1. Introduction
Microbes are living organisms that are perceived only on a microscopic scale. They are found almost everywhere on earth, either in unicellular form or in a colony of cells [1]. The most common examples of microbes are fungi, bacteria, and viruses, of which the first two are primarily responsible for outbreaks on textile materials [2]. However, textiles can also be processed to achieve antiviral properties, thus helping to prevent the spread of viruses [3]. Although studies on the antiviral properties of textiles are still in the emerging phase, the antifungal and antibacterial properties of textiles have been widely investigated.
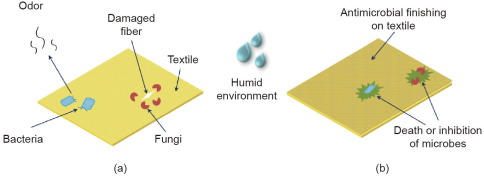
Antimicrobial finishing can protect textiles from microbial damage and enhance durability, while protecting the wearer from microbial infections. Fig. 1 shows a schematic representation of microbial disturbances on unfinished textile material compared with the textile finished with an antimicrobial agent. In addition to fungi and bacteria, the significance of and opportunity for using textiles to hamper the spread of viruses has come to the forefront during the current pandemic, if the textiles are provided with an antiviral finish. Therefore, antimicrobial finishing on textiles is becoming a matter of high importance and has received significant attention.
 Fig. 1. Schematic representation of (a) microbial attack on unfinished textile and (b) antimicrobial activity of finished textile toward the microbes.
Fig. 1. Schematic representation of (a) microbial attack on unfinished textile and (b) antimicrobial activity of finished textile toward the microbes.To date, several methods have been used for the application of antimicrobial agents to textiles. A convenient method of antimicrobial finishing is to use the finishing agent in the dope solution and form the textile fibers through a spinneret and coagulation bath during spinning [4]. This method produces a very durable finish because the finishing agent becomes an integral part of the fiber itself. However, this method can only be applied to regenerated or synthetic fibers, not natural fibers. The pad–dry–cure technique is an extensively used method for applying finishes to textiles made from either natural or synthetic fibers. Nevertheless although this is a practical method with industrial-scale use, it has some major drawbacks. For example, padding produces 70%–100% wet pickup, which requires the evaporation of waterthrough an energy-intensive drying process. In addition, during evaporation, migration of the finishing agent occurs on the fabric surface, which often results in uneven finishing [2]. Another common technique of applying the finish is exhaustion or impregnation, which also requires water as a medium and further drying [5]. Some advanced methods for antimicrobial finishing have also been reported, including the sol–gel technique and layer-by-layer deposition [6], [7]. The sol–gel technique is a method in which the fabric is impregnated in a sol (a colloidal solution that gradually changes into a gel form) and then passes through a subsequent padding and drying-washing system [7]. Layer-by-layer deposition is a technique in which fabric is dipped into a cationic polymer solution in one step and an anionic polymer solution in another step to deposit polymer layers sequentially [6]. Rinsing and drying of the fabric are required between the dipping operations in this technique. Although these methods are comparatively more effective than traditional wet finishing, the use of water and energy for drying is still a matter of concern. A few relatively dry techniques for antimicrobial finishing have been reported in the literature. For example, the deposition of ultraviolet-cured chitosan on cotton and silk to confer antimicrobial properties has been reported, where the fabric was dipped in a chitosan solution containing a photo initiator and then dried prior to the curing process [8]. In another study, in situ generation and deposition of zinc oxide nanoparticles (ZnONPs) on cotton were reported, where the fabric was immersed in a zinc acetate water/ethanol solution in a sonication flask for irradiation for 30 min (20 kHz, 750 W), followed by washing and drying [9]. Although the antimicrobial properties attained were encouraging, these methods are not completely dry or energy-efficient, given the necessity for water and subsequent drying.
The requirement of a large amount of water (followed by wastewater generation) and subsequent energy-intensive drying processes in conventional processes implies that an efficient and environmentally friendly alternative solution is of great interest to the industry. In this context, using plasma in textile finishing is a unique approach that can significantly reduce the use of water and energy, as plasma treatment does not require water or drying, which result in considerable water and energy savings [10], [11]. Fig. 2 illustrates the advantage of using plasma in antimicrobial finishing, compared with conventional wet techniques, such as the pad–dry–cure method. Plasma-assisted antimicrobial finishing of textiles is achieved by polymer deposition or graft polymerization. Polymer deposition involves the incorporation of an antimicrobial finishing polymer by spreading it in the form of a thin film on the textile substrate, whereas graft polymerization is the integration of the antimicrobial finish with the textile surface [10], [12], [13]. In addition, even if the plasma is used as a pretreatment process before finishing, the essential functional properties of the textiles (e.g., uptake) can be greatly improved, and a similar finishing effect can be achieved using less water and time.
 Fig. 2. Schematic illustration of the (a) pad–dry–cure method and (b) typical plasma application for antimicrobial finishing of textiles.
Fig. 2. Schematic illustration of the (a) pad–dry–cure method and (b) typical plasma application for antimicrobial finishing of textiles.Considering the importance of antimicrobial finishing on textiles and the radical improvement in finishing efficacy when plasma is involved, plasma-assisted antimicrobial finishing on textiles is a topic of utmost interest. However, existing relevant reviews are based on either the antimicrobial finishing agents used on textiles or achievable functionalities and efficiency when using plasma. To date, no detailed review has focused on the importance of plasma-assisted antimicrobial finishing on textiles. Therefore, the main aim of this paper is to focus on and discuss the use of plasma for antimicrobial finishing on textiles and to investigate the rationale and future opportunities. To achieve this, the mechanism of microbial attack, types of antimicrobial agents, methods of antimicrobial testing, and plasma and its mechanisms are discussed, followed by a critical evaluation of plasma-assisted antimicrobial finishing on various textile materials.
2. Mechanism of microbial attack on textiles
Microbes (mostly fungi and bacteria) can propagate on textile materials during their use and storage. A humid environment and the tendency of textiles to absorb moisture promotes the growth of microorganisms [14]. Because of their comparatively higher moisture absorption ability, textiles from natural fibersare more susceptible to microbe attack than synthetic fibers [15]. Microbes can cause visual and functional alterations, as well as cause unhygienic issues on textiles that can be harmful to the wearer. As mentioned above, among microbes, fungi and bacteria are the most detrimental to textile materials. Among these, fungi are mostly responsible for discoloration, stains, and fiber damage. Although bacteria are not as damaging as fungi, they produce an unpleasant odor and can lead to a slimy texture on the textile surface [2].
The effect of microbes on textile materials is usually related to two main phenomena: assimilation and degradation. Assimilation is the stage when microbes (i.e., fungi and bacteria) use textiles as their nutrition source [16]. Carbohydrates in plant-based fibers (e.g., cotton) and proteins in animal fibers(e.g., wool) act as a nutrient source for microbes. For example, cotton is more prone to fungal attack than it is to attack by bacteria, whereas wool is more prone to bacterial attack than it is to attack by fungi [2].
Microbes can attack the whole material or only a single component, such as dirt present on the surface or any additive incorporated in textiles, that is suitable for their growth. Microbes produce extracellular enzymes for collecting food, which causes physical changes in textile properties, such as loss of strength and flexibility [17]. Plant fibers (e.g., cotton, jute, hemp, and flax) are more prone to attack by cellulase enzymes produced by cellulolytic fungi [2]. Moreover, many fungi can produce pigments and are thus capable of causing discoloration. Because of their structure, which is similar to that of cellulose, regenerated cellulose fibers (e.g., viscose, acetate, and triacetate) are also susceptible to microbial attack. However, protein fibers, such as wool and silk, have higher resistance to fungi than plant fibers. Nevertheless, in adverse conditions, damage can occur because of the actions of proteolytic (protein-degrading) enzymes created by fungi and bacteria [17]. Synthetic fibers, such as polyester (PET), nylon, and acrylic, are quite resistant to microbes. However, the presence of additives in their chains, such as antistatic agents, lubricants, and thickeners, and the existence of dirt, soil, and dust can encourage the growth of microbes. In addition, the body waste of the wearer, that is, sweat and sebum, promotes the growth of microbes in textiles [2].
Small spots or bubbles seen on the textile surface often occur during the initial stage of microbe growth. Then, the degradation stage becomes dominant via the penetration of microbes inside the cavities of fibers and breakage of bonds, which adversely affects the physical properties (e.g., mass loss), mechanical properties (reduction of breaking strength), and chemical properties (e.g., degradation of cellulose or protein) [16].
3. Types of antimicrobial finishing agents
Various antimicrobial finishing agents have been proposed over the years to prevent microbial attacks on textiles. These include metallic compounds and nanoparticles, organic compounds (quaternary ammonium compounds (QACs), polybiguanides, N-halamines, and triclosan), natural polymers, and dyes, such as chitosan and plant extracts. Table 1 lists the most common types of antimicrobial agents used in textile applications and their advantages and disadvantages [15], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35].
Table 1. Common types of antimicrobial agents used in textiles and their merits and demerits.
| Type | Action | Merits | Demerit | Applications | References |
|---|---|---|---|---|---|
| Ag and other metals | Producing reactive oxygen species, demolition of protein, lipid, and DNA | Effective and durable | Chance of depletion | Cotton, wool, silk, polyester, nylon, and regenerated cellulose | [15], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27] |
| QACs | Formation of complex with microbes, denaturing protein, disturbing DNA to reduce propagation | Effective and durable | Often hazardous | Cotton, polyester, nylon, and wool | [26], [27], [28], [29] |
| Polybiguanide | Damaging lipids, leakage of cytoplasmic sources | Effective and durable | Large amount required | Cotton, polyester, and nylon | [26], [27] |
| Triclosan | Prohibiting lipid biosynthesis, cell membrane integrity depletion | Effective and durable | Breaks into toxic dioxin | Polyester, nylon, polypropylene, cellulose acetate, and acrylic | [26], [27], [30] |
| N-Halamines | Binding with microbes, preventing enzymatic and metabolic processes | Effective and durable | Needs regeneration, can cause odor | Cotton, polyester, nylon, and wool | [27], [31] |
| Chitosan | Blocking protein synthesis, obstructing transportation of solutes toward cells | Eco-friendly | Poor durability, opposing effect on handle | Cotton, polyester, and wool | [26], [32], [33], [34], [35] |
Heavy metals, such as silver, copper, and zinc, as well as metal oxides, such as titanium dioxide and metal salts, have been commonly used in the antimicrobial finishing of textile materials. These metals or metallic compounds show effective antimicrobial performance because of their ability to prevent DNA replication of microbes, cause irregular operation of the cytoplasmic membrane, discharge intracellular constituents, and break protein linkages [15]. These compounds are toxic to microbes at very low concentrations and can make microbes inactive or kill them. Among these inorganic compounds, silver is most commonly used in textiles because of its low toxicity toward humans [36]. There are a number of silver-based commercial antimicrobial finishing products available on the market that can be directly applied to textiles, for example, AlphaSan (Milliken, USA), MicroFresh (O’Mara, USA), toxicity concern associated with metal nanoparticless, Canada) [2].
Recently, the application of metallic compounds in textiles for antimicrobial activity has been improved by using metallic nanoparticles. This is because nanoparticles have distinct physicochemical properties and have a high surface area to volume ratio, which acts favorably during contact with microorganisms[37]. Although silver nanoparticles (AgNPs), TiO2 nanoparticles (TiO2NPs), ZnONPs, and copper nanoparticles (CuNPs) are the most frequently reported antimicrobial finishing agents used on textiles, AgNPs remain the optimal material for this purpose. Although there is a toxicity concern associated with metal nanoparticles when using small particle sizes (< 10 nm) that can penetrate human skin, the application of AgNPs has been widely investigated because of their low toxicity and wide spectrum of antimicrobial activity [38], [39]. Many studies have reported an approximately 99.9% reduction in microbes when AgNPs are used in key textile fibers, such as cotton, wool, silk, polyester, and polyamide [18], [19], [20], [21], [22], [23], [24], [25]. Comparatively, other nanoparticles for antimicrobial finishing on textiles have been reported mostly on cotton, with some exceptions [39].
In addition to the inorganic sources stated above, various organic compounds are also extensively used in textiles for antimicrobial finishing. For example, QACs are widely used for this purpose. The antimicrobial performance of QACs is contingent on their alkyl chain length, with approximately 12–18 carbons commonly showing good antimicrobial activity [2]. The cations in QACs assist their attachment to the negatively charged textile fiber surface as well as the formation of a QAC/microbe complex with an electronegative cell membrane [28], [29], [40]. The formation of the QAC/microbe complex disrupts the protein activity and functions of microbes [40]. It also reduces the propagation ability of microbes by disturbing their DNA [41].
In addition to QACs, polybiguanides, N-halamines, and triclosan are three other groups of synthetic organic antimicrobial agents used in textiles. Polybiguanides are composed of cationic biguanides separated by long hydrocarbon chains [30]. A longer polymer chain of polybiguanides delivers more cationic sites, which results in higher antimicrobial activity. Among the polybiguanides used, polyhexamethylene biguanides (minimal inhibitory concentration of 0.5–10 parts per million (ppm)) are the most commonly used antimicrobial finishing agents in textiles [2]. N-halamines are heterocyclic compounds with one or two halogens (X) covalently bonded with nitrogen (N) [31]. In the presence of water, the halogen ion is replaced by a hydrogen ion (N–H), which then binds with microbes and stops their metabolic and enzymatic processes. Because the application of N-halamines leaves a large amount of free halogen (commonly chlorine) on the fabric surface, a further reduction process is required to avoid discoloration and unpleasant odor from the fabric. Triclosan (2,4,4-trichloro-2-hydroxydiphenyl ether), an organic synthetic polymer, is an antimicrobial agent suitable for inhibiting the growth of a wide variety of microbes [30]. Because of its small molecule size, triclosan is exhausted into textile materials such as disperse dyes. The slow release of triclosan during the use of textiles leads to antimicrobial activity [2].
Although the synthetic antimicrobial agents mentioned above have been proven to be effective and durable on textiles, one of their drawbacks is the hazardous effect toward human health and the surrounding environment. In this respect, some natural polymers and dyes could be considered because of their environment-friendly nature and confirmed antimicrobial activity. Among the natural antimicrobial agents, chitosan is possibly the most widely used polymer with proven activity against microbes [30]. Chitosan is a deacetylated chitin, which is the second most abundant polysaccharide after cellulose [35], [42]. Because the amine groups in chitosan provide positive charges, they interact with the negative charges in microbes and change the cell surface and permeability of microbes, while producing leakage in their molecules. This can inhibit the synthesis of messenger RNA (mRNA) proteins via penetration into the nuclei of the microorganisms. Furthermore, chitosan can form chelates with metal ions, which are essential for microbial growth. Chitosan has been frequently investigated on cotton, silk, polyester, viscose, and so forth, and as an antimicrobial finish (even as nanoparticles), and its durability on textiles can be further enhanced using cross-linking agents [32], [33], [34], [35]. However, because it is effective only at higher concentrations and has a propensity to alter other important properties of textiles (e.g., handle and permeability), it is still not recommended as a commercial finishing agent on the same level as synthetic antimicrobial polymers [2].
Various natural dyes and extracts with antimicrobial activity are also used in textile finishing. The antimicrobial performance of natural dyes and extracts largely depends on the chemical structure and functional groups, as well as the presence of tannins (a naturally occurring polyphenol) in their composition. Natural dye sourced from gull nuts (Quercus infectoria) showed higher reduction (97.4%–99.5%) of the microbes’ colony forming units compared with a commercial synthetic antimicrobial agent (96.2%–98.4%) [43]. In addition, many other natural dyes, such as turmeric (Curcuma longa), pomegranate (Punica granatum), amala (Mallotus philippinensis), cutch (Acacia catechu), myrobalan(Terminalia chebula), and pine cones, have shown antimicrobial performance when used on textile materials such as cotton and wool [43], [44], [45]. The use of mordants (metal salts) resulted in a further increase in the durability of natural dyes and antimicrobial performance. In addition to dyes, various natural extracts and polymers, such as neem (Azadirachta indica), aloe vera (Aloe barbadensis), ginkgo (Ginkgo biloba), alginate, cinnamon essential oil, and propolis, have also been effectively used for antimicrobial finishing of textiles [46], [47], [48], [49], [50].
However, the major problem in using natural compounds is their initial composition as they are usually a complex mixture of various elements, and the composition varies when collected from different sources. In addition, for natural polymers, the required concentration for the inhibition of microbes is often high, and thus other properties of textiles could be altered. Therefore, a combination of natural materials with synthetic polymers, such as alginate/QACs, chitosan/AgNPs, and chitosan/ZnONPs, have also been considered as antimicrobial finishing agents, showing positive results [33], [49], [51].
4. Methods of testing antimicrobial properties of textiles
Several standard methods are available for testing the antimicrobial performance of textiles. These techniques allow microbes to grow in the presence of a textile substrate and then assess the resistivity of the substrate toward that particular microbe (using fungi, bacteria, or viruses). Although standards are available for antifungal, antibacterial, and antiviral testing of textiles, the former two are widely practiced because of their greater impact on the physical and chemical properties of textiles resulting from the influence of fungi and bacteria [2].
These testing methods can be divided into qualitative and quantitative test methods. Qualitative methods present a fast and easy approach to determine antimicrobial activity, particularly when the test samples are large in quantity and require screening, whereas quantitative methods are generally cumbersome processes that consume a lot of material [52].
Qualitative test methods are also known as agar diffusion methods because the textile material is placed on nutrient agar plates (containing bacterial cells) during the experiment [53]. ISO 20645:2004 [54], AATCC 147:2004 [55], and JIS L 1902:2008 [56] are some of the best-known examples of agar diffusion testing. In the ISO 20645 method, the test sample is placed between two layers of agar plates, where the upper layer is inoculated with microbes and the lower layer consists of agar only. In the JIS L 1902 method, the test sample is placed on one agar layer containing microbe cells, and in the AATCC 147 method, the agar layer is streaked with microbes before the test sample is place on it [52]. These methods measure the antimicrobial activity by observing the halo formation on test samples, that is, the places where microbes do not grow. Although a larger halo size could be an indication of higher antimicrobial performance, quantification is not usually performed using these methods [38].
The quantitative methods of antimicrobial activity testing are also known as absorption or suspension tests. These methods can provide values for antimicrobial performance, depending on the reduction in microbial growth [2], [57]. Generally, in quantitative methods, a bacterial inoculum is placed directly on the test sample, leading to the adsorption of all liquid on the textile material. After incubation for a certain period, the bacteria are removed from the test sample by successive dilution and the total bacterial number is determined. The antimicrobial performance of the test sample is calculated as a percentage of bacterial reduction compared with a control sample that is not treated (finished) with an antimicrobial agent [52]. ISO 20743:2007 [58], JIS L 1902:2008 [56], and AATCC 100:2004 [59] are some common examples of quantitative methods that are commonly used to test the antimicrobial properties of textiles.
5. Plasma treatment
5.1. Function of plasma
Plasma is composed of a high concentration of reactive species and is capable of altering the physicochemical properties of a polymer surface [11]. Because it is distinct from solid, liquid, or vapor states, plasma is referred to as the fourth state of matter. The plasma state is achieved by applying energy to a neutral gas, for example, heating or exposure to electromagnetic fields [11], [60]. Thus, a quasi-neutral mixture of various active species, such as electrons, ions, neutrons, radicals, photons, excited molecules, and metastable atoms, is produced with high energy. Hence, when plasma is guided to the surface of a material, it dissociates ranges of chemical bonds on the surface (usually at a depth < 10 nm) and thus alters the functionality. In addition, the application of plasma produces various simultaneous recombination mechanisms, which can be used to deposit a polymer layer on the textile surface through grafting or coating [61]. Fig. 3 shows a schematic representation of the functional properties and polymer grafting on a textile surface using plasma treatment [60].
 Fig. 3. Schematic illustration of (a) achieving functional property and (b) polymer grafting on a textile surface (i) during and (ii) after plasma treatment.
Fig. 3. Schematic illustration of (a) achieving functional property and (b) polymer grafting on a textile surface (i) during and (ii) after plasma treatment.By choosing a suitable gas, or a combination of gases (mostly O2, H2, N2, NH3, air, and inert gases), and selecting the correct plasma parameters (e.g., pressure, energy, time, and gas flow rate), definite alteration of the textile surface can be achieved [62]. For example, the correct use of plasma parameters and treatment conditions can improve the wettability, chemical reactivity, chemical and mechanical resistance, and adhesion properties of a material [63]. Depending on the plasma environment and parameters, four key phenomena (simultaneous and interconnected) are commonly observed on a textile substrate: etching, chain scission, formation of radicals, and polymer deposition [60].
Etching is the elimination of particles from the textile surface within a very narrow limit (i.e., a few hundred angstroms) without changing the bulk characteristics [64]. This phenomenon is also connected to the deposition of polymers on textiles because the etched particles are ionized in the plasma environment and deposited on the surface, and they thus continue to undergo recombination [65]. The collision of the active species with the molecules on the textile surface leads to chain scission and abstraction of atoms (by etching). In addition, new groups are created on the surface when plasma radicals interact with the surface radicals, causing the surface to become functionalized (Fig. 3(a)) [65]. Furthermore, chain cross-linking occurs through the recombination of radicals. When a finishing polymer (such as antimicrobial finishing) is introduced into the plasma chamber, the polymer is grafted onto the textile substrate through the simultaneous cross-linking of plasma radicals (as shown in Fig. 3(b)) [60].
5.2. Plasmas used in the processing of textiles
Plasma can be applied either at high or low temperatures. Because most textile fibers are heat-sensitive, only low-temperature plasma (also referred to as cold plasma) is applied to textiles [66], [67], [68], [69], [70]. Plasma treatment can be conducted through direct current (DC) or alternating current (AC) using low-frequency (1–500 kHz), radio-frequency (RF) (commonly 13.56 or 27.12 MHz), and microwave (commonly 915 MHz or 2.45 GHz)) energy [66], [71].
Plasma can be produced either at low pressure in a closed chamber or at atmospheric pressure [72]. Low-pressure (1–100 Pa) plasma is usually applied using RF or microwave energy [11]. Although this technique can produce a uniform effect and has good reproducibility with respect to textiles, while consuming a small amount of gas, it is difficult to adapt to large-scale continuous production of textiles because the sample size is limited to the size of the closed chamber [66], [71]. Therefore, the application of plasma under atmospheric conditions is considered more practical for continuous textile line production [72].
As illustrated in Fig. 4, four types of atmospheric-pressure plasmas are commonly used in textiles, namely, corona discharge (CD), dielectric barrier discharge (DBD), atmospheric-pressure glow discharge (APGD), and atmospheric-pressure plasma jet (APPJ), as listed in Table 2 [11], [61], [63], [73]. In the CD method, the electrodes used are dissimilar: One is needle-like, and the other is like a cylinder or plate [11]. Plasma exists in an area of extending gas near the needle tip called the ionization region. The plasma species diffuses toward the other electrode, producing a drift region (Fig. 4(a)). The distance between the two electrodes is usually very small and suitable for thin textiles [61]. However, the ionization in this method is very weak and has an inhomogeneous effect on the surface. Hence, the use of CDs in textiles is currently very limited.
 Fig. 4. Schematic illustration of different atmospheric-pressure plasma treatments on textiles: (a) CD, (b) DBD, (c) APGD, and (d) APPJ.
Fig. 4. Schematic illustration of different atmospheric-pressure plasma treatments on textiles: (a) CD, (b) DBD, (c) APGD, and (d) APPJ.Table 2. Common methods of atmospheric plasma treatment in textile processing.
| Application method | Characteristics | Common parameters | References |
|---|---|---|---|
| Corona discharge | Weak ionization, inhomogeneous effect | Near 1 mm inter-electrode spacing | [11], [61] |
| Dielectric barrier discharge | Mostly uniform or filamentous effect | 20 kV (AC) | [11] |
| Atmospheric-pressure glow discharge | Uniform and homogenous effect, requires expensive helium (He) gas | High frequency (2–60 MHz) and low voltage (~200 V) | [11] |
| Atmospheric-pressure plasma jet | Uniform toward any shape though only toward one side of textiles | RF, 100–250 V | [63], [73] |
DBD is an upgraded technique (compared with CD), where an insulating material is coated by at least one of two electrodes that are placed 2–5 mm apart (Fig. 4(b)). The applied voltage for the common DBD technique is approximately 20 kV (AC) [11]. Although two forms of DBD can occur, homogeneous or filamentous, uniform and homogeneous surface treatment can be achieved by careful selection of the treatment conditions. The third technique, APGD, is usually conducted at a high frequency (2–60 MHz) and low voltage (~200 V) using two bare electrodes (uncoated metal) placed at a distance of a few millimeters (Fig. 4(c)) [11]. Although this technique commonly produces a uniform and homogenous effect on textiles, a shortcoming is the necessity to use expensive He gas to avoid arc formation between the electrodes. To offset this problem, other gases are sometimes used (e.g., Ar and N2) as alternatives [71], [73]. Finally, the APPJ technique is the most recent inclusion, in which two electrodes are placed in a concentric manner (Fig. 4(d)). The gas passes through the electrodes, and the applied energy (RF, 100–250 V) converts the gas into plasma. Plasma then exits toward the surface of the textile substrate through a nozzle. The APPJ technique can be uniformly applied to any shaped textile [11], [73].
In addition to those mentioned above, there are a few other types of plasma application techniques commonly used in textile finishing, including vapor deposition and inductively coupled plasma [74], [75]. Plasma vapor deposition is the projection of a continuous vapor phase toward a textile substrate to achieve a thin film layer. This thin layer is mostly achieved in textile industriesby plasma sputter deposition in the presence of a magnetic field (also known as a magnetron sputter deposition system) (Fig. 5(a)) [74]. The textile substrate is placed on the anode, and a metal or alloy target is held with the cathode. The magnetic field is placed parallel to the target near the cathode to trap electronsnear the target. Commonly, argon (Ar) gas is used for this purpose; Ar breaks down with the application of energy and forms an abnormal glow discharge. The presence of a magnetic field increases the plasma density and the number of sputtered atoms that hit the target, resulting in a higher deposition on the textile substrate.
 Fig. 5. Schematic diagrams of (a) magnetron sputter deposition system and inductively coupled plasma in (b) cylindrical and (c) planar geometries using RF energy.
Fig. 5. Schematic diagrams of (a) magnetron sputter deposition system and inductively coupled plasma in (b) cylindrical and (c) planar geometries using RF energy.The other type of plasma, that is, inductively coupled, can be either cylindrical (Fig. 5(b)) or planar (Fig. 5(c)), and is commonly powered by RF energy [75]. The cylindrical form is maintained by the axial electrostatic or electromagnetic field of the primary coil. However, in a planar coil system, a flat helix is coiled from the axis to the outer radius, and multipole magnets are used outside to enhance the outward plasma uniformity. Although inductively coupled plasma can be used either at low pressure or atmospheric pressure, most previous studies have used low-pressure conditions to achieve a higher plasma density.
5.3. Textile characterizations for assessing plasma modification
Common methods for evaluating textile modification by plasma treatment include both physical and chemical analyses. The application of plasma commonly disrupts the surface of textiles (often called plasma etching) at a very narrow depth. This can be observed through the morphological analysis of the textile surface, for example, scanning electron microscopy (SEM) images [35]. However, because the changes in the surface are nominal, they are sometimes not perceived in SEM images. In such cases, other techniques for analyzing surface topography, such as scanning probe microscopy, are useful, providing numerical values of the surface roughness [68].
The chemical changes in textiles by plasma treatment largely depend on the chemical structure of the textile itself and the plasma gas used. Different plasma gases can produce different functionalities on the textile surface by introducing new chemical groups. For example, oxygen (O2)-containing plasma can produce new C O or O–C–O groups on cellulosic fibers, whereas nitrogen-containing plasma can produce C–N or O
O or O–C–O groups on cellulosic fibers, whereas nitrogen-containing plasma can produce C–N or O C
C NH groups [76]. Fluorine-containing gases (e.g., C2F6, C3F6, and SF6) are often used to produce a thin hydrophobic layer on a textile substrate [60]. The changes in chemical structure can be investigated using various techniques, including Fourier transforminfrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS). The application of plasma on textiles affects the surface energy, wettability, and color (nominal change caused by etching, which alters the light reflection pattern). Hence, plasma modifications on textiles are also often assessed by investigating the lightness of the surface, surface energy measurement, and capillary height measurement [60], [77], [78].
NH groups [76]. Fluorine-containing gases (e.g., C2F6, C3F6, and SF6) are often used to produce a thin hydrophobic layer on a textile substrate [60]. The changes in chemical structure can be investigated using various techniques, including Fourier transforminfrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS). The application of plasma on textiles affects the surface energy, wettability, and color (nominal change caused by etching, which alters the light reflection pattern). Hence, plasma modifications on textiles are also often assessed by investigating the lightness of the surface, surface energy measurement, and capillary height measurement [60], [77], [78].
6. Applications of plasma treatment in antimicrobial finishing of textiles
6.1. Grafting and polymerization of antimicrobial compounds
Unlike common processes such as pad–dry–curing, which are employed for the grafting of various compounds on textiles, plasma treatment can activate the substrate and monomers to induce the grafting reaction without heating or immersion in a chemical bath, reducing water and energy consumption and minimizing environmental pollution [79]. Polymerizable precursors such as vinyl, aromatic, cyclic, and saturated hydrocarbons or metalorganic compounds can be directly polymerized on the surface of a substrate with the aid of plasma. If the polymerizable precursors cannot be directly used in the plasma chamber, reactive gases (such as O2 or N2) or non-reactive gases (such as Ar and He) can be used for surface activation. Free radicals are created on the surface during the collision of energetic species present in the plasma medium with the polymeric chains of the substrate [80], [81]. Vinyl monomers can react with free radicals produced by plasma and undergo graft polymerization on activated substrates. Usually, the surface of the textile substrate is activated by plasma, and free radicals are produced on the surface. Then, the activated substrate is subjected to the vinyl monomer solution by immersion, padding, or spraying methods. Heating the plasma-treated substrate, the monomer-treated substrate may enhance the grafting yield. The deposition of the monomer on the textile substrate by padding, evaporation, or spraying enables the process to be carried out continuously in line with atmospheric-pressure plasma treatment, reducing the use of chemicals, energy, and time [12], [26].
QACs containing vinyl groups can be grafted onto textile fibers using plasma and impart antimicrobial functionality. Plasma treatment creates free radicals on the surface of the fibers. Peroxy radicals are formed upon exposure of the initially formed radicals to O2 or air, which initiates the grafting polymerizationof vinyl monomers on the substrate. Addition of Mohr’s salt (Fe(NH4)2(SO4)2·6H2O) can prevent homopolymerization in the solution, increasing the grafting yield. Cornelius et al. [12] employed atmospheric plasma (DBD and APPJ) to graft (3-acrylamidopropyl) trimethyl ammonium chloride (as a vinyl QAC) on polypropylene (PP) and cotton nonwoven fabrics. The process sequence was plasma–pad–dry–plasma. The grafting was confirmed by XPS, SEM, and acid dye adsorption investigations. The mechanism of the grafting process is shown schematically in Fig. 6. This process resulted in a high grafting yield and uniformity without the use of heat or wet solvents [12].
 Fig. 6. Schematic mechanism of grafting of (3-acrylamidopropyl) trimethyl ammonium chloride on PP nonwoven initiated by DBD plasma [12]. HV: high voltage.
Fig. 6. Schematic mechanism of grafting of (3-acrylamidopropyl) trimethyl ammonium chloride on PP nonwoven initiated by DBD plasma [12]. HV: high voltage.Diallyldimethylammonium chloride (DADMAC) was grafted onto PP nonwoven fabric by pre-activation and post-treatment by APGD plasma (99% He/1% O2). The samples were subjected to a process including plasma pre-activation, padding with DADMAC and pentaerythritol tetraacrylate (as a cross-linker to increase the durability of the coating), drying, plasma-induced graft polymerization, and rinsing. A schematic diagram of the process and free radical mechanism of plasma-induced graft polymerization is shown in Fig. 7. The grafting of poly-DADMAC on PP fibers was confirmed by SEM, FTIR, and time-of-flight secondary ion mass spectrometry (TOF-SIMS). The sample prepared under optimized conditions showed good antimicrobial activity against Staphylococcus aureus (S. aureus) and Klebsiella pneumoniae (K. pneumoniae) bacteria [10]. The same process was applied to a nylon/cotton (50%/50%) blend fabric, and a 99.9% reduction in bacterial growth was reported. The high degree of cross-linking led to the high durability of the antibacterial coating prepared by this method [13].
 Fig. 7. Schematic presentation of plasma-induced graft polymerization: (a) process and (b) mechanism of attachment of DADMAC on PP nonwoven by plasma activation.
Fig. 7. Schematic presentation of plasma-induced graft polymerization: (a) process and (b) mechanism of attachment of DADMAC on PP nonwoven by plasma activation.Arik et al. [82] examined the effect of Ar DBD plasma treatment (130 W, 40 s) on the finishing of cotton with commercial antimicrobial products based on Ag, dichlorophenol, triclosan, and diphenyl alkane derivatives using a pad–dry–cure procedure. The energy-dispersive X-ray spectroscopy (EDX) results confirmed that the Ag content of the plasma-treated cotton was higher than that of the untreated sample. The antibacterial activity and durability were improved significantly by plasma treatment, and it was more effective for the loading and durability of diphenyl alkane derivatives [82].
Plasma treatment can enhance the adsorption and attachment of chitosan to textile fibers. Naebe et al. [35] used helium and He/O2 atmospheric-pressure plasma to attach chitosan to cotton fabric using a pad–dry–cure process. The surface energy and thickness of the chitosan coating improved after the plasma treatment. The finished fabric showed significant antimicrobial activity against Gram-negative Escherichia coli (E. coli) bacteria (Fig. 8 (a)). However, the results indicated that plasma treatments alone and without any further chitosan coating were also capable of reducing bacterial activity (Fig. 8) because of the formation of active agents, such as helium metastable species (He*), O, and OH [35]. Low-pressure oxygen plasma treatment (800 W, 27.12 MHz, 75 Pa, 30 s) of viscose fabric enhanced the loading of chitosan and conferred excellent antibacterial activity to the finished fabric [83]. Haji et al. [84], [85], [86], [87], [88], [89] showed that O2 plasma treatment enhanced the attachment of chitosan to cotton and wool fibers and improved the dyeability and antibacterial activity of the fabrics. DBD plasma increased the number of O2-containing groups on the surface of the PET fabric and enhanced the deposition of chitosan by immersion in chitosan acetate solution (2% (w/v)) and vacuum drying. The chitosan-coated sample showed high antimicrobial activity against E. coli and S. aureus (Fig. 8(b)) [90]. Similar results were obtained when the Ar/O2 plasma-treated PET samples were coated with chitosan polymers and oligomers using a pad–bake procedure [91]. The activation of nylon fabric with an APPJ at a power of 1000 W, using air as the processing gas, improved the grafting of chitosan on the fabric and enhanced the antimicrobial activity of the coated fabric. Two types of chitosan, that is, chitosan oligomers (molecular weight Mw = 12 000 Da) and chitosan polymers (Mw = 170 000 Da), were used for grafting. The plasma-treated fabrics were immediately impregnated in a chitosan solution for 6 min and cured at 95 °C for 8 min, followed by washing. Surface activation, introduction of new O2-containing groups, and grafting of chitosan onto nylon fibers were confirmed by XPS analysis. Fabric samples grafted with chitosan polymer showed higher antibacterial activity than the samples grafted with the oligomer. The antibacterial activity and hydrophilicitywere further improved by plasma post-treatment [92]. The antibacterial activity of chitosan-based antimicrobial finishes depends on the molecular weight, degree of deacetylation of chitosan, and pH of the medium. Although chitosan has a broad antimicrobial spectrum, its efficiency is reduced at alkaline pH. The introduction of a permanent positive charge by chemical modification of chitosan improves its antibacterial activity, regardless of the pH of the medium [93], [94].
 Fig. 8. Antibacterial activity of (a) (i) an inoculum only after 0 h, (ii) inoculum only after 1 h, (iii) control cotton fabric, (iv) chitosan-treated cotton fabric, (v) helium plasma-treated cotton fabric, (vi) helium plasma/chitosan-treated cotton fabric, (vii) He/O2 plasma-treated cotton fabric, and (viii) He/O2plasma/chitosan-treated cotton fabric; and (b) DBD plasma/chitosan-treated polyester fabric. DD: degree of deacetylation. Reproduced from Refs. [35], [90]with permissions of Springer Nature, © 2016, 2010.
Fig. 8. Antibacterial activity of (a) (i) an inoculum only after 0 h, (ii) inoculum only after 1 h, (iii) control cotton fabric, (iv) chitosan-treated cotton fabric, (v) helium plasma-treated cotton fabric, (vi) helium plasma/chitosan-treated cotton fabric, (vii) He/O2 plasma-treated cotton fabric, and (viii) He/O2plasma/chitosan-treated cotton fabric; and (b) DBD plasma/chitosan-treated polyester fabric. DD: degree of deacetylation. Reproduced from Refs. [35], [90]with permissions of Springer Nature, © 2016, 2010.Guanidine-based antimicrobial agents are well-known for their excellent antimicrobial and biocidal action because of their cationic character, which causes electrostatic interactions with the bacteria or organism cell membrane, leading to their death. Yim et al. [95] used a DBD system for the deposition of 1,1,3,3-tetramethylguanidine (TMG) on ultra-high-molecular-weight polyethylene (UHMWPE) film through an atmospheric-pressure plasma-enhanced chemical vapor deposition (AP-PECVD) process. The precursor aqueous solution was heated to 25 °C, and the vapor was transferred to the space between the electrodes using helium as the carrier gas. Plasma treatment was conducted at 330 W for 30–180 s (90 kHz). The coating of the surface with the island-like guanidine polymer was confirmed by SEM, atomic force microscopy (AFM), attenuated total reflection (ATR)–FTIR, and XPS analyses. The samples coated under optimal conditions showed a complete reduction in E. coliand S. aureus growth. The advantage of this process is the cationic and alkaline nature of the guanidine compound leads to antibacterial activity at all pH ranges [95]. Table 3 provides an overall comparison of the antimicrobial finishing achieved through various plasma treatment techniques on various textile fibers [10], [13], [35], [36], [38], [57], [80], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139], [140], [141].
Table 3. Common plasma treatment techniques for antimicrobial finishing on different textile fibers and reported outcomes.
| Substrates | Antimicrobial agents | Type of plasma | Method of application | Properties | References |
|---|---|---|---|---|---|
| PP | DADMAC | APGD (He/O2) | Pre-activation and post-treatment | Good antibacterial activity | [10] |
| Nylon/cotton | DADMAC | APGD (He/O2) | Pre-activation and post-treatment | 99.9% reduction in bacterial growth | [13] |
| Cotton | Ag, dichlorophenol, triclosan and diphenyl alkane derivative | DBD (Ar) | Pre-activation and pad–dry–cure | Improved antibacterial activity and durability | [82] |
| Cotton | Chitosan | DBD (He and He/O2) | Pre-activation and pad–dry–cure | Significant antimicrobial activity | [35] |
| Viscose | Chitosan | Low-pressure O2 plasma | Pre-activation and pad–dry | Excellent antibacterial activity | [83] |
| Cotton and wool | Chitosan | Low-pressure O2 plasma | Pre-activation and pad–dry | Improved dyeability and antibacterial activity | [84], [85], [86], [87], [88], [89] |
| PET | Chitosan | DBD (air) | Immersion–vacuum drying | High antibacterial activity | [90] |
| PET | Chitosan | DBD (Ar) | Pre-activation and pad–cure | Good biocompatibility and antibacterial activity | [91] |
| Nylon | Chitosan | APPJ (air) | Pre-activation and pad–cure | Good biocompatibility and antibacterial activity | [92] |
| UHMWPE | TMG | DBD | Plasma-enhanced chemical vapor deposition | Excellent antibacterial activity | [95] |
| PET | Alkyldimethylbenzylammonium chloride (ADBAC) | DBD (air) | Pre-activation and immersion | Good antimicrobial efficacy with increased shelf life | [96], [97] |
| PP mesh | Ampicillin | Low-pressure Ar plasma | Pre-activation, grafting with tetra ethylene glycol dimethyl, loading of ampicillin by pad–dry–cure | Antibacterial activity used for hernia repair | [98] |
| PET and PP | Octenidine | DBD (air) | Ink-jet printing of nanoparticles | Continuous antibacterial finishing | [99] |
| Nylon | Poly(N-vinylpyrrolidone) (PVP)-coated AgNPs | DBD (air) | Spray and exhaustion | Antibacterial activity using very low amount of AgNPs | [100] |
| Nylon | AgNPs | DBD (air) | Pre-activation, immersion, and curing | Enhanced hydrophobicity, long-term antibacterial effect | [36], [101] |
| PET | AgNPs | Low-pressure air plasma | Pre-activation, immersion, and curing | Durable antibacterial activity | [102] |
| Cotton | Ag nitrate | Corona (air) | Pre-activation-immersion-pad–dry–cure | Enhanced loading and durability of antibacterial effect | [103] |
| Cotton | CuNPs and ZnONPs | Corona (air) | Pre-activation–exhaustion-drying | Improved self-cleaning and antibacterial activity | [104] |
| Bamboo | AgNPs | DBD (air) | Pre-activation-in-situsynthesis of AgNPs using microwave | Excellent antibacterial activity protection and antibacterial activity | [105] |
| Cotton | AgNPs | Low-pressure CF4plasma | Simultaneous reactive dyeing and nano-finishing (exhaustion) | Good antibacterial activity without affecting the color of the fabric | [106] |
| PET | Ag nano-gel and chlorhexidine | DBD (CO2) | Immersion and drying | Excellent antibacterial and healing properties | [107] |
| PP | AuNPs | DBD and diffuse coplanar surface barrier discharge (DCSBD) (air) | Immersion and drying | Good antibacterial activity | [108] |
| Viscose | Ag+ and Cu2+ ions | DBD (air) | Immersion and drying | Enhanced antimicrobial activity, improved by ageing | [109] |
| Cotton/PET | Ag+ ions | DBD (air) | Immersion and drying | Enhanced antimicrobial activity | [110] |
| Cotton | AgNPs | Sputtering | Simultaneous sputtering of AgNPs and plasma deposition of hexamethyldisiloxane (HMDSO) | Hydrophobic, durable antibacterial activity with controlled release of nanoparticles | [38] |
| PET | AgNPs, CuNPs, and ZnONPs | APPJ (O2/N2) | Embedding of NPs between two plasma-polymerized HMDSO layers | Hydrophobic, durable antibacterial activity with controlled release of nanoparticles | [57], [111], [112] |
| Cotton | AgNPs | APPJ | Sputtering | Good antibacterial activity | [113] |
| Modal | ZnONPs | APGD | Pre-activation and in-situ synthesis of ZnO NPs | Wash-fast antibacterial property | [114] |
| Cotton | ZnONPs | Low-pressure O2 plasma | Pre-activation-immersion–pad–dry–cure | UV protection | [115] |
| Cotton | 5,5-Dimethyl hydantoin (DMH) | APPJ (N2) | Pad–plasma–dry–cure | Regenerable antibacterial activity | [80], [116], [117], [118], [119] |
| Bamboo | Extract of Aloe barbadensis miller leaves and Rosa damascene flowers | Low-pressure air plasma | Pre-activation–pad–dry–cure | Wash-fast antibacterial property | [120] |
| Banana | Green tea and tulsi extracts | DBD (air) | Pre-activation–immersion–dry–cure | Enhanced antimicrobial activity | [121] |
| Cotton | Thymol | Low-pressure plasma (O2 and N2) | Pre-activation and exhaustion | Great antibacterial activity durable after 50 washing cycles | [122] |
| Cotton | Neem leaf extract | Low-pressure plasma (air or O2) | Pre-activation and pad–dry–cure in presence of citric acid | Excellent and durable antifungal and antibacterial activity | [123], [124], [125], [126], [127] |
| Cotton | Neem oil vapor | Low-pressure plasma (air) | Pre-activation and vapor treatment | Good antibacterial activity | [128] |
| Cotton | Onion skin extract | Low-pressure O2 plasma | Pre-activation and exhaustion dyeing | Good antibacterial activity | [129] |
| Wool | Berberine | Low-pressure O2 plasma | Pre-activation followed by β-cyclodextrin grafting and dyeing | Significant antibacterial activity | [130] |
| Wool | Berberine | DBD (air) | Exhaustion dyeing | Good antibacterial activity | [131] |
| Cotton | Ag | Low-pressure plasma | DC magnetron sputtering | Durable antibacterial and UV protective | [132] |
| PET | Ag/TiO2 | Low-pressure plasma | DC/RF sputtering | Structural coloration, significant antibacterial activity and improved antistatic and UV protection properties | [133] |
| Cotton | Ag and Zn | Low-pressure plasma (Ar) | Magnetron sputtering | Biocompatibility, wound healing and antimicrobial activity | [134] |
| PET | Ag | Hollow discharge cathode plasma (Ar) | Sputtering | antibacterial activity | [135] |
| PET/silk | Cu | Low-pressure plasma (Ar) | Sputtering | Conductivity and antibacterial activity | [136] |
| Cotton | Titanium oxynitride (TiON)/Cu | Low-pressure plasma (Ar, O2, and N2) | Co-sputtering | Good antibacterial activity | [137] |
| Cotton | Ag and SiO2 | Low-pressure plasma (Ar) | Co-sputtering | High antibacterial activity | [138] |
| PP | AgNPs | DBD (H2/Ar) | In-situ reduction of Ag ions by plasma | Self-cleaning property | [139] |
| Cotton | AgNPs | DBD (H2/Ar) | In-situ reduction of Ag ions by plasma | High antimicrobial activity with good fastness | [140] |
| Cotton | AgNPs | APGD (Ar) | In-situ reduction of Ag ions by plasma | Significant antimicrobial activity | [141] |
6.2. Loading improvement of antimicrobial agents through surface activation
Surface activation or functionalization by plasma treatment can be considered a fast and economical pretreatment process for promoting the loading of non-polymerizable antimicrobial agents on textile substrates. Usually, the loading and fastness properties of antimicrobial agents such as QACs, nanoparticles, and drugs on textiles, especially those made of hydrophobic fibers, are low. Surface treatment is needed to improve the wettability, surface tension, and reactivity of textile fibers.
QACs possess advantages such as thermal stability, low human toxicity, and oxidative properties (without deterioration of the physical properties of textiles), which has led to the widespread application of these compounds in antimicrobial finishing of textile surfaces at low cost and with adequate germicidal performance. Song et al. [97] employed a DBD plasma treatment to improve the interaction between alkyldimethylbenzylammonium chloride (ADBAC) as a QAC and cotton, PET, and cotton/PET wipes. DBD plasma treatment increased the adsorption of ADBAC on PET and cotton/PET wipes because of the interaction with the plasma-generated O2-containing groups on their surfaces. Although the adsorption of ADBAC on the untreated cotton sample was higher than that on untreated PET and cotton/PET samples, the amount of ADBAC on the surface of cotton decreased after DBD plasma treatment, which may have been due to the penetration of ADBAC inside the fibers. Therefore, the plasma-treated PET sample showed the highest antimicrobial activity compared with the other fabrics because it had the highest concentration of ADBAC on the surface. The antibacterial activity of the plasma-treated cotton sample was at its lowest level because of the penetration of ADBAC into the bulk of the hydrophilic cotton fibers, reducing its concentration on the surface Fig. 9 [97]. Overall, DBD plasma treatment improved the antimicrobial activity and increased the shelf life of the antibacterial efficacy of PET-containing wipes [96].