CTAB molecules on the particles’ surface. They performed zeta potential analysis to address the adsorption of CTAB on the particles (Fig. 1a). As can be inferred from Fig. 1a, the absolute value for zeta potential of silica particles with initial surface charge of – 40 mV at pH = 9.5 decreases with an increase in CTAB concentration and at 10−2 M CTAB NPs become uncharged. This change in zeta potential of particles is resulted from the adsorption of surfactant monomer on the particles leaving alkyl chains exposed to solution, indicating hydrophobization of NPs, which is depicted schematically in Fig. 2a and b. This condition corresponds to the most stable dodecane in water emulsion. It is worth mentioning that in this case the degree of NPs hydrophobization is not that much to invert the emulsion type from oil in water to water in oil emulsion. However, double inversion of emulsion due to cationic surfactant adsorption has been also reported. First from oil in water to water in oil due to monolayer adsorption of cationic surfactant on silica NPs and then with a further increase in surfactant concentration from water in oil to oil in water due to increase in hydrophilicity of NPs resulted from bilayer surfactant adsorption (Binks and Rodrigues, 2007b, Binks and Rodrigues, 2009, Binks et al., 2013). At higher CTAB concentrations, the zeta potential of the particles positively increases due to further adsorption of surfactant through chain –chain interaction to form bilayer on the particles (Binks et al., 2007a). This bilayer-coated particles become hydrophilic again, which is resulted from the exposure of head-groups of surfactants to solution (depicted schematically in Fig. 2c and d).
 Fig. 1. Zeta potential of a) 2 wt. % silica NPs at pH = 3.0 and 9.5 (the initial zeta potential in the absence of CTAB is equal to – 40 mV at pH = 9.5) (Binks et al., 2007a) b) 0.5 wt. % silica NP (the initial zeta potential in the absence of CTAB is equal to – 25.2 mV at pH = 6.1 (Zhu et al., 2015), as a function of CTAB concentration.
Fig. 1. Zeta potential of a) 2 wt. % silica NPs at pH = 3.0 and 9.5 (the initial zeta potential in the absence of CTAB is equal to – 40 mV at pH = 9.5) (Binks et al., 2007a) b) 0.5 wt. % silica NP (the initial zeta potential in the absence of CTAB is equal to – 25.2 mV at pH = 6.1 (Zhu et al., 2015), as a function of CTAB concentration. Fig. 2. Schematic depiction of cationic surfactant adsorption on the surface of negatively-charged silica NP with increasing surfactant concentration: a) partial monolayer adsorption at low surfactant concentration, b) full monolayer adsorption of surfactant molecules at just right surfactant concentration result in the highest degree of NP hydrophobization, c) partial bilayer adsorption of surfactant molecules at higher surfactant concentration making NP less hydrophobic, d) full bilayer adsorption of surfactant molecules render NP hydrophilic again.
Fig. 2. Schematic depiction of cationic surfactant adsorption on the surface of negatively-charged silica NP with increasing surfactant concentration: a) partial monolayer adsorption at low surfactant concentration, b) full monolayer adsorption of surfactant molecules at just right surfactant concentration result in the highest degree of NP hydrophobization, c) partial bilayer adsorption of surfactant molecules at higher surfactant concentration making NP less hydrophobic, d) full bilayer adsorption of surfactant molecules render NP hydrophilic again.Zhu et al., also used zeta potential measurement (Fig. 1b) to address electrostatic adsorption of CTAB molecules on the surface of negatively-charged silica NPs which in turn results in NPs wettability alteration (Zhu et al., 2015). This CTAB adsorption on the surface of negatively charged silica NP was also reported by Lan et al. (2007a) and Ma et al. (2010). Based on their results, CTAB molecules adsorb on the surface of silica NPs (with initial zeta potential of – 36 mV at pH = 5.4) through monolayer adsorption mechanism so that the surface charge of 1 wt. % silica NP solutions becomes zero at CTAB concentration of 0.42 mmol g−1 (Lan et al., 2007a). At this CTAB concentration, silica NPs mostly aggregate with particle size as large as more than 4 000 nm. At higher CTAB concentrations, bilayer adsorption of CTAB molecules on NPs surface results in a positively increase in NPs zeta potential accompanying with more repulsion in between leading to reduction in NPs size.
Lan et al., also tried to quantify this CTAB-assisted hydrophobization of silica NPs using three phase contact angle measurements (Lan et al., 2007a). As shown in Fig. 3a, contact angle increases with an increase in CTAB concentration and reaches the maximum of 85° at CTAB concentration of 0.46 mmol g−1 and then decreases with a further increase in CTAB concentration. This highest degree of hydrophobization has been resulted from the maximum amount of CTAB molecules adsorbed on NPs through monolayer adsorption. In this case, nearly all the surface areas of NPs are covered by CTAB head-groups with hydrophobic chains pointed out to the solution which in turn justifies enhancement in NPs’ tendency to sit at oil-water interface (Lan et al., 2007a). At higher CTAB concentrations, bilayer-coated particles again become hydrophilic due to the exposure of CTAB head-groups to the solution. Binks and Whitby also reported the effect of CTAB to alter wettability of initially hydrophilic silica NPs (Binks and Whitby, 2005). They measured the contact angle at dodecane-water interfaces on a hydrophilic glass substrate. As shown in Fig. 3b, the contact angle with an initial value of less than 20° increases with an increase in CTAB concentration up to CMC (1 mM) and then decreases with a further increases in CTAB concentration. In this regard, Binks and Whitby reported the maximum adsorption of n-Dodecyltrimethylammonium bromide (DTAB) onto silica at DTAB concentration equal to CMC (1.5 × 10−2 M = 0.46251 wt. % (McGrath, 1995)) and under alkaline conditions (Binks and Whitby, 2005). Cui et al., also addressed the hydrophobization of initially hydrophilic silica NP (with particle diameter between 7 and 40 nm) due to the adsorption of cationic surfactants (such as dodecyltrimethylammonium bromide (DTAB) or cetyltrimethylammonium bromide (CTAB) or the gemini surfactant (II-14-3)) (Cui et al., 2010b). Based on their study, un-modified silica nanoparticles with an extreme hydrophilicity cannot stabilize toluene in water emulsion. However, partially hydrophobized silica NPs act as an efficient emulsifier. In addition, they reported very high hydrophobicity of the silica nanoparticles resulted from the effect of double-chain cationic surfactant (di-C12DMAB) leading to the inversion of emulsion type from oil in water into water in oil emulsion (Cui et al., 2010b).
 Fig. 3. Three-phase contact angle of a) a pressed circular disk of silica NP at the paraffin oil-water interface (Lan et al., 2007a) b), CTAB solution (at pH = 6) on a hydrophilic glass substrate under dodecane (Binks and Whitby, 2005), as a function of CTAB concentration.
Fig. 3. Three-phase contact angle of a) a pressed circular disk of silica NP at the paraffin oil-water interface (Lan et al., 2007a) b), CTAB solution (at pH = 6) on a hydrophilic glass substrate under dodecane (Binks and Whitby, 2005), as a function of CTAB concentration.In the other study, Fuerstenau and Jia recognized four distinct regions for the adsorption of cationic surfactant (dodecylpyridinium chloride (DPC)) on the surface of negatively-charged and finely ground quartz (Fuerstenau and Jia, 2004). They concluded that at low surfactant concentration (region 1) the adsorption is governed by electrostatic interaction. In this case, surfactants adsorb individually on the quartz surface and there is no chain-chain interaction. In this region, the hydrophobicity of quartz surface is slightly increased (Fuerstenau, 2002). With increasing surfactant concentration (region 2) patches or hemi-micelles are formed at the interface. In this case, decrease in the absolute value of zeta potential and sharp increase in contact angle justifies the effect of surfactant adsorption as monolayer to hydrophobize quartz. In this and subsequent regions, adsorption is mainly due to chain-chain association. Region 3 starts at surfactant concentration required to neutralize the surface charge of quartz eliminating electrostatic attraction between surfactant-coated quartz and free surfactants. Therefore, in this region adsorption occurs only due to hydrophobic chain interaction leading to formation of bilayer. In this case, electrostatic repulsion may force already adsorbed surfactant to orient their head-groups towards solution leading to reduce quartz hydrophobicity reflected in decrease in contact angle. In region 4 which occurs at surfactant concentration around the bulk CMC, the bilayer is completed and the hydrophilic head of adsorbed surfactant is exposed to the solution.
2.1.1.1.2. Effect of cationic surfactants along with NPs to alter interfacial properties
Binks et al., measured the surface tension at different initial values of CTAB concentration with and without 2 wt. % NPs (Fig. 4a) (Binks et al., 2007a). As can be inferred from Fig. 4a, the surface tension in the presence of NPs is larger than the corresponding values in the absence of NPs, indicating that a fraction of cationic surfactant adsorbed on the surface of negatively-charged NPs and is not available at the interface (Binks et al., 2007a). Ahualli et al., also reported larger surface tension for the system containing 5 wt. % silica NP along with CTAB compared to that of only contains CTAB (Ahualli et al., 2011). As shown in Fig. 4b, the surface tension in the presence of NPs is independent of surfactant concentration and its value is close to that of pure water without any surfactant justifying the CTAB adsorption on silica NP leaving no free surfactant at the interface.
 Fig. 4. Effect of negatively charged silica NP on the surface activity of cationic surfactant (CTAB): a) Binks et al., 2007a b) Ahualli et al., 2011.
Fig. 4. Effect of negatively charged silica NP on the surface activity of cationic surfactant (CTAB): a) Binks et al., 2007a b) Ahualli et al., 2011.In this regard, Biswal et al., reported 49 mN/m for the IFT of n-hexane-water system which remains almost constant (they did not present any value) due to different loadings of only silica NP (Biswal et al., 2016). As can be inferred from Fig. 5a, NPs enhance the efficiency of CTAB to further reduce IFT. This effect has been attributed to the hydrophobization of negatively-charged silica NPs due to CTAB adsorption through electrostatic attraction. In this case, hydrophobic NPs can move to the interface and as a result enhance IFT reduction (Biswal et al., 2016). It is worth mentioning that at high enough surfactant concentration the enhancing effect of NPs is eliminated, because at this condition IFT has been already reduced to a large extent due to existence of a sufficient number of surfactant molecules at the interface (Fig. 5a). Ravera et al., also reported an increase in NPs hydrophobicity with increasing CTAB concentration justifying the enhancement in particles affinity for the interface (Ravera et al., 2006a, Ravera et al., 2006b). Therefore, the number of CTAB-covered NPs increases with an increase in CTAB concentration. In this case, CTAB-covered NPs can act as a carrier of CTAB molecules to the interface where surfactant molecules can be released to further reduce IFT (Ravera et al., 2006a). However, the opposite behaviour has been reported by Ahualli et al. (2011). As shown in Fig. 5b, the interfacial tension in a system containing CTAB solution and 1-octadecene increases due to addition of 5 wt. % silica NP. They attributed this trend to the NPs agglomeration resulted from adsorption of CTAB molecules which in turn, hinders CTAB-covered NPs’ approach to the interface. Therefore, one can conclude that although surfactant-assisted NPs agglomeration may have some enhanced effect in IFT reduction (as reported by Ravera et al. (2006a) and Biswal et al. (2016)) and emulsification (as reported by Binks et al. (2007a)) it may have a detrimental effect on interfacial properties as reported by Ahualli et al. (2011).
 Fig. 5. Effect of negatively charged silica NP on the surface activity of cationic surfactant (CTAB) to alter IFT between aqueous phase and a) n-hexane (Biswal et al., 2016) b) 1-octadecene (Ahualli et al., 2011).
Fig. 5. Effect of negatively charged silica NP on the surface activity of cationic surfactant (CTAB) to alter IFT between aqueous phase and a) n-hexane (Biswal et al., 2016) b) 1-octadecene (Ahualli et al., 2011).In this regard, Lan et al., also reported a range for the concentration of silica NPs and CTAB within which NPs and surfactant molecules can interact with each other to further reduce IFT between paraffin oil and aqueous phase compared to pure CTAB (Lan et al., 2007a). Based on their results, at CTAB concentrations lower than 0.01 mM with increasing NP concentration up to 1 wt. % the IFT decreases. However, further increase in NP concentrations to 2 and 5 wt. % leads to increase in IFT. Furthermore, at higher CTAB concentrations (greater than 0.01 mM) IFT increases with an increase in NP concentration. Therefore, they reported a concentration ranges of less than 0.1 mM CTAB and between 0.01 and 1 wt. % for silica NPs within which just enough CTAB molecules can adsorb on NPs’ surface to render them appropriately hydrophobic to adsorb on the interface and enhance IFT reduction (Lan et al., 2007a).
In a more recent study, Saien and Gorji investigated the effect of hydrophilic magnetite NP along with different concentrations of CTAB on IFT of the system containing n-hexane and water (Saien and Gorji, 2017). Based on their results, there is no change in IFT of the system due to different NP loadings (1.0 × 10−4 - 1.0 × 10−2 wt. %) of only NPs justifying their high hydrophilicity and the maximum IFT reduction of 89.3% was achieved with 0.75 mM CTAB without NP. They reported enhancement in IFT reduction due to addition of 1.0 × 10−3 wt. % NP to different concentrations of CTAB at neutral, acidic and basic environments. At neutral pH, hydroxyl groups on the NPs surface can bond to CTAB molecules through hydrogen bonds, rendering NPs less hydrophilic so that they can approach the interface and affect IFT. At acidic conditions, the surface charge of NPs is positive and there is a repulsion between them and CTAB molecules which in turn pushes surfactant molecules back to the interface to reduce IFT. Under basic pH conditions, NPs of negative charge can attract CTAB cationic head-groups. Therefore, this assembly with hydrophobic surfactant tail on its surface tend to migrate towards the interface and reduce IFT. Since they reported the highest IFT reduction in alkaline conditions one can conclude that the attractive interaction between NPs and surfactants is more efficient than the repulsive one to decrease IFT. Furthermore, they indicated that either acidic or basic condition is more effective than neutral pH from NPs and CTAB interaction point of view to reduce IFT.
2.1.2. Anionic surfactants
2.1.2.1. Anionic surfactants + positively charged NP
There are some articles addressing the enhancement in oil in water emulsion stability due to the effect of positively charged NPs mixed with anionic surfactants (Binks and Rodrigues, 2007a, Moghadam and Azizian, 2014, Cui et al., 2008). In this case, surfactants can be adsorbed on NPs due to charge attraction to form an agglomeration. This particle agglomeration adsorbs on oil-water interface to form a very stable emulsion (Binks and Rodrigues, 2007a).
In one study, Binks and Rodrigues reported a long term n-dodecane in water emulsion stability (6 months) without a change in average emulsion drop diameter and any oil release due to the addition of positively charged and hydrophilic alumina-coated silica NP (2 wt. %) to different concentrations (1, 5, 10, 20, 30, 50, 100, 400 mM) of SDS. They used a homogenizer with a 1 cm head operating for 2 min at 11000 rpm to mix equal volumes of aqueous phase (containing different loadings of SDS and NP) and n-dodecane (total 10 cm3). In this study, the highest stability was achieved at 30 mM SDS concentration around which NPs are the least stable and most flocculated. It is worth mentioning that emulsions prepared with only NPs at all concentrations between 0.1 and 5 wt. % are very unstable and complete phase separation was reported within several minutes of preparation (Binks and Rodrigues, 2007a). In this case, NPs are very hydrophilic and tend not to adsorb at the oil-water interface leading to a very rapid phase separation.
In another study, the increase in n-decane in water emulsion stability due to addition of 0.01 wt. % of positively charged ZnO NP to high concentrations of SDS (4, and 8 mM) at neutral pH has been reported (Moghadam and Azizian, 2014). On the other hand, stability of n-decane in water emulsion at low SDS concentrations (0.01, 0.4, 1 mM) is decreased after 24 h in the presence of 0.01 wt. % of ZnO NPs compared to pure SDS. It is worth mentioning that they used ultrasonication technique (60 s) using an ultrasonic probe to mix equal volumes of n-decane and water containing SDS and ZnO NP.
2.1.2.1.1. Stabilization mechanism
2.1.2.1.1.1. Anionic surfactant effect to alter wettability of positively-charged NP
The enhancement in emulsion stability due to the addition of positively charged NPs to anionic surfactant was attributed to the surfactant-assisted wettability alteration of NPs. At low SDS concentrations, SDS molecules are adsorbed on the positively charged and hydrophilic NPs through monolayer adsorption with the hydrophobic tail exposed to solution rendering NPs hydrophobic with more tendency to sit on the oil-water interface. Binks and Rodrigues reported an increase in hydrophobicity of NPs due to addition of SDS (Binks and Rodrigues, 2007a). Based on their work, this effect is increased with an increase in SDS concentration up to an optimum SDS concentration of 30 Mm (Binks and Rodrigues, 2007a). At higher SDS concentrations, there is a bilayer adsorption of SDS molecules on NPs surface leaving the hydrophilic sulfate head group exposed to solution. In this case, NPs again become hydrophilic with less tendency to sit at the oil-water interface. These adsorption mechanisms are schematically depicted in Fig. 6.
 Fig. 6. Schematic depiction of anionic surfactant adsorption on the surface of positively-charged silica NP with increasing surfactant concentration: a) partial monolayer adsorption at low surfactant concentration, b) full monolayer adsorption of surfactant molecules at just right surfactant concentration result in the highest degree of NP hydrophobization, c) partial bilayer adsorption of surfactant molecules at higher surfactant concentration making NP less hydrophobic, d) full bilayer adsorption of surfactant molecules render NP hydrophilic again.
Fig. 6. Schematic depiction of anionic surfactant adsorption on the surface of positively-charged silica NP with increasing surfactant concentration: a) partial monolayer adsorption at low surfactant concentration, b) full monolayer adsorption of surfactant molecules at just right surfactant concentration result in the highest degree of NP hydrophobization, c) partial bilayer adsorption of surfactant molecules at higher surfactant concentration making NP less hydrophobic, d) full bilayer adsorption of surfactant molecules render NP hydrophilic again.The aforementioned mechanism for the adsorption of anionic surfactant on the surface of positively charged NPs can be quantified through zeta potential measurements. Upon addition of SDS to NPs dispersion, the zeta potential of initially positive NPs decreases in magnitude, becomes zero, and negatively increases with a further increase in SDS concentrations, which is reported by different researchers (Fig. 7). At low SDS concentrations, monolayer adsorption of SDS molecules results in a decrease in NP's zeta potential up to a point where the zeta potential of the NPs becomes zero. At this SDS concentration, SDS-coated NPs become uncharged with the least repulsion in between. Therefore, NPs have the highest tendency to agglomerate. At this point, there is the most number of SDS molecules adsorbed on the surface of NPs through monolayer adsorption, schematically depicted in Fig. 6b, leaving the most number of surfactant hydrophobic tail exposed to the solution which in turn, maximizes the NPs hydrophobicity. At this condition where NPs have the highest hydrophobicity and there is the highest degree of flocculation, NPs can sit on the oil-water interface to stabilize the oil in water emulsion. At higher SDS concentrations, SDS molecules adsorb further on NPs surface through bilayer adsorption, schematically depicted in Fig. 6d, leaving hydrophilic sulfate head group exposed to the solution. This bilayer-coated silica NPs have negative charges and their negative charge increase with a further increase in SDS concentration, as shown in Fig. 7. At these concentrations, NPs again get hydrophilic with lower tendency to sit at the oil-water interface. Therefore, one can conclude that at an optimum anionic surfactant concentration, there is the highest degree of surfactant-assisted hydrophobization of NPs, which corresponds to the highest degree of NPs flocculation, which in turn enhances the stability of oil in water emulsions. Binks and Rodrigues reported instability and flocculation of the mixture of silica NPs due to addition of SDS at concentration of around CMC (Binks and Rodrigues, 2007a). Based on their work, the degree of this flocculation increases with a further increase in SDS concentration up to a maximum around 20 mM, beyond which NPs again get more stable (Binks and Rodrigues, 2007a). It is worth mentioning that the highest stability of n-dodecane in water emulsion was observed at 30 mM SDS concentration around which the flocculation of silica NP is the highest (Binks and Rodrigues, 2007a). The enhancement in toluene in water emulsion stability due to flocculation of hydrophilic clay particles is also addressed by Ashby and Binks who used salt as a flocculating agent instead of surfactant (Ashby and Binks, 2000).
 Fig. 7. Zeta potential of a) 2 wt. % silica NPs at pH = 3.5 (the initial zeta potential in the absence of SDS is equal to + 43 ± mV) (Binks and Rodrigues, 2007a) b) 1 wt. % AlOOH without pH adjustment (the initial zeta potential in the absence of SDS is equal to + 55.4 mV) (Yang et al., 2017), as a function of SDS concentration.
Fig. 7. Zeta potential of a) 2 wt. % silica NPs at pH = 3.5 (the initial zeta potential in the absence of SDS is equal to + 43 ± mV) (Binks and Rodrigues, 2007a) b) 1 wt. % AlOOH without pH adjustment (the initial zeta potential in the absence of SDS is equal to + 55.4 mV) (Yang et al., 2017), as a function of SDS concentration.Wettability alteration of Aluminium hydroxide oxide (AlOOH) NPs due to the addition of just right concentration of SDS is also reported by Yang et al. (2017). They reported the SDS molecule adsorption onto AlOOH NP (with initial surface charge of +55.4 mV) either through electrostatic attraction between SDS hydrophilic group (OSO3−) and NPs at low SDS concentrations or hydrophobic interaction at high SDS concentration. Based on their work, the zeta potential of 1 wt. % ALOOH NPs gets zero at SDS concentration of around 5 mM, resulting the most NPs flocculation (Fig. 7b). They attributed the rapid decrease in NPs zeta potential to the monolayer adsorption of SDS rendering NPs hydrophobic resulting from exposure of alkyl chains to the solution. Further SDS adsorption on the NPs surface through hydrophobic interaction between alkyl chains of free surfactant and already adsorbed ones make NPs hydrophilic again. At higher SDS concentration hydrophobic interaction is a dominant mechanism for surfactant adsorption on the surface of NPs rather than electrostatic attraction.
In another study, Cui et al., also reported a sequential wettability alteration of hydrophilic CaCO3 NP with slightly positive charge at pH = 8.4 (isoelectric point of particles is reported to be 9.3) due to addition of SDS (Cui et al., 2008). Based on their work, although NPs are hydrophilic and tend not to spontaneously settle at the interface, there is a temporarily stabilization of toluene or n-octane in water emulsion due only to the effect of CaCO3 NPs (in this article, there is nothing mentioned to define temporarily stable emulsion). It is worth mentioning that they used a homogenizer operating at 5 000 rpm for 3 min to mix an equal volume of oil and nanofluid. Therefore, one can guess that the emulsion prepared with hydrophilic NPs may be created due to the high amount of energy put in the system which in turn, breach NPs to the surface of emulsion droplets which was depicted by the presented micrographs. Based on their work, the size of emulsion droplets decreases with an increase in NP concentration. In these cases, coalescence was reported to be very appreciable at particle concentration lower than 2 wt. %. However, upon addition of 0.1 wt. % SDS to CaCO3 NP dispersion (2 wt. %) the improvement in oil in water emulsion stability was reported. They performed sensitivity analysis on SDS concentration and used different concentrations (0.1, 0.6, 1, 3, 6, and 100 mM) of SDS along with 2 wt. % CaCO3 NP. Based on their results, when SDS concentration is lower than 0.6 mM SDS molecules can be adsorbed on positively charged CaCO3 NPs through electrostatic attractions between NPs and anionic head of SDS. In this case, the external surface of NPs are covered by the hydrocarbon chain of adsorbed SDS, rendering NPs less hydrophilic which in turn results in a notable improvement in stability of toluene or n-octane in water emulsion compared to the case when there is only NPs. The zeta potential of CaCO3 NP (2 wt. %) gets zero due to addition of 0.6 mM SDS and negatively increases with a further increase in SDS concentration. At the medium SDS concentrations (0.6–3 mM) along with 2 wt. % CaCO3 NP, Cui et al., reported an emulsion phase inversion from toluene in water to water in toluene (Cui et al., 2008). To further understand this emulsion phase inversion, they investigated the effect of different concentrations of pure SDS (0.01, 0.1, 0.6, 1, and 3 mM) to stabilize oil in water emulsion. They reported that these emulsions are all oil in water emulsion and initial SDS concentration of at least 3 mM is required to stabilize toluene or n-octane in water emulsion against coalescence. On the other hand, since the emulsions prepared with only NPs are also oil in water type they concluded that the aforementioned emulsion phase inversion should be resulted from the interaction of SDS molecules and NPs leading to maximum NPs hydrophobization which in turn inverts the system to have a continuous oil phase containing water droplets. At even higher SDS concentrations (more than 6 mM) where NPs zeta potential becomes more negative, there is another emulsion phase inversion from water in toluene to toluene in water emulsion resulted from bilayer adsorption of SDS molecules on NPs surface rendering them hydrophilic again. These emulsions are not stable to coalescence compared to the systems containing only SDS or mixtures of 2 wt. % NP and low concentration of SDS (less than 0.6 mM). So it seems that bilayer-coated NPs are not as efficient emulsifier as monolayer-coated NPs, which is also reported by previous researchers (Binks and Rodrigues, 2007a, Yang et al., 2017).
Cui et al., also investigated the effect of different types of surfactant as an in-situ surface activation agent to change wettability of positively charged CaCO3NPs (Cui et al., 2010a). Based on their results, neither cationic surfactant (Cetyltrimethylammonium bromide (CTAB)) nor non-ionic surfactant (OP-10) can change wettability of CaCO3 NPs. However, the electrostatic interactions between positively charged NPs and anionic surfactant such as Dioctyl sodium sulfosuccinate (Aerosol OT, AOT), which is a double chain anionic surfactant, and SDS result in monolayer adsorption of SDS molecules at the particle-water interface (quantified by zeta potential measurements similar to the data presented in Fig. 7) rendering NPs partially hydrophobic with more tendency to sit at the interface which in turn enhances foam stability (Cui et al., 2010a). Based on their work, SDS is more efficient than AOT as a NP surface activation agent.
This surfactant-assisted hydrophobization of NPs was also confirmed by three phase contact angle measurement, as shown in Fig. 8. As can be inferred from Fig. 8a, the three phase contact angle of NPs (with an initial value of 18.5° in the absence of SDS, which justifies NPs hydrophilic nature) increases with an increase in SDS concentration to a maximum value of 55.5° at SDS concentration of around 5 mM (Yang et al., 2017), at which NPs zeta potential gets zero (Fig. 7b). At this point, monolayer adsorption of SDS molecules onto NPs surface renders them hydrophobic. Yang et al., reported an enhancement in foam stability at this condition where NPs have the highest affinity to settle at the interface (Yang et al., 2017). With a further increase in SDS concentration, the contact angle decreases due to bilayer adsorption of SDS molecules (Fig. 8a) with hydrophilic head exposed to solution rendering NPs again hydrophilic. To measure the data presented in Fig. 8a, the mixture of 1 wt. % AlOOH NP and different concentrations of SDS were centrifuged at 6 000 rpm for 30 min to separate the particles, which then washed with deionized water to remove SDS that is not adsorbed on NPs. Afterwards, the particles were heated up to 75 °C for 6 h. Then the tablets were made by compressing (under 16 MPa) the dried particles, and sessile drop technique was applied to measure the contact angle and wettability of the tablet.