1. Introduction
Titanium and its alloys are clinically preferred biomaterials for bone implants due to their superior biocompatibility and corrosion resistance [1]. Titanium (Ti) has been extensively used for manufacturing hard tissue implants to replace and/or correct missing or diseased tissues in clinical applications such as total joint replacements, fracture fixation (plates, nails or screws) and dental implants. It is reported that over 327,000 hip replacements were performed in 2009 in the United States alone, and the orthopaedic implant market is estimated at $33 billion with projected increases of 7.1% per year [2,3]. Notwithstanding relatively high success rates, around 4.1%~7% implants (knee/hip arthroplasty) face challenges mainly due to excessive inflammation, poor osseointegration and bacterial infection, which can result in complete implant failure requiring revision surgery [4,5]. These challenges may be addressed by augmenting the bioactivity of Ti based implants via surface modification. This can be achieved by altering the surface topography at the macro, micro and nanoscales [6].
Macroscale (millimetres to micrometres) directly relates to implant geometry, with threaded screws and macro-porous surfaces being commonly utilized in the clinic. Reports have demonstrated that macro-roughness features lead to an increase both in early bone apposition and implant fixation by improving mechanical interlocking between the macro-rough features and surrounding bone [7,8]. However, the direct bone-implant contact can be maximized by micro-roughness (1–10 μm), which can be produced by sandblasting, acid etching, plasma spraying and other surface modification techniques [9]. Furthermore it has been theoretically calculated that the ideal implant surface features at the micro-scale should be 1.5 μm long and 3–5 μm wide [10,11]. Despite numerous studies demonstrating that micro-roughness features can augment bone-to-implant contact and torque removal resistance [12,13], it is noteworthy that the initial fate of host tissues and cells is not directly affected by microscale surface roughness [14]. Rather, biomolecular interactions, protein adsorption and bone cell behaviour can be influenced by nanostructures (1–100 nm) [[15], [16], [17]]. In this context, nanoscale topography has been regarded as a promising surface modification strategy for Ti based implants [18].
Recently, nanoscale features (TiO2 nanopores or nanotubes) generated by the self-ordering electrochemical anodization (EA) technique, have drawn considerable attention as a means of improving the bioactivity and therapeutic potential of conventional Ti implants [19]. EA enables cost-effective fabrication of well-ordered titania nanotubes (TNTs) on titanium surfaces with great control over TNTs characteristics, achievable by varying the anodization parameters [20]. The influence of TNTs on cellular functions (mesenchymal stem cells, hematopoietic stem cells, endothelial cells, osteoblasts, and osteoclasts) including adhesion, proliferation and differentiation has been extensively investigated in the context of bone/dental implant applications [21]. A critical finding is that nanotube dimensions and other characteristics (wettability, crystal structure, loading of drugs/proteins, etc.) significantly dictates cell functionality [22]. Furthermore, due to the geometry of TNTs (resembling tiny test tubes, open at top, closed at bottom), they can act as a drug reservoir for the local delivery of drugs (antibiotics, proteins, anti-inflammatory or anti-cancer drugs) directly inside the implant micro-environment, with the ultimate aim of improving the therapeutic efficacy of conventional implants [23,24].
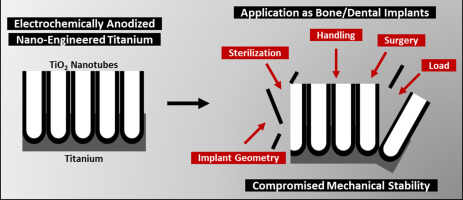
Despite significant advancements achieved in the domain of TNTs modified implants, many research gaps are challenging the successful translation of TNTs from research to clinical testing and integration into the commercial implant market. For example, the critical issue of the mechanical properties and stability of TNTs remains underexplored. The mechanical characteristics of a titanium implant surface modification such as TNTs, including elastic modulus, hardness and fracture strength, play a decisive role in its long-term success, especially in bone/dental implant scenarios requiring surgical placement and survival in load bearing conditions. Indeed, in the presence of cracks and delamination due to mechanical stress and abrasion, severe host immune-inflammatory reactions may occur, which can lead to complete implant failure. Highlighting this research gap, in this review article we elaborate on the underlying factors determining the mechanical stability of TNTs modified anodized Ti implants (Fig. 1). This review article details the in-depth understanding of the interface between TNTs and the Ti implant substrate, identifying issues that may affect mechanical stability and integrity. Furthermore, it incorporates a critical discussion of the various strategies used to augment mechanical stability, as well as implantation studies performed ex vivo and in vivo highlighting the survival of TNTs based implants.
 Fig. 1. Schematic representation of the parameters influencing the mechanical stability of titania nanotubes (TNTs) fabricated via electrochemical anodization.
Fig. 1. Schematic representation of the parameters influencing the mechanical stability of titania nanotubes (TNTs) fabricated via electrochemical anodization.2. Understanding the interface between nanotubes and the titanium substrate
In 1999, pioneering work by Zwilling et al. reported the fabrication of self-organized TNTs on Ti via EA in HF based electrolytes [25]. This first generation of TNTs could be fabricated up to a limited length of around 500 nm, with considerable sidewall inhomogeneity [26]. Future research focused on generating TNTs with lengths up to several micrometers and extremely smooth homogenous sidewalls by varying the pH values, anodizing voltage/time and introducing organic electrolytes [[27], [28], [29]]. However, for the orthopaedic/dental implant setting, which is continuously under load bearing, the stable adhesion of bioactive or drug incorporated TNTs to the underlying Ti implant substrate is essential to ensure long term success in what is often a compromised bone micro-environment. This section of the review will provide an overview of the fabrication mechanisms and critical factors affecting TNTs geometry and composition for an improved understanding of the mechanical stability at the interface between nanotubes and the implant substrate (Fig. 1).
2.1. Mechanism of electrochemical anodization of Ti
Briefly, EA is performed by exposing a two-electrode system to DC voltage in an appropriate fluoride-based electrolyte, with target Ti as the anode and platinum/Ti as the cathode. Under optimized conditions, the as-fabricated TNTs are vertically oriented on the Ti surface with closed bottoms and open tops (Fig. 1). The growth mechanism of self-ordered TNTs is the result of three simultaneous reactions, which can also be related to different regions observed in the current–time curves as shown in Fig. 2(a). The formation of TNTs is schematically depicted in Fig. 2(b). The anodization process starts when Ti is oxidized to Ti4+ [Eq. (i)] to form a compact TiO2 layer [Eq. (ii)] accompanied by the transport of Ti4+ ions outward and O2– ions inward with the assistance of an electric field.(i)(ii)
 Fig. 2. Mechanism of electrochemical anodization of Ti to fabricate titania nanotubes (TNTs). (a) Typical current–time characteristics in fluoride containing electrolytes compared with fluoride-free solution, (b) schematic depiction of the formation of TNTs, and (c) steady state growth stage: TNTs dissolution rate (v1) = formation rate (v2). Reproduced with permission from [29], © Elsevier 2007.
Fig. 2. Mechanism of electrochemical anodization of Ti to fabricate titania nanotubes (TNTs). (a) Typical current–time characteristics in fluoride containing electrolytes compared with fluoride-free solution, (b) schematic depiction of the formation of TNTs, and (c) steady state growth stage: TNTs dissolution rate (v1) = formation rate (v2). Reproduced with permission from [29], © Elsevier 2007.Anodic oxidation processes typically follow Eq. (iii), where U is the applied voltage and d represents the thickness of the oxide. That is, the field keeps on decreasing with the increase in film thickness until it is not sufficient to promote ion transport and the finite film thickness forms.(iii)
The presence of fluoride ions can significantly influence the anodization process [20]. On one hand, fluoride ions chemically dissolve TiO2 or directly react with Ti4+ arriving at the oxide–electrolyte interface to form water-soluble [TiF6]2−complexes [Eqs. (iv), (v)]. The ability to form these soluble complexes results in the permanent chemical dissolution of compact TiO2, thereby leading to the transformation of the compact layer to hollow porous and tubular structures. On the other hand, the small ionic size makes fluoride ions easier than O2– to get through the TiO2 layer, thus forming an approximately 15 nm thick fluoride-rich layer at the metal-oxide interface [28]. This layer is very important for nanotube growth and significantly influences the adherence of TNTs to the Ti substrate, which will be discussed in Section 2.2.(iv)(v)
According to the typical current-time curve for self-organized TNTs in aqueous electrolyte with intermediate fluoride concentrations (0.1–1 wt%), the EA process can be divided into three stages [20,29]. In the first stage, the current initiates exponential decay due to the formation of a compact oxide barrier layer. In stage two, random nanopores start to grow which increases the active area, and thereby the current rises. In stage three, the current density reaches a steady level which promotes the self-organization of TNTs. The tubes grow continually with time until the etching rate at the top of the tube is equivalent to the formation rate at the bottom, as depicted in Fig. 2(c) [30]. It is noteworthy that the TNTs morphology turns into a v-shape with extended anodization time since chemical dissolution occurs at the entire tube length, which means that the walls of the nanotube tops are significantly thinner than the bottoms [31]. In the following sections we will explore the various EA parameters that can contribute towards the fabrication of robust TNT structures.
2.2. Composition of anodization electrolyte
To understand the interface between TNTs and Ti, visualization of the bottom of nanotubes at the point of attachment to the Ti substrate is crucial, which can be obtained by imaging the fractured/peeled-off anodized films (TNTs), easily achievable by mechanical, physical or chemical methods. This closed and dome-like bottom of the TNTs is a thin titania layer separating the TNTs from Ti, and is defined as the barrier layer (BL), which commonly exists in nanoporous alumina[32]. Owing to the rounded BL, surface dimples are encountered on the metallic surface, giving it a nano-templated topography. Electrolyte composition, including water content and fluoride concentration, play an important role in controlling the geometrical parameters of the TNTs, including the BL-Ti interface [33].
The water content of the electrolyte (supplying oxygen for the oxidation of Ti) significantly affects both the growth rate and the chemical dissolution speed of TNTs. According to Eq. (ii); field-enhanced oxidation occurs at the BL-Ti interface, where the oxygen ions (O2−) from the electrolyte combine with Ti4+ to form TiO2 along the direction of the nanotube growth [34]. Valota et al. have shown that the addition of water to fluoride/glycerol electrolytes results in a thicker barrier layer, while also enhancing the expansion factor, which induces the volume difference and stress between the TNTs layer and the Ti substrate [35,36]. The expansion factor is determined by the volume ratio of oxide to the consumed metal and is called the Pilling–Bedworth ratio (PBR) [37]. Slightly increasing the water content (for systems with water >2%) in organic electrolyte accelerates the dissolution rate of the nanotubes, which in turn can result in decreased nanotube length and increased diameters [[38], [39], [40]]. The most striking characteristic of TNTs formed in aqueous electrolyte is the ripple on the sidewall, and this ring-like structure is occasionally seen in organic electrolytes with low water content (<2.5%) [29,36]. Occurrence of ripples on the TNT sidewall is the result of competition between the tube growth rate at the bottom and the tube splitting speed at the cell boundaries [41]. Thus, in organic electrolyte systems with limited water content, where the chemical dissolution rate is further decreased by anodization, the nanotubes can be longer and smoother. Furthermore, in long-duration anodization, etching of TNT tops becomes apparent, which results in needle-like or grass-like inhomogeneous structures (collapsed and bundled tops), which may compromise the overall stability of the structures. Because this so-called “nanograss” often aggregates during drying to resist capillary actions, it enhances internal stress in the oxide layer and generates an upward force at the nanotube bottom, thereby decreasing the bond strength between TNTs and the metal substrate [42].
As previously mentioned, fluoride ions in the electrolyte strongly influence the anodization process by chemically dissolving TiO2 and binding ejected Ti4+ at the oxide/electrolyte interface. It is noteworthy that as a result of their fast migration rate [33], fluorides accumulate at the nanotube bottoms and cell boundaries in the form of soluble fluoride species (Ti O
O F or Ti
F or Ti F complexes) to form an additional fluoride-rich interface layer separating the barrier layer and the Ti substrate during the growth phase of TNTs [29]. This has been demonstrated by X-ray photoelectron spectroscopy sputter profiles from the bottom side of lifted-off TNTs as seen in Fig. 3(a) [28,43]. Shimizu et al. demonstrated that the existence of a soluble fluoride-rich layer underneath the nanotube bottoms resulted in poor adhesion of the resultant anodic films [44]. Thus, eventual detachment of the anodic film from the substrate can be observed [45]. After dislodgement of TNTs, the majority of the remnant flakes of the fluoride-rich layer are visible on the dimples of the Ti substrate, as shown in Fig. 3(b) [28]. Therefore, methods involving an additional fluoride sedimentation procedure in EA [46], or annealing to reduce fluoride content in the overall nanotube layer are aimed at enhancing the adhesion strengths of the TNT films [47]. The fluoride-rich layer is a key reason for the transition of porous structures to nanotubes as the formed layer at the bottom of the nanotubes can move to cell boundaries by a flow mechanism, which is prone to chemical dissolution. This process is schematically depicted in Fig. 3(c) [43].
F complexes) to form an additional fluoride-rich interface layer separating the barrier layer and the Ti substrate during the growth phase of TNTs [29]. This has been demonstrated by X-ray photoelectron spectroscopy sputter profiles from the bottom side of lifted-off TNTs as seen in Fig. 3(a) [28,43]. Shimizu et al. demonstrated that the existence of a soluble fluoride-rich layer underneath the nanotube bottoms resulted in poor adhesion of the resultant anodic films [44]. Thus, eventual detachment of the anodic film from the substrate can be observed [45]. After dislodgement of TNTs, the majority of the remnant flakes of the fluoride-rich layer are visible on the dimples of the Ti substrate, as shown in Fig. 3(b) [28]. Therefore, methods involving an additional fluoride sedimentation procedure in EA [46], or annealing to reduce fluoride content in the overall nanotube layer are aimed at enhancing the adhesion strengths of the TNT films [47]. The fluoride-rich layer is a key reason for the transition of porous structures to nanotubes as the formed layer at the bottom of the nanotubes can move to cell boundaries by a flow mechanism, which is prone to chemical dissolution. This process is schematically depicted in Fig. 3(c) [43].
 Fig. 3. Presence of a fluoride-rich barrier layer at the interface of titania nanotubes (TNTs) and the underlying substrate: (a) XPS (X-ray photoelectron spectroscopy) depth profile from the barrier layer of detached TNTs confirming the existence of a fluoride-rich interface layer (reproduced with permission from [28], © WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim 2008), (b) SEM images showing the remaining flakes of fluoride-rich layer both on the bottom of the removed TNTs and at the Ti substrate (reproduced with permission from [20], © WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim 2008). (c) Schematics of nanotube formation in the presence of fluoride in the electrolyte (reproduced with permission from [43], © Elsevier 2011).
Fig. 3. Presence of a fluoride-rich barrier layer at the interface of titania nanotubes (TNTs) and the underlying substrate: (a) XPS (X-ray photoelectron spectroscopy) depth profile from the barrier layer of detached TNTs confirming the existence of a fluoride-rich interface layer (reproduced with permission from [28], © WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim 2008), (b) SEM images showing the remaining flakes of fluoride-rich layer both on the bottom of the removed TNTs and at the Ti substrate (reproduced with permission from [20], © WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim 2008). (c) Schematics of nanotube formation in the presence of fluoride in the electrolyte (reproduced with permission from [43], © Elsevier 2011).2.3. Aging of electrolyte
Electrolyte optimization influences the quality and morphology of anodized films, and interestingly aging of organic electrolytes is often used in practice to fabricate highly ordered TNTs, but remains underexplored to date. Aging of electrolyte is the repeated anodization of dummy titanium prior to the fabrication of TNTs on the target substrate. Only a few studies have reported titanium anodization in aged electrolytes, using fluoride containing organic electrolytes with dimethyl sulfoxide (DMSO) [40] and ethylene glycol or glycerol [42,[48], [49], [50], [51]]. With regard to the effects of previously used electrolyte on TNT dimensions, it has been reported that the previously used DMSO-based electrolyte decreased the length and diameter of TNTs while thickening the tube walls [40]. But in an aged low water content (<2.0%) ethylene glycol based electrolyte, the length, pore diameter and wall thickness of TNTs were higher relative to those in fresh electrolyte, which may be ascribed to the anhydrous nature of ethylene glycol [42]. However, these studies all demonstrated a greatly improved adhesion between TNTs and the underlying Ti substrate. In this section, the effect of electrolyte ageing on the growth rate of TNTs, formation of barrier layer and growth-induced internal stress such as PBR are discussed in detail with focus on the integrity and adherence strength of the TNT film.
It is noteworthy that most studies ignore the hygroscopic nature of the ethylene glycol based electrolyte which may explain the conflicting results obtained with respect to conductivity and growth rates of nanotubes upon ageing of the electrolyte [51]. More recently we have optimized the ageing of the electrolyte in an enclosed electrochemical cell, thereby avoiding any chance of moisture incorporation inside the electrolyte [51]. Repeated anodization or ageing of the electrolyte continuously alters its chemical composition, leading to an increase in [TiF6]2− content, while the oxygen bearing anionic species are gradually depleted, which further reduces electrolyte conductivity [49]. Meanwhile the formation/dissolution rate of oxide is also hindered [Eqs. (ii), (iv)], thereby impeding the growth rates of TNT formation [52]. Thus it is reasonable to infer that a thicker oxide BL at the base of nanotubes is formed under these conditions. Compared with fresh electrolyte, the time to reach the anodization equilibrium phase (with the low and stable value of current density, denoted as teq) is delayed for aged electrolyte due to reduced conductivity, which was confirmed by current-time monitoring and electrolyte conductivity measurements [49,51]. Furthermore, applied anodization voltage is the sum of potential difference (PD) at the metal-oxide interface, across the oxide, oxide-electrolyte interface, and across the electrolyte. Assuming that changes of PD at the interfaces are negligible, PD across the electrolyte increases as the consequence of conductivity reduction, which means a decrease in the PD across the oxide layer [42]. This, in turn, reduces the formation of [TiF6]2−complexes and hence causes delayed teq. This can also be linked to the formation of a thicker BL. In this regard, lower growth rates combined with reduced conductivity can result in a thicker compact BL layer representing better contact between the resulting nanotubes and the underlying substrate, and thereby improving the stability of the nanotubes. Changes of electrolyte features and characteristics of as-anodized TNTs in fresh and aged electrolytes are shown in Fig. 4. Furthermore, internal stress develops along with anodic oxidation due to the volume increase of oxide (PBR) [50]. The magnitude of this growth-induced compressive stress depends on the actual fraction of oxide formed at the metal/oxide interface, and the high stress may yield fragile anodic layers [53]. The decreased growth rate of aged electrolyte can reduce the compressive internal stress at the barrier layer-metal substrate interface, thereby yielding stable and well-adherent TNTs. To summarize, with the use of aged electrolyte, a thicker BL with a more compact structure and improved stability can be obtained, leading to an improved stability of TNTs.
 Fig. 4. Schematic representation of changes in nanotube and electrolyte characteristics for fresh and aged electrolyte anodization. Reproduced with permission from [51], © American Chemical Society 2015.
Fig. 4. Schematic representation of changes in nanotube and electrolyte characteristics for fresh and aged electrolyte anodization. Reproduced with permission from [51], © American Chemical Society 2015.2.4. Sterilization without compromising stability
Prior to undertaking in vitro cell studies and implantations in vivo, it is crucial to sterilize implants, without compromising the biocompatibility, surface chemistry and mechanical stability. However, studies have suggested that the inconsistent biological performances, especially for the similar range of nanotube dimensions, may be due to varied sterilization procedures utilized, without appropriate investigation of the related changes to the implant surfaces [23]. Furthermore, most studies investigating TNTs modified implant applications do not detail the sterilization methodology. Recently, a few studies have aimed to elucidate the relationship between effective sterilization and biocompatibility [[54], [55], [56]]. Moreover, it is well accepted that any seemingly small change on the surface of implants may influence cellular behaviour, and hence optimizing and investigating the various techniques used for sterilization is crucial. Table 1 summarizes the pros and cons of various sterilization methods with respect to effective cleaning of TNTs-modified implants.
Table 1. Methodology and research gaps of commonly used sterilization methods for titania nanotube (TNT) modified implants.
| Sterilization technique | Methodology | Research gaps | Ref |
|---|---|---|---|
| Autoclaving | High temperature/pressure treatment, in presence of adequate moisture (wet) or in the absence of moisture (dry) |
|
[56,57,64] |
| Ethanol Immersion | Immersion in absolute or 70% ethanol |
|
[54,55] |
| UV Irradiation | UV induced sterilization based on decomposing contaminants, which may be enhanced by photocatalytic activity of TiO2. | Parameters unexplored: amplitude, wavelength, irradiation time, intensity | [54,65] |
| Plasma Treatment | Sputtering plasma to decontaminate | Precise optimization of plasma conditions (pressure, gas composition and flow rate etc.) | [62,63] |
In a pioneering study, Zhao et al. reported that ultraviolent (UV) and ethanol treatment enhanced the wettability of TNTs compared to autoclaving, whereas UV irradiation induced the best in vitro primary rat calvarial osteoblast responses (owing to effective removal of surface contaminants, especially hydrocarbons) [54]. Later Kummer et al. compared the role of sterilization on bacterial growth on various implant surfaces, including flat Ti and TNTs [55]. Autoclaving resulted in the highest amount of S. aureus and S. epidermidisgrowing on implant surfaces compared to UV and ethanol treatment. This was ascribed to the incorporated hydrophobic carbon impurities onto the samples, which may alter surface chemistry and hence bioactivity. Moreover, it was reported that smaller diameter (20 nm) TNTs and heat treatment induce subtle changes in surface chemistry, roughness, wettability and crystallization, that contribute towards antibacterial efficacy [55]. Furthermore, Oh et al. found that air entrapment into nanotubes during dry autoclaving dramatically increased the in vitro osteogenic functionality of MC3T3-E1 mouse osteoblasts after 24 h incubation [56].
Considering that high temperature and pressure treatments such as autoclaving may compromise the stability of TNTs, more investigations are needed in this regard to ensure the maintenance of nanotube stability. Recently, Junkar et al. reported surface morphology alterations of TNTs sterilized by autoclave, UV irradiation, and H2O2/O2 plasma techniques. Significant changes were observed after autoclaving, whereby TNTs structures (15, 50, 100 nm in diameter) were damaged to some extent at both the top and bottom. Severe destruction was observed on smaller diameter TNTs, where the open mouth of the TNTs collapsed while the bottom delaminated. However, the different sterilization methods did not influence the wettability of freshly prepared and sterilized TNTs [57]. Interestingly, in this study only freshly fabricated TNTs were used, as it was reported that ageing turns TNTs more hydrophobic within three weeks [58]. Furthermore, UV sterilization, suggested to be the most favourable sterilization technique, also represents a minimally invasive technique with respect to nanotube stability.
It is worth noting that high temperature/pressure in the presence of moisture, such as inside the steam autoclaving setup, may induce TNTs crystallization [59]. In this context, Yu et al. investigated the mechanism of TiO2 phase transition by comparing crystallization and morphology changes, using various sterilization treatments including: calcination (annealing at 450 °C in air for 3 h), vapor-thermal (autoclaving at 180 °C without direct contact of TNTs and water), and hydrothermal (autoclaving at 180 °C in wet environment). It was shown that water can act as a phase transformation catalyst to facilitate the crystallization process, which is schematically described in Fig. 5(A) [59]. The water catalysed crystallization reaction tends to first occur at the interface of TNTs and the Ti implant due to the lowest activation energy of interface nucleation [60]. At the same time, titania in the tubular wall remains amorphous, which has higher solubility and surface energy compared to the anatase titania at the bottom. As the hydrothermal time increases, the size of anatase crystallites at the interface grows and amorphous titania are rapidly dissolved, which leads to the eventual destruction of nanotube structures and formation of aggregated anatase nanoparticles [61]. The aforementioned mechanism is further confirmed by the TEM images of TNTs treated by hydrothermal treatment [Fig. 5(B)]. On the contrary, calcination and vapor-thermal treatments induced a different crystallization mechanism without damaging the TNTs. However, slight deformation is occasionally observed at the top of TNTs sterilized by UV irradiation or plasma sputtering, which can etch the TNTs and modify the surface activity [57,62,63]. However, their influence on mechanical stability of TNTs is not well understood. Further advancements in this domain can be realised by varying the experimental parameters such as the wavelength, irradiation time, and intensity for UV treatment, and pressure, gas composition and flow rate for plasma treatment, which needs to be optimized for improved and reproducible outcomes.
 Fig. 5. Effect of autoclaving on crystallization of nanotubes: (A) scheme showing transformation of amorphous nanotubes to anatase, and (B) TEM images showing the morphology evolution of TNTs hydrothermally treated at 180 °C for (a) 0 min, (b) 15 min, (c) 30 min, and (d) 2 h. Reproduced with permission from [59], © American Chemical Society 2010.
Fig. 5. Effect of autoclaving on crystallization of nanotubes: (A) scheme showing transformation of amorphous nanotubes to anatase, and (B) TEM images showing the morphology evolution of TNTs hydrothermally treated at 180 °C for (a) 0 min, (b) 15 min, (c) 30 min, and (d) 2 h. Reproduced with permission from [59], © American Chemical Society 2010.2.5. Influence of underlying titanium substrate topography
The geometry, topography and chemistry of the target Ti metal substrate can influence the characteristics, including the mechanical stability, of the fabricated TNTs film [51]. For instance, Ti surface pretreatments (polishing/pre-anodizing in fluoride-free electrolyte) have been utilized to alter the formation of nanotubular or nanoporous morphologies, whereby in such systems fabrication of nanopores is favored on the polished Ti surfaces [66]. With progress in EA techniques, it has been recognized that polished Ti substrates yield an improved alignment of TNTs with reduced surface defects (weak spots or poor mechanical stability) [67]. To address this, various surface smoothening treatments including mechanical, chemical and electro-polishing have been applied to reduce Ti substrate roughness, and the resultant effect on the growth/morphology of TNTs has been investigated [68]. As reported by Smith et al., chemical polishing (immersion in HF/HNO3/H2O mixture) smoothened the surface, but it also resulted in unavoidable micro-scale defects [69]. Both mechanical polishing (grinding by abrasive papers) and electro-polishing (anodizing in perchloric acid electrolyte) of Ti resulted in a desirable smoothness for anodization, while the electro-polished sample had the lowest roughness [68,70].
Furthermore, chemical pitting by F− from the electrolyte takes place at the oxide layer, which is formed at the very beginning of the EA process. This F− attack is most prone to occur at the defect sites because of the relatively higher electrolyte distribution. Via the direction of pitting holes, nanopores are generated unevenly on the rough as-received surfaces, which are followed by self-ordering of nanotubes underneath, and meanwhile, the porous layer is gradually dissolved [71]. By contrast, on the as-polished surfaces the nanopores are formed randomly and retained. As described by Shin et al., the electro-polished smooth Ti surface was beneficial to the formation of a thin and uniform nanoporous layer, and highly ordered TNTs [72]. For mechanically polished Ti, Xing et al. found that the upper nanopores on the polished surface were rough and mainly aligned along the scratch lines [70]. The nanoporous top layer has relatively low solubility due to the contained anatase or rutile titania, which effectively acts as etching protection for the underlying nanotubes [66,71]. In the absence of this porous layer, chemical dissolution takes place at the nanotube mouth leading to the formation of a disordered “nanograss” structure as the reaction time continues, which is mechanically unstable when removed out of the electrolyte, often leading to the tubes clumped as bundles (compromised stability) [73]. When these two structures are subjected to external compression forces (nanoindentation), the nanopores are more stable than nanotubes, as they are connected with the surrounding pores, which enables sharing/distribution of the applied force, while the individual nanotubes are successively fractured due to the absence of mechanical interactions [74]. Interestingly, these more mechanically robust nanoporous structures have not as yet been applied towards enhancing the bioactivity of Ti based implants.
With the progress in EA, a more commonly used two-step anodization approach was developed for fabricating highly ordered TNTs arrays [75]. The first-step anodization provides well-ordered hexagonal imprints by removing the as-formed TNTs, which serve as the template for the growth of TNTs in the second-step in order to generate hierarchical top-porous/bottom-tubular titania nanostructures [70]. The novel two-step Ti anodization notably outperformed the conventional one-step anodization due to the greatly improved size uniformity and alignment of TNTs, which have been reported to have enhanced photo-electrochemical properties, while being rarely investigated in the biomaterial domain [76,77]. The two-step anodized TNTs are closely packed with surrounding tubes and covered with a smooth nanopore array, and as a result the mechanical stability may be improved. Besides, repeated anodizations of a Ti substrate may totally eliminate the need to perform surface smoothening in the first place, however this hypothesis remains unexplored.
3. Strategies to augment mechanical stability of TNTs
Long-term mechanical stability of the biomaterial coating is crucial for the life-long success of bone/dental implants. Mechanical properties, including hardness, elastic behaviour and adherence strength, are particularly important and must be investigated for all implant surface modifications. Implant coating delamination/deformation may occur during surgical placement or upon post-implantation loading. Indeed, following implantation, material destruction or bone resorption may take place when there is a mismatch in the mechanical properties of the bone and the implant. For instance, the elastic modulus of human cortical and trabecular lamellar bone is 13.5–25.8 GPa, which is above many of the reported modulus values of commonly used biomaterials and their surface modifications [78]. Notably, as-anodized TNTs can easily be peeled off by mechanical bending [31,94], and hence it is crucial to augment their mechanical properties. Table 2 summarizes the modified anodization conditions and physical/chemical treatments post-fabrication utilized to augment the mechanical stability of TNTs, and these will be examined in detail in the remainder of the review. Furthermore, the mechanical stability of the TNTs can be assessed using a variety of methods, and these are also outlined in Table 2. Among these, the nano-indentation technique is appropriate for testing mechanical properties, including the elastic modulus and hardness of TNTs modified Ti implants, due to the low load (1 mN) and small displacement (1 nm) employed in this method [79]. In addition, axial fatigue, tensile, scratch and wear testing are also utilized to measure the adhesion strength of TNTs to the substrate [31,46,84,85,87,88,95,96].
Table 2. Overview of techniques used to enhance the mechanical stability of titania nanotubes (TNTs). (EG: ethylene glycol).