1. Introduction
The main goal of tissue engineering is the fabrication of functional replacements for impaired organs or tissues. Scaffolds play an important role in providing the necessary support for cells to proliferate and maintain their differential function. Moreover, scaffold architecture defines the ultimate shape of the new bone and cartilage [1]. An ideal scaffold should have four characteristics: (a) three-dimensional and highly porous structure with an interconnected network for cell growth and flow transport of nutrients and metabolic waste (b) biocompatible and bioresorbable with controllable degradation and resorption rate matching cell/tissue growth in vitro/in vivo (c) appropriate surface chemistry for cell attachment, proliferation and differentiation (d) suitable mechanical properties to match those of the tissues at the site of implantation [2], [3], [4]. One type of materials which attempt to fulfill many of these requirements are the composites of biodegradable polymers reinforced with bioactive materials. Biocomposites has gained much attention in bone scaffolds because of their composition and structural similarity with natural bone in addition to their distinctive functional properties such as excellent mechanical properties, high surface area, enhanced bioactivity and controlled resorption rates [5], [6], [7]. Composite scaffolds can be produced in a variety of forms such as manufacturing a composite material in a porous form, coating a ceramic scaffold with a polymer, and coating a tough polymeric scaffold with a bioactive layer [8]. A range of techniques can be used to develop porous structure, including electrospinning [9], solvent casting and particular leaching [10], super critical gas foaming [11], emulsion freeze-drying or thermally induced phase separation (TIPS) [12], and solid free-form fabrication (SFF) [13].
The common artificial polymers used include poly(lactic acid) (PLA), polycaprolactone (PCL), polyglycolic acid (PGA), and polyhydroxybuterate (PHB) and several of their co-polymers [14]. PLA belongs to the family of aliphatic polyesters often fabricated from α-hydroxyacids which includes polyglycolic acid or polymandelic acid, and are regarded biodegradable and compostable. PLA is a linear aliphatic thermoplastic, high-modulus, high-strength polymer which can be produced from renewable resources such as sugar, corn, potatoes and cane for use in either industrial packaging field or the bioresorbable/biocompatible medical device market [15], [16], [17]. PLA is one of the few synthetic and biodegradable polymers with an extensive FDA approval history [18], [19]. It has interesting physical properties along with biocompatibility, suitable biodegradability and innocuous degradation products [20]. These properties are affected by stereochemical structure which can be tailored by polymerizing a controlled mixture of the L- or D-isomers to obtain high molecular weight amorphous or crystalline polymers [15], [21], [22]. Poly(l-Lactic acid) (PLLA) is well-known for its excellent mechanical properties among bioabsorbable polymers [23]. PLA, however, suffers from some shortcomings including low cell adhesion caused by its hydrophobic property, and inflammatory reactions in vivo due to its degradation product, lactic acid [24]. Moreover, PLA has a low degradation rate of between 10 months and 4 years owing to the hydrophobic methyl group in the backbone. Therefore, the addition of bioactive fillers into PLA matrix can buffer the localize pH decrease due to PLA degradation products, modulate degradation rate, and enhance cell adhesion, mechanical properties and osteoconductivity [25], [26].
A bioactive material is defined as a material that undergoes specific surface reactions, when implanted into the body, resulting in the formation of an hydroxyapatite-like layer that is responsible for the formation of a firm bond with hard and soft tissues [27]. Ceramic nanomaterials including hydroxyapatite (HA) and related calcium phosphates (CaP) and bioactive glasses (BG) in particular Bioglass® are the common bioactive fillers used in biocomposites. These nanomaterials are considered bioactive since they bond to bone and improve bone tissue formation [28]. HA (Ca10(PO4)6(OH)2) has a similar composition with natural bone along with high mechanical strength, osteoinductivity, osteoconductivity and biodegradability. In addition, its medical products such as screws, plates and rods form a strong bond to natural bone in vivo. The incorporation of HA can regulate the pH of the biomaterials which prevents the inflammatory reactions and inducts the growth of bone [29], [30]. Tricalcium phosphate (Ca3(PO4)2, TCP), another CaP material, is widely used in the medical field instead of HA due to its high dissolution properties [31]. TCP can be classified as α-TCP and β-TCP. α-TCP is more reactive in aqueous systems in comparison with β-TCP, and can be hydrolyzed to HA [18], [32]. Although the solubility of TCP materials is much higher than HA, from a crystallographic point of view, HA is more analogous to natural bone tissue apatite than TCP and so it represents a better structural material for bone growth [33]. Biphasic calcium phosphate (BCP), formed by the combination of HA and β-TCP, can be also used as bone substitution material and filler for composite fabrication [31]. Amorphous calcium phosphate (ACP) is another potential CaP candidate for composite reinforcements. ACP has great remineralization ability and high solubility in comparison with other CaP materials, and it plays an important role in the process of tissue mineralization [34].
BG, a system of SiO2–CaO–Na2O–P2O5, such as Bioglass® 45S5 has a great ability to bond strongly with hard and soft tissues and to foster the growth of bone cells [35], [36], [37]. BG has an amorphous structure and its structure and chemistry can be modified over a wide range by changing glass composition, or thermal or environmental processing history [27], [38]. When implanted into the body, BG undergoes specific reactions, which results in the formation of ACP or crystalline HA phase on the surface of the glass leading to strong bonding with the surrounding tissue, and release ions that activate expression of osteogenic gens [38], [39]. The gene regulating effect of the dissolution products of Bioglass® has been confirmed by researchers [32]. The size of the crystals formed on the BG surface is in nanometer scale identical to the crystal phase of bone mineral with an anisotropy mimicking the architecture of mineralized bone [40]. Phosphate-based glass materials (PG), a ternary-based P2O5–CaO–Na2O glass system, also have potential for use as biomaterials since their chemical composition is close to that of natural bone. The use of PG offers a more controlled rate of dissolution in comparison with silica containing glasses. However, simple PGs do not have enough chemical durability for biomedical applications [41], [42].
The present study is organized in the following manner: each section provides a comprehensive study on composites of PLA containing either HA, or BGs, or other CaP materials. Finally, a summary of the main PLA/CaP and PLA/BGs scaffold fabrication techniques and properties is presented as a table. This review will consider the methods of fabrication, mechanical properties, in vitrobioactivity, in vitro cell culture and in vivo testing of improved PLA-based biocomposites with focusing on one of their main potential application, bone tissue engineering.
2. Poly(lactic acid)/hydroxyapatite composites
PLA/HA composites have attract much attention due to their suitable osteoconductivity, osteoinductivity, high mechanical strength and biodegradability [43], [44], [45]. Nanocomposites of PLA/HA fabricated by electrospinning method showed an improved mechanical strength, and were suitable nanofibers for bone tissue regenerations [46]. According to Deng et al. [47], the introduction of HA particles in PLLA/HA hybrid electrospun scaffolds restrained inflammation from the acid release by autocatalytical acceleration of PLLA. Human osteosarcoma MG-63 cell adhered and spread out on the hybrid scaffolds surface. However, the degradation rate of PLLA/HA scaffold was reduced significantly in comparison with pure PLLA since the dissolving of HA particles blocked off the entry of water. Jeong et al. [48] produced PLA/HA nanofibers in which HA particles were homogeneously distributed. The scaffold containing 16.7 wt% HA showed the highest tensile strength (0.262 MPa) compared with other samples including pure PLA (0.063 MPa). In vitro study indicated that MC3T3-E1 cells maintained viability and proliferated for up to 21 days. PLA/HA composite membranes reinforced with PLA fibers showed superior protein absorption kinetics as compared to PLA-PLA fiber membranes [49]. In a recent study, fibrous PDLLA/HA composites were formed from in situgrowth of HA within ultrafine fiber. The interactions between PDLLA and formed HA and high HA loadings improved the tensile strength and Young's modulus up to 8.2 and 63.5 MPa, respectively, which were significantly higher than those of blend electrospun composites with HA inoculation. Moreover, the non-stoichiometric HA particles on the fiber could maintain desirable cell-substrate interactions, provide conducive conditions for cell proliferation and stimulate to undergo osteogenic differentiation [50].
3D resorbable scaffolds with high porosities (~ 97%) can be fabricated by TIPS method to generate controlled microstructures as scaffolds for tissues. Pore morphology, microporosity, mechanical properties, bioactivity and degradation rates of TIPS produced foams can be controlled by varying the polymer concentration in the solution, volume fraction of secondary phase, quenching temperature and solvent and polymers used [51], [52], [53]. Ma et al. [54]reported that PLLA/HA composite scaffolds fabricated by TIPS method possessed higher osteoblast survival rate, more uniform cell distribution and growth, enhanced new tissue formation, and improved bone specific gene expression in addition to superior mechanical properties compared with PLLA scaffolds. The incorporation of HA particles in PLLA increased protein adsorption which is of the importance in evaluating a scaffold for tissue engineering because the adhesion and survival of cells can be modulated by protein pre-adsorption on the substrate. In addition, compressive modulus enhanced significantly from 4.3 MPa to 8.3 MPa in comparison with pure PLLA [55]. Zhang et al. [56] reported the preparation of highly porous PLA/HA composites through in situ formation of apatite onto PLA foams in simulated body fluid (SBF). The compressive modulus of the composite foams was higher than that of pure PLA foam, which increased with incubation time in SBF. In another study, rode shaped HA was used to fabricate HA filled PLLA composite scaffolds by TIPS method. The obtained scaffolds exhibited highest compressive strength (8.67 MPa) with 85% porosity that is comparable to the high end of compressive strength of cancellous bone [57].
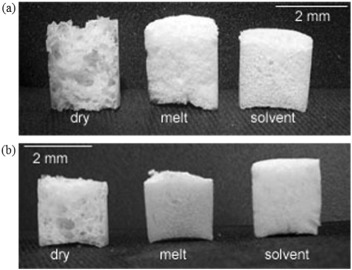
The use of organic solvents is the major drawback of TIPS method since they present potential toxicity which cannot be neglected [58]. Therefore, employing solvent-free processes such as gas foaming method for the fabrication of composite scaffolds has attracted attention. Mathieu et al. [59], [60] prepared composite foams of PLA/HA and PLA/β-TCP, by supercritical CO2 foaming which demonstrated anisotropy in the morphology. The scaffolds showed a longitudinal modulus up to 1.5 times greater than the transverse modulus and viscoelastic behavior with enhanced modulus for higher strain rate. Biocompatibility studies demonstrated that the scaffolds supported cell growth, proliferation and differentiation of human primary osteoblasts cells. In another study, dry, solvent, and melt processes were investigated to mix HA or β-TCP fillers with PLA as a first step prior to supercritical foaming to fabricate porous composite structures. The dry process resulted in a ceramic particle network around polymer pellets, while melt and solvent techniques led to a homogeneous dispersion filler in the polymer and intimate blending of both components. According to mechanical tests, modulus of the foams prepared by melt extrusion was closer to the theoretical ones. In addition, a homogeneous foam morphology was achieved by solvent and melt methods while dry mixed composites induced large variations in pore size (Fig. 1). Since the main drawback of the solvent method is the risk of toxic organic solvent residues, thus, melt extrusion was considered for the fabrication of homogeneous ceramic/polymer blend [61]. Montjovent et al. [62] evaluated the biocompatibility of PLA and PLA/HA foams produced by supercritical gas foaming after a melt extrusion of ceramic/polymer mixture. When human fetal and adult osteoblasts seeded on scaffolds, they proliferated and differentiated in their immediate vicinity. Both adult and fetal cells could spread on the scaffold surfaces independent of the presence or absence of ceramic fillers. After 4 weeks of culture, fetal cells penetrated inside the scaffolds better than did adult bone. In addition, the inner colonization was more noticeable with composite scaffolds than with pure PLA foams. The alkaline phosphatase (ALP) assays indicated that pure PLA induced a lower enzymatic activity level in comparison to PLA/HA. Ren et al. [63] prepared PLA/HA composites by in situpolymerization and then fabricated highly porous scaffolds using a novel method, supercritical CO2/salt-leaching technique. The porosity of the scaffolds was ~ 83% and the compressive strength increased by 39.2% compared to pure PLA. The cytotoxicity of the scaffolds was in grade I according to ISO 10993-1, indicating great cytocompatibility of the PLA/HA scaffolds. In vivo evaluation demonstrated excellent biocompatibility and reduced inflammatory response of the tissue in comparison with pure PLA.
 Fig. 1. Comparison of foam morphology after dry, melt and solvent mixing of PLA with (a) HA, and (b) β-TCP. (From Ref. [61].)
Fig. 1. Comparison of foam morphology after dry, melt and solvent mixing of PLA with (a) HA, and (b) β-TCP. (From Ref. [61].)The influence of HA amount and particle size on the mechanical properties and bioactivity of PLA/HA composites have been the subject of several studies. Lin et al. [64] reported the effect of HA amounts on the mechanical strength and cell proliferation of PLA composites. The ultimate compressive strength and compressive elastic modulus of PLA increased from 44 to 80 MPa and from 1 to 1.7 GPa, respectively, by increasing HA content from 0 to 40 wt%. However, the trend was different for bending modulus and it decreased from 3.2 to 2.3 GPa with increasing HA content from 0 to 30 wt% followed by an increase to 4.2 GPa at 40 wt% of HA, indicating that the mechanical properties can be controlled by adjusting PLA/HA ratio. When the amounts of HA was < 20 wt%, the osteoblast cells did not grow enough to fill the surface of the substrates while at 30 wt% of HA a significant difference in the proliferation was observed, demonstrating the ability of controlling cell proliferation in PLA/HA composites by HA level. Rizzi et al. [65] prepared simple PLA/HA mixtures containing 50-μm sintered or submicron nonsintered HA by solvent casting method. Cell culture experiments HOBs demonstrated a positive initial cell interaction. Cells on the PLA/sintered HA exhibited a rough macrotexture because of the 50-μm sintered particles, assumed an elongated morphology by bridging between adjacent particles while the composite containing submicron HA displayed a more conventional spread shape of cells. However, no significant difference in cell activity between the composites was observed after 24 h. According to Tanodekaew et al. [66] the scaffolds with smaller pore size and higher HA content promoted the ALP activity at the early culture. However, after longer period of incubation all of the scaffolds supported the proliferation and differentiation of cells to the same extent. Kothapalli et al. [67] prepared PLA/HA composites containing different HA amounts by solvent casting and particulate leaching method. Increasing in HA amount enhanced the compression strength and the modulus of elasticity from 0.29 to 0.44 MPa and from 4.7 to 9.8 MPa, respectively, while it reduced the values of porosity from 92% to 86%.
A major issue for the fabrication of scaffolds in tissue engineering is the development of processing techniques flexible enough to fabricate materials with adequate porosity, a wide range of mechanical properties and bioresorption rates matching those of calcified tissues. To this aim, the nanocomposite membrane mats of PLA/HA were prepared by air jet spinning method as a novel composite fabrication process with high production rate. The mechanism of the process is illustrated in Fig. 2. The surface morphologyshowed highly interconnected bonded fibers because of the high fabrication rates. In addition, the difference in the kinetic energies between HA particles and PLA molecules resulted in effective embedding of HA particles in the fibers. It was also found that the addition of only 0.1 g HA to the PLA increased the tensile strength by 13.5%. Moreover, the PLA/HA composite scaffolds prepare by the novel method illustrated significant cell ingrowth and proliferation compared to the plain PLA scaffolds [68].
 Fig. 2. Schematic drawing of the air jet spray process. (From Ref. [68].)
Fig. 2. Schematic drawing of the air jet spray process. (From Ref. [68].)Danoux et al. [69] prepared dense composite materials containing PLA/HA by using extrusion process, a manufacturing technique that does not require the use of solvents. The implants were designed such that an artificial pore was created between the plates of extruded materials (Fig. 3). Despite the dense structure, the composite represented proliferation of hMSCs in in vitro and in vivo canine model. The PLA/HA composite also up-regulated the expression of ALP resulting in heterotopic bone formation.
 Fig. 3. Schematic representation with dimensions of dense composite materials prepared by Danoux et al. [69].
Fig. 3. Schematic representation with dimensions of dense composite materials prepared by Danoux et al. [69].The combination of indirect SFF technologies, which is current scaffold manufacturing methods, and image-based design provides the means to control scaffold composition and architecture. This control allows for the fabrication of scaffolds which are optimized for biological and mechanical function by precise manipulation of scaffold porosity, shape and internal pore structure, which facilitates the developing of biomimetic scaffolds and scaffolds for complex biomechanical applications including perfused tissues and interfaces between soft and bone tissues [70]. PLA/HA scaffolds with controlled microstructures (pore sizes ranging between 200–500 μm) were produced using robotic assisted deposition at room temperature. The schematic diagram of the process is illustrated in Fig. 4. The incorporation of HA enhanced the stiffness of the scaffolds but they were not brittle and could be machined even for HA contents up to 70 wt%. After 20 days of immersion in SBF, the stiffness of hybrid scaffolds did not degrade considerably while pure PLA scaffolds became much stiffer and the Young's modulus increased from 3 to 76 MPa. The results indicated that the robotic assisted deposition could be a fast and economical method for the fabrication of “on demand” scaffolds in tissue engineering [71].
 Fig. 4. (a) Schematic diagram of the robocasting process. The liquid flow through the nozzle and the syringe displacement are controlled by the computer. (b) During the process, the inks swell after leaving the capillaries and the evaporation of the solvent creates a solid skin which favors the consolidation of the printed line. (c) Low magnification picture of PLA/70 wt% HA scaffold. (d) Three dimensional image of PLA/70 wt% HA grid taken by synchrotron X-ray computed tomography. (From Ref. [71].)
Fig. 4. (a) Schematic diagram of the robocasting process. The liquid flow through the nozzle and the syringe displacement are controlled by the computer. (b) During the process, the inks swell after leaving the capillaries and the evaporation of the solvent creates a solid skin which favors the consolidation of the printed line. (c) Low magnification picture of PLA/70 wt% HA scaffold. (d) Three dimensional image of PLA/70 wt% HA grid taken by synchrotron X-ray computed tomography. (From Ref. [71].)Using SFF method, Schek et al. [72] manufactured biphasic composite scaffolds to generate bone and cartilage simultaneously in discrete regions and provide the development of a stable interface between cartilage and subchondral bone (Fig. 5). The biphasic composite scaffolds were differentially seeded with porcine articular chondrocytes in the polymeric phase and human gingival fibroblasts transduced with adenovirus expressing bone morphogenetic protein 7 (BMP-7) in the ceramic phase. A thin film of PGA was placed between phases before assembly to prevent cell migration between them. After ectopic implantation in mice, the biphasic scaffolds promoted the simultaneous growth of cartilage, bone and a mineralized interface tissue. In the ceramic phase the pockets of tissue were generated containing blood vessels, marrow stroma, and adipose tissue. Therefore, the aforementioned method is a promising strategy for the generation of functional articular surfaces.
 Fig. 5. PLA was utilized to join the ceramic and polymer phases of the composite scaffold. One face of the ceramic phase was coated with a thin film of PGA (a). The film was then removed and PLA was applied to the surface (b). The PLA sponge was pressed onto the HA scaffold, allowing the solubilized PLA to adhere (c). PLA rods were extruded on two opposite sides of the scaffold to stabilize the scaffold (d). (From Ref. [72].)
Fig. 5. PLA was utilized to join the ceramic and polymer phases of the composite scaffold. One face of the ceramic phase was coated with a thin film of PGA (a). The film was then removed and PLA was applied to the surface (b). The PLA sponge was pressed onto the HA scaffold, allowing the solubilized PLA to adhere (c). PLA rods were extruded on two opposite sides of the scaffold to stabilize the scaffold (d). (From Ref. [72].)Over the years there has been an increasing interest in self-reinforced composites which are made by melting a small part of the surface of the fibers under a low pressure and then compression at higher pressure. Upon cooling, the recrystallizing melt acts as the matrix to bind the fibers. These composites possess a strong bond between the fibers and the matrix and improved mechanical properties because of the identical chemical structure of the two [73], [74], [75]. Charles et al. [74] developed PLA/HA self-reinforced composites for bone-fixation devices. The incorporation of HA led to a gradual increase in the flexural modulus from 8.3 to 9.7 GPa. Notably, the procedure used successfully provided composites with flexural moduli near the lower range of bone which could have a possible clinical use for load bearing bone-fixation devices.
The interface adhesion of HA particles and PLA is significantly important and influences the properties of the PLA/HA composites. In vitro biological evaluation demonstrated that the existence of modified HA in nanocomposites provides favorable environment for protein adsorption and cell adhesion and proliferation [76]. Silane modification of HA particles in PLLA/HA nanofibrous scaffolds not only increased the PLA/HA interfacial strength but also improved protein adsorption capacity by 21% compared with the scaffold containing untreated HA, which indicates that more cells can attach and survive on PLLA/silanized HA during initial culture period [77]. Surface modification of HA with silane derivatives also improved bending strength by 27.8% [78] and compressive strength of the composites by 70.7% [79]. Li et al. [80] used ring-opening polymerization of lactide to modify the surface of HA particle. It was revealed that modified HA particles were well dispersed in polymeric matrix in comparison with unmodified HA particles, and the novel composite exhibited better mechanical properties and thermal stability as compared with PLA/HA composite. Qiu et al. [81] modified the surface of HA by surface grafting reaction of l-lactic acid and ring-opening polymerization of l-lactide. The highest grafting contents of l-lactic acid and PLA were about 33 wt% and 22 wt%, respectively. PLLA modified composites showed a more enhancement of tensile strength and modulus in comparison with l-lactic acid modified composite, while the latter one exhibited better ductility. The tensile strength and modulus of PLLA modified composite were 67 MPa and 2.1 GPa, respectively, while those of unmodified PLLA/HA composites were 45 MPa and 1.7 GPa, respectively. The elongation at the break of l-lactic acid modified composite containing 15 wt% HA was 44%, in comparison with 6.5% of unmodified PLLA/HA containing 15 wt% HA. In another study, the HA nanoparticles were surface-grafted with PLLA and then blended with the polymer. The results indicated that at a low content of surface grafted HA, the samples exhibited the highest tensile strength (~ 75 MPa). Further, the novel composite showed an improved cell compatibility due to the biocompatibility and uniform distribution of the surface-grafted HA on the film surface [43]. Electrospun composite fibers composed of poly(l-lactic acid)-grafted hydroxyapatite (PLLA-g-HA) nanoparticles and PLA matrix also exhibited an improvement in mechanical properties. The increase in PLA-g-HA content in the scaffold led to an increase in in vitro degradation rate due to the improved wettability of the composite fibers and the escape of the nanoparticles from the fiber surface during incubation [82]. The tensile strength of PLLA/PLLA-g-HA composite was 55 MPa while that of PLLA/HA composite was 40 MPa [83]. Surface modification of HA particles using a surfactant hydroxystearic acid is another technique to disperse HA in the matrix and creating continuous fibers during the electrospinning stage. Initial osteoblast cellular assays showed excellent cell attachment and proliferation and improved expression of ALP at 7 days of culturing [84]. Xiao et al. [85] modified PLA surfaces with poly(α-methacrylic acid) (PMMA) via photooxidization and UV induced polymerization. The presence of modified PLA in PLA/HA composites resulted in controlling the morphology, size and distribution of HA crystals over the organic phase and obtaining PLA/HA composite with suitable interfacial interaction. Wang et al. [86] developed a calcium-phosphate/phosphonate hybrid shell on the surface of HA particles to introduce a greater amount of reactive hydroxyl groups on it. PLA was then grafted on HA using surface-initiated polymerization via non-ionic surface hydroxyl groups. The tensile strength of PLA/PLA-g-HA were found to be over twice that of PLA/HA composite. Therefore, the aforementioned approach is beneficial to fabricate bioresorbable composites with improved mechanical properties that are in the range of natural bones.
Interconnected porous HA scaffolds are widely used in bone tissue engineering due to their ability to support the adhesion, transfer, proliferation and differentiation of cells. However, the poor mechanical strength of HA scaffolds restricts their applications in load-bearing conditions [87], [88]. According to Zhao et al. [87] one possible solution is coating HA composites with PDLLA to enhance the mechanical strength of HA scaffold. The PDLLA-coated composite scaffolds exhibited significant improvement in both mechanical and biological properties while maintaining the 3D interconnected porous structure. Tian et al. [89] also produced PLLA/HA scaffolds through immersing as-sintered HA scaffold into pre-prepared PLLA solution. Although compressive strength increased from 0.34 to 2.46 MPa, a pore volume fraction of 70% was achieved which is lower than what is ideal for bone tissue scaffolds.
According to the studies mentioned above, conventional PLA/HA composites do not seem to be able to mimic the hierarchical structure and mechanical properties of bone. On the other hand, novel techniques such as SFF and jet spray can be used to make micro- and macro-structures that mimic porous bone and compressive strength larger than porous bone. Moreover, surface functionalization of HA (such as surface grafting with PLLA), elimination of solvent in mixing process (melt mixing), adjusting the particle size and PLA/HA ratio affects the morphology, interaction between nanoparticle and polymer, and consequently, enhances mechanical properties and cell adhesion and proliferation.
3. Poly(lactic acid)/bioactive glass composites
The PLA/BG composites associate advantages of both constituting phases, mechanical strength of the polymer matrix, ease of processing and the bone growth ability promoted by BG. Accordingly, Vergnol et al. [90] investigated the ability of PDLLA/Bioglass® composite to be used as a bone fixation device. The addition of inorganic particles accelerated the degradation rate of the composite in simulated physiological conditions. In vitro assays demonstrated an absence of cytotoxicity for both polymer and composite materials, and in vivo investigation on rabbits revealed that the presence of Bioglass® triggered bone osseointegration especially during first month of implantation. Wilda et al. [91] developed PDLLA/Bioglass® films by solvent casting method for the culture of annulus fibrous cells in vitro. The results indicated that the composite films provide a suitable substrate for annulus cells and promote the production of extracellular matrix containing plentiful sulphated glycosaminoglycan and collagen. According to Cao et al. [92] the compressive strength and water contact angle of PLLA/mesoporous bioglass (MBG) composite fabricated by solution casting were significantly improved from 26 to 61 MPa and 84° to 34°, respectively. Moreover, the large surface area and high pore volume of MBG in the composites provided more chances for interaction between cells and composite surface, which resulted in the enhanced cell adhesion and growth in PLLA/MBG composites. In addition, the ALP activity of MC3T3-E1 considerably increased by increasing the MBG content.
Nanofibrous materials are highly regarded in biomedical fields, including tissue regeneration matrices and cell supporting substrates [93]. Accordingly, Noh et al. [94] produced a composite nanofiber of PLA filled with BG nanoparticlesusing electrospinning method. Addition of BG nanofiller up to 10 wt% greatly improved in vitro bone-bioactivity by inducing calcium phosphate mineral on the fiber surface. Further, osteoblastic cells cultured on the nanocomposite fiber demonstrated favorable cellular adhesion and growth. Macro-mesoporous BG/PLA nanofibers synthesized by electrospinning method displayed suitable hydrophilic property, controlled drug release and fast HA mineralization performance [95]. Yunos et al. [96], [97] fabricated PDLLA fibrous coatings on Bioglass® using electrospinning method, which resulted in the introduction of a rough fibrous topography on the polished surface of substrate. Immersion in SBF created a uniform HA covering of fibrous structure in 14 days. Chondrocyte cells could attach, proliferate and migrate within the fibrous PDLLA layer. Indeed, this process provides a convenient rout to develop a controlled mineralized fibrous topography on BG substrates for enhanced cell attachment in bone tissue engineering. Kim et al. [98] reported on the novel glass nanofiber-reinforced PLA composites in which the nanofibers were generated via electrospinning process with an average diameter of ~ 320 nm (the fabrication process in shown in Fig. 6). The novel composites induced rapid formation of HA layer on the surface under a simulated physiological medium. The in vitro bioactivity was improved by the increase in nanofiber content (from 5 to 25%). Moreover, osteoblastic cells exhibited favorable cell attachment and proliferation behavior on the nanocomposites, and the incorporation of filler considerably enhanced further differentiation and mineralization behaviors of cell.
 Fig. 6. Scheme of the process introduced to prepare BG nanofiber and its nanocomposite with PLA. The optical images of the produced materials at each step are shown below. (From Ref. [98].)
Fig. 6. Scheme of the process introduced to prepare BG nanofiber and its nanocomposite with PLA. The optical images of the produced materials at each step are shown below. (From Ref. [98].)According to a study by Blaker et al. [99], in the presence of Bioglass® particles, poly(α-hydroxyexter)-based composites should not be processed at elevated temperatures since thermal processing may lead to the production of carboxylate salt by-products and oligomeric fragments and reduction in mechanical properties arising with molecular weight reduction. Therefore, the processing of PLA/Bioglass® foams by low temperature techniques, such as TIPS, are not detrimental to the polymer. The properties of PLA/Bioglass® composite scaffolds such as in vitro degradation behavior, bioactivity and mechanical properties can be controlled by tailoring the concentration of BG [100]. Hence, the influence of Bioglass® content on the characteristics of PDLLA/Bioglass® composite foams fabricated by TIPS method has been widely studied. It was found that the pore volume of the composites decreased from 9.5 to 5.7 cm3/g by increasing filler content from 5 to 40 wt%, but the overall pore morphology did not alter very much by varying polymer/BG ratio. Conversely, the compressive modulus of the composites was significantly improved from 14 to 21 MPa [101], [102], [103]. Blaker et al. [104] investigated long-term in vitro degradation of PDLLA/Bioglass® in SBF. The incubation of foams in phosphate-buffered saline at 37 °C showed that the addition of Bioglass® to polymer foams enhanced the water absorption and weight loss in comparison with pure PDLLA. Indeed, in the foams containing higher filler concentration, a burst was even observed in weight loss during the first two weeks of incubation. However, the polymer molecular weight was found to decrease more quickly and to a large extent in the absence of bioactive glass. This delayed degradation rate in the composites might be due to the dissolution of ions from Bioglass®, which leads to a buffering effect of the incubation medium [102], [103]. El-Kady et al. [105] also reported the possibility to modulate degradation rate of PLA-based composite scaffolds by varying their BG content. Indeed, the introduction of bioactive glasses to PLA scaffolds increases in vitro degradation rate and can compensate the released acidity during the degradation of the polymer; therefore, it provides a method to avoid possible inflammatory reaction. In another study it was revealed that after 28 days of incubation of samples in SBF at 37 °C, the foams filled with the higher content of Bioglass® developed a continuous layer of HA while the formation of HA for the samples containing lower amount of filler was localized to the Bioglass® particles. The size of HA domains formed in the sample containing 40 wt% Bioglass® was estimated between 10 and 20 μm. Cell culture studies using MG-63 exhibited the affinity of cells to attach to the foam substrates and it was found that the increase in the amount of Bioglass® leads to the enhanced cell adhesion and growth [106], [107], [108]. Yang et al. [109]also observed the adhesion and growth of hBMCs on PDLLA/Bioglass® foams. In vivo analysis revealed widespread bone collagen formation throughout each of the scaffolds, indicative of bone regeneration. According to Lu et al. [110], PDLLA/Bioglass® foams supported attachment, growth and osteogenic differentiation of ADSCs. Increase in Bioglass® content resulted in the improvement in ALP activity and formation of new bone. In another study, the PDLLA/Bioglass® foams were able to provide a suitable microenvironment for bovine annulus fibrosus cells, in which cell proliferation and the production of sulphated glycosaminoglycans, collagen type I and collagen type II were enhanced by the increment of Bioglass® content [111], [112]. It seems that the addition of Bioglass® to PDLLA stimulates osteoblast differentiation and mineralization of extracellular matrix, indicating the osteoinductive capacity of the composites [113]. Kim et al. [114] reported on PLA/BG nanofibers fabricated by freeze-drying method. The glass nanofibers were evenly embedded within the matrix while preserving the scaffold porous structure. SBF test demonstrated quick induction of bone mineral-like apatite on the surface of the composite scaffold, which was not readily seen in neat PLA. MSCs adhered well onto the composite structure during culture period. The incorporation of BG significantly stimulated ALP activity and expression of bone associated-gens such as collagen I, ALP, osteopontin and osteocalcin; therefore, the composite scaffolds provide suitable conditions for osteogenesis of MSCs.
Recently, Kim et al. [115] produced PLA/BG macroporous and nanofibrous scaffolds by camphene-assisted phase-separation method (Fig. 7). The results indicated highly porous and nanofibrous structure with porosities of 90–95% depending on BG content and pore sizes of over hundreds of micrometer. The nanofibrous scaffolds showed improved hydrophilicity and more hydrolytic degradation as the BG content increased. SBF immersion test exhibited a substantial improvement in bioactivity with the incorporation of filler, and Ca/P ratio changed greatly from 2.22 at day 1 to 1.66 at day 7 of immersion, in a manner of matching well with the stoichiometry of HA with immersion time.
 Fig. 7. Scheme of preparation of PLA/BG nanofibrous scaffolds: (a) Camphene is mixed with a solution containing PLA/BG. (b) Mixture is shaped in a mold containing NaCl. Freezing below − 20 °C leads to the solidification of solvents and camphene, and simultaneously, phase separation occurs between materials and camphene. (c) Freeze-drying allows for the sublimation of solvents and camphene. Camphene sublimation creates nanofibrous structures and salt-leaching in distilled water generates macropores in scaffolds. (From Ref. [115].)
Fig. 7. Scheme of preparation of PLA/BG nanofibrous scaffolds: (a) Camphene is mixed with a solution containing PLA/BG. (b) Mixture is shaped in a mold containing NaCl. Freezing below − 20 °C leads to the solidification of solvents and camphene, and simultaneously, phase separation occurs between materials and camphene. (c) Freeze-drying allows for the sublimation of solvents and camphene. Camphene sublimation creates nanofibrous structures and salt-leaching in distilled water generates macropores in scaffolds. (From Ref. [115].)The application of Bioglass® particles both as coatings and fillers in scaffolds adds to the possibilities of tailoring of the time dependent mechanical properties and the rate of in vitro resorption of composite scaffolds for the required application [116]. Boccaccini et al. [117] prepared highly porous PDLLA foams filled with Bioglass® particles using TIPS process and coated the composite foams with homogeneous layers of Bioglass® particles. HA particles were formed rapidly in coated foams after only 7 days of immersion in SBF indicating their bioactivity, which further grew to form a thick (~ 10 μm) and uniform HA layer on the scaffold. In non-coated samples, however, the formation of HA was less pronounced and slower than in Bioglass®-coated scaffolds.
Highly porous, bioactive and biodegradable BG scaffolds are widely used in bone tissue engineering [118], [119], [120]. To improve the mechanical properties, in particular, the fracture toughness, BG scaffolds are coated by various biodegradable polymers including PLA. Bretcanu et al. [121]investigated PDLLA-coated BG scaffolds which were obtained by the foam replication technique. PDLLA-coated samples exhibited higher compression strength and fracture strength as compared to uncoated scaffolds. Upon immersion of the scaffolds in SBF for 4 weeks, the mechanical strength of uncoated composite foams decreased to a large extent (form 0.3 to 0.03 MPa) due to the transformation of the crystalline phase to an amorphous calcium phosphate. On the other hand, the mechanical strength of PDLLA-coated foams was well-maintained even after immersion in SBF for 8 weeks. Moreover, HOBs were seeded onto coated and uncoated foams which showed progressive spreading of initial cell attachment on both types of foams [122], [123]. The attachment and proliferation of HOS-TE85 on scaffolds confirmed the effectiveness of PDLLA-coated scaffolds to support HOS cell function, indicating their potential as osteoconductive substrates for bone tissue engineering [121], [124]. Bioglass® scaffolds coated with 5 wt% PDLLA showed ~ 40% improvement in compressive strength and demonstrated the porosity of 80% [125]. Chen et al. [126] coated surgical sutures with a PDLLA/Bioglass® composite film followed by a second PDLLA coating. The adhesion strength of novel coatings was remarkably improved in comparison with Bioglass® particles on suture surfaces. In addition, the PDLLA layer provided paths for SBF fluid to contact BG and stimulate bioactivity; meanwhile, it sustained the mechanical integrity of coating.
Advanced composite scaffolds containing BG demonstrating osteogenic and angiogenic properties indicate an appropriate solution for the regeneration of complex tissue structure defects such as at soft-hard tissue interfaces [127]. Accordingly, Gerhardt et al. [128] investigated angiogenic properties of micron-sized (m-BG) and nano-sized (n-BG) Bioglass® filled PDLLA composites both in vitro and in vivo. Human fibroblasts produced 5 times higher vascular endothelial growth factor on composite films containing 20 wt% m-BG or n-BG than on neat PDLLA films. After 8 weeks of implantation (Fig. 8), composite scaffolds were well-infiltrated with newly formed tissue and exhibited higher vascularization and higher blood vessel to tissue ratio. Therefore, the composite films provide potential for enhanced bone formation arising from the increased vascularization of the construct.
 Fig. 8. Implantation of PDLLA scaffolds with and without Bioglass® particles, (a) PDLLA control scaffolds after 8 weeks of implantation. (b) m-BG containing scaffold implanted in the left upper abdominal site and n-BG in the lower right abdominal region after 8 weeks. (From Ref. [128].)
Fig. 8. Implantation of PDLLA scaffolds with and without Bioglass® particles, (a) PDLLA control scaffolds after 8 weeks of implantation. (b) m-BG containing scaffold implanted in the left upper abdominal site and n-BG in the lower right abdominal region after 8 weeks. (From Ref. [128].)To improve filler-polymer interaction and homogeneous dispersion of particles in the polymeric matrix, Bioglass® was surface modified using esterification reactions with dodecyl alcohol by Gao et al. [129]. The results revealed that the films containing modified inorganic particles have more uniform particle distribution and improved tensile strength (from ~ 10 to 12 MPa) in comparison with unmodified sample. Moreover, the hydrophilicity of modified composite films was greatly improved. In vitro cellular assessment of BMSCs after hydrolytic treatment of BG showed the highest proliferation rate. SBF immersion experiment, however, confirmed that the modification did not affect the bioactivity of sample [129], [130]. In another study, PLLA was grafted onto the surface of BG nanoparticles using the coupling of diisocyanate. The surface modified nanoparticles were able to be dispersed uniformly within PLLA matrix. In addition, the novel composites had greater tensile strength and easier apatite deposition in SBF in comparison with the unmodified ones, but the tensile modulus for these two kinds of composites were almost the same [131].
Niemelӓ et al. [132], [133] manufactured self-reinforced composites of PDLLA/BG by twin-screw extruder followed by self-reinforcing. The self-reinforcing process enhanced mechanical properties of the composites, changed the initial brittle composite to ductile one and modified the composite structure, generating both interior and exterior porosity. It was shown that the bending strength, bending modulus, shear strength, compression strength and torsion strength decreased with the increment of BG content in self-reinforced composites while the strain at maximum bending increased. In vitrodegradation revealed that degradation of glass containing composites was slower in terms of mechanical properties and molecular weight loss compared with plain matrix polymer. However, the degradation was more pronounced in vivo than in vitro, indicating that the soft tissue environment (enzymes and implant movement) facilitates the degradation process [134]. The self-reinforced composites also retained their original shape longer than did the plain matrix polymer. It was concluded that self-reinforced composites containing 20–30 wt% BG have the potential to be used as small bone fracture fixations [132], [133]. Tuomo et al. [135] showed that the mechanical strength and fixation properties of self-reinforced PDLLA/BG composite rods are suitable for the fixation of cancellous bone osteotomies in rats.
Guided tissue/bone regeneration has emerged as a therapeutic modality by using a barrier membrane to prevent the faster growing soft tissue cells from the defect space and regenerate periodontal ligament, cementum and bone [136], [137]. Caridade et al. [137] fabricated biodegradable membranes based on PDLLA and Bioglass® for using in guided tissue/bone regeneration that show asymmetric bioactivity, in which the osteoconductive particles are well integrated and deposited preferably in one side of the membrane. The asymmetric distribution of the particles was prepared during the processing of the membrane by a solvent casting technique. Only the Bioglass® rich face of the composite membranes represented osteoconductivity and induced the precipitation of HA in SBF. Composite membranes improved cell adhesion and proliferation in addition to cell differentiation, mineralization and production of extracellular matrix and calcium nodules. Indeed, the smooth side richer in PDLLA prevented cell invasion to other side and the opposite porous side containing higher amounts of Bioglass® allowed both cell growth and bioactivity [137], [138].
In the final analysis, it has to be mentioned that optimizing biocomposites of PLA/BG requires understanding their structure and properties. In vitro and in vivo studies on BGs show that BG particles can perform better than ceramic particles. However, the fabrication techniques of PLA/BG composite scaffolds affect their mechanical properties and bioactivity. Utilizing low temperature techniques particularly TIPS method, tailoring the composition and particle size of BG, varying filler content in polymer, surface treatment of BG and using drug-loaded particles can improve compressive modulus, modulate degradation rate of the composite, enhance mineralization of extracellular matrix, and increase cell adhesion and proliferation.
4. Poly(lactic acid)/other calcium phosphate composites
The combination of TCP with resorbable polymers like PLA could enhance the biocompatibility and osteointegration of bone substitute materials with improved mechanical properties. Hence, numerous authors have applied this promising concept [139], [140]. Ignatius et al. [141] mixed and sintered PDLLA and α-TCP at a temperature of 145 °C. The mixing ratio was 55 wt% PDLLA and 45 wt% α-TCP. The obtained composite had an interconnecting macro porosity of 42%. The initial compression strength of the composite was 12.5 MPa which corresponds to that of cancellous bone, and elastic modulus was 645 MPa which was about two times higher than that of cancellous bone. During degradation in vitro, the mechanical stability of the scaffold was constant for 12 weeks, and decreased by 25% until 26 weeks. After that time point the scaffold degraded continuously until 52 weeks. In another study by Ignatius et al. [142] the biocompatibility and in vivo degradation of the PDLLA/α-TCP were examined in a loaded implant model in sheep. After 6 months of implantation, the interconnecting pores of the composite were filled with newly formed bone and soft tissue (~ 14% and ~ 30% of the implant, respectively) and only a mild inflammatory tissue reaction was seen. Two years after implantation, however, a strong inflammatory response and a significant reduction in strength was observed simultaneously with the degradation of PDLLA.
Porous PLA/CaP composites were produced by Bolay e al. [143] using co-grinding in a tumbling ball mill to disperse the mineral filler within polymer and hot molding the mixture with a pore-forming agent. It was found that the incorporation of 30 wt% filler and 70 wt% pore-forming agent produce scaffolds with 2.2 MPa resistance and 60% porosity. The incorporation of 20 wt% of β-TCP into PLA improved the modulus of elasticity by 21% and reduced the flexural strength by 15% [144]. PLA/β-TCP microspheres fabricated by Lin et al. [145]demonstrated a slight difference in degradation rates with pure PLA microspheres. This indicates that β-TCP was entrapped and spread in the whole microsphere and consequently, the rate of calcium release from the microspheres depended on the polymer degradation rate. Histological examination of PLA/β-TCP microspheres in rabbit's condyle model showed excellent repairing and recover of the osteocyte tissues on the wounded sites within 1 month of application.
Numerous studies have shown favorable effects with the incorporation of CaP materials in electrospun materials with minimal cytotoxicity, enhancement of cellular proliferation and increased mineralization [146], [147]. McCullen et al. [147] evaluated the increasing β-TCP content in PLA/β-TCP electrospun composite scaffolds on mechanical properties and proliferation, osteogenic differentiation and calcium production of ADSCs. Tensile strength of the neat scaffold was 847 kPA while the addition of β-TCP reduced this value to an average of 350 kPA. During the culture of ADSCs, cellular viability was extremely high with minimal dead cells on all scaffold combinations. In addition, cellular DNA was enhanced in a temporal manner for all scaffolds during 18 days in culture, however, the scaffolds containing 10 wt% β-TCP induced the greatest hASC proliferation for the day 12 timepoint. Electrospun PLA/20 wt% β-TCP demonstrated enhanced osteogenic differentiation and increased cell-mediated mineralization as compared with the pure PLA scaffolds. Dinarvand et al. [148] coated electrospun PLA scaffolds with HA, BG and TCP and studied the bone formation induced by these scaffolds in a rat model. Histological and digital mammography experiments indicated that PLLA/HA-BG scaffolds induced a considerably higher level of reconstruction compared with that observed in defects treated with PLLA/TCP. Furthermore, PLLA/HA-BG scaffolds synergistically improved bone regeneration higher than that observed in PLLA/HA and PLLA/BG. In another study, ACP nanospheres and HA nanorods were separately hybridized with PLA to produce composite nanofibers by electrospinning. The addition of ACP nanospheres and HA nanorods in PLA reduced tensile strength of the nanofibers from 1.6 MPa to 1 MPa and 0.9 MPa, respectively. The in vitro mineralization exhibited a poor mineralization ability of the PLA nanofibers while PLA/ACP and PLA/HA composite scaffolds showed favorable mineralization behaviors in SBF. The in vivo biocompatibility and bone defect repair were investigated through using PLA/ACP and PLA/HA as bone defect fillers. The results showed that the collagen constituent in the bone defects treated with PLA/ACP and PLA/HA composite nanofibers was more obvious compared with pure PLA, indicating high in vivobiocompatibility of the composites [149]. Ma et al. [150] also hybridized ACP nanoparticles with PDLLA using electrospinning and obtained PDLLA/ACP composite nanofibers by optimizing the experimental conditions and different architectures including the nanofibrous mesh and tube. The increment in ACP content resulted in the acceleration of mineralization of PDLLA/ACP composite nanofibers. The adhesion and spreading behavior of MG-63 cells on the composite nanofibrous mesh surface occurred at the beginning of the cell/composite interaction, which is an important factor that affects the further proliferation and differentiation of the cells. The porous three-dimensional PLLA/ACP scaffold incorporated with basic fibroblast growth factor could successfully resurface the defect with cartilage and regenerate the subchondral bone in rabbit model [151].
CaP glasses are well suited for bone repair since they have a chemical composition similar to that of the mineral bone phase, and their degradation can be adjusted by modifying their chemical formulation. The presence of CaP glass particles in polymer matrix allows the penetration of the aqueous fluid into the interior of the composite through PLA/CaP glass interface at the surface and the formation of surface microcracks, which accelerates degradation of the polymer chains [152]. PLA/CaP glass composite fabricated by solvent casting and salt leaching method showed an interconnected structure with a porosity as high as 97% and an increased surface roughness in comparison with pure PLA matrix. The incorporation of CaP glass particles to PLA not only improved the compressive modulus from 74.5 to 120 kPa but also enhanced cell viability. In addition, CaP particles induced the formation of a CaP precipitate which improves the interaction between the material and bone tissue. Cell culture showed that the MG-63 cells seeded in pure PLA exhibited a very flat and extended morphology, however; the cells seeded in rough surfaces showed a more rounded or cuboidal shape with long cytoplasmic extensions [153], [154], [155]. To examine the effects of solvent in the preparation method on the properties of scaffolds, scaffolds of PLA/CaP glass were prepared via solvent casting and salt leaching, and phase separation by Charles-Harris et al. [156]. The stiffness and porosity of solvent cast composite scaffold were 0.19 MPa and 95%, respectively, however; those of phase separated scaffolds were 7.1 MPa and 90%, respectively. The MG-63 cell cultures showed that the phase separated scaffolds induced less proliferation during first week of culture, however, from then on the proliferation rates were similar for both types of scaffolds. Moreover, MG-63 cells in the solvent cast scaffolds spread towards their interior while the cells in phase separated scaffolds remained on their surface and formed a thick layer there. The level of ALP activity of solvent cast scaffolds attained its maximum at day 14 while that of phase separated scaffolds reached its maximum at day 7, and then decreased. Hence, the phase-separated scaffolds sustained more and earlier cell differentiation as compared with the solvent cast ones.
To solve the problems associated with residual organic solvent and cell compatibility, Jung et al. [157], [158] developed a new process to produce PLA/calcium metaphosphate (CMP) composites for bone tissue engineering using press-and-baking method. The scheme of the novel sintering method is illustrated in Fig. 9. The tensile strength of the composites made by the novel method and by solvent casting having the 90% porosity were 0.72 and 0.13 MPa, respectively. In vitro experiments with osteoblasts demonstrated that the composites fabricated by press-and-baking method had higher efficiency of cell seeding and enhanced osteogenic differentiation as compared to the composites fabricated by solvent casting method. In vivo assessments in mice model showed appropriate interactions with transplanted osteoblasts and well-formed tissues ingrowth in the composites made by the novel method. Therefore, the scaffolds made without solvent have the more chance of direct interaction between cell and ceramic and consequently, might have an enhanced cell proliferation and differentiation as compared with the scaffolds made with solvent [159], [160]. Similarly, Kang et al. [161] used compression molding and particulate leaching to fabricate PLLA/β-TCP composite scaffolds. The scaffolds possessed high porosity with interconnected structure and high compressive strength in the range of 1.03–9.5 MPa. According to in vitroosteoblasts culture studies and immersion in SBF, the PLLA/β-TCP scaffolds had good abilities of cell adhesion and bone-like apatite formation.
 Fig. 9. The scheme of the novel press-and-baking method. (From Ref. [158].)
Fig. 9. The scheme of the novel press-and-baking method. (From Ref. [158].)It has been shown that the use of freeze-drying method to fabricate scaffolds leads to obtaining a uniform large porous structure and better mechanical properties compared to solvent casting and porogen leaching method [162]. Haimi et al. [163] fabricated PLA/β-TCP scaffolds using freeze-drying method. Cell culture evaluation indicated that ADSCs culture on PLA/β-TCP composite scaffolds produced higher DNA content and ALP activity relative to pure PLA scaffold. Later, the freeze-dried scaffolds of PLA/β-TCP containing different filler ratios (5, 10 and 20 wt%) were prepared to investigate the effect of different filler ratios on the properties of the polymer. The study displayed that the amount of β-TCP did not influence the pore distribution or pore size in the scaffolds. In addition, after 26 weeks of hydrolysis, all the samples lost only 5% of their weight. In order to enhance cell attachment on the porous surface, β-TCP was allowed to disperse only at the bottom of the scaffolds to produce a porous bottom surface. Therefore, the porous bottom of scaffolds and the enhanced osteogenic differentiation potential obtained with the incorporation of β-TCP into PLA may encourage the growth of bone cells [163], [164]. Cao et al. [165] reported that freeze-dried composite scaffolds containing 30 wt% and 50 wt% β-TCP exhibited enhanced ingrowth of new bone, and possessed similar biological performance in terms of osteogenesis. However, the scaffold with 50 wt% filler was shown to be very brittle which cannot be used in bone repair.
Supercritical gas foamed composite scaffolds have suitable architecture and properties for bone tissue regeneration applications. Using this method, porous scaffold based on PLA containing 5 wt% β-TCP was obtained. The porosity and elastic modulus of the composite were ~ 83% and ~ 121 MPa, respectively, while those of neat PLA scaffold were ~ 83% and ~ 50 MPa, respectively. Cell culture evaluation with human fetal and adult osteoblasts indicated that both foams supported adhesion, intense proliferation and differentiation of cells in vitro. The incorporation of β-TCP led to a stronger production of Gla-osteocalcin for adult bone cells and a higher ALP activity for fetal bone cells. The in vivobehavior of PLA/β-TCP foams in combination with human fetal cells in rat model demonstrated that the degradation rate was coupled to the rate of tissue regeneration which resulted in a structural integrity of the constructs [62], [166].
In recent years, efforts to fabricate scaffold structures using conventional methods such as solvent casting and particulate leaching, freeze drying, and gas foaming have been found to be sub-optimal for tissue regeneration. Therefore, rapid prototyping techniques have been developed with the aid of computer-aided design/manufacturing to manufacture scaffolds that satisfy specific requirements for tissue engineering applications [167]. A new direct rapid prototyping process called low temperature deposition manufacturing (LDM) was proposed by Xiong et al. [168] to fabricate PLLA/TCP composite scaffolds with ~ 90% porosity. The computer-aided manufacturing process was based on the layer-by-layer manufacturing principle of SFF. The mechanical properties of the PLLA/TCP scaffolds were close to those of human spongy bone and much lower than those of human compact bone. The histological assessments showed that the novel scaffolds had good biocompatibility and bone conductivity and they were entirely degraded 24 weeks after implantation without any inflammatory reactions. Serra et al. [169] used a nozzle-based rapid prototyping system to combine PLA and CaP glass to prepare 3D biodegradable scaffolds with two patterns (orthogonal and displaced double layer) (Fig. 10). Morphological studies confirmed the interconnected porosity, uniform distribution of CaP glass particles and a controlled and repetitive architecture of the scaffolds. Mechanical tests showed that the compressive modulus of PLA scaffolds was 92 MPa for orthogonal design and 28 MPa for the displaced double layer one, whereas those of PLA/CaP glass were 99 MPa and 44 MPa, respectively, indicating that the compressive modulus highly depends on the scaffold geometry and the presence of glass particles. According to biological evaluation, all of the scaffolds showed positive biological response; however, only the scaffold with CaP glass particles displayed well-spread cells. Therefore, the success of 3D scaffolds is dependent on the combination of appropriate materials with right design and production technique, which leads to obtaining tailored 3D structure adapted to specific needs [170]. Using the rapid prototyping method, Almeida et al. [171] fabricated PLA and chitosan-based orthogonal scaffolds consisted of CaP glass and analyzed the secretion of both pro- and anti-inflammatory cytokines by monocytes/macrophages in contact with scaffolds. The result showed that PLA-based scaffolds induced higher production of IL-6, IL-12/23 and IL-10 and lower production of TNF-α in comparison with chitosan composite scaffolds.
 Fig. 10. SEM image of PLA/CaP glass: (a) orthogonal pattern, top view; (b) orthogonal pattern, cross-section view; (c) displaced double layer pattern, top view; (d) displaced double layer pattern, cross-section view. (From Ref. [169].)
Fig. 10. SEM image of PLA/CaP glass: (a) orthogonal pattern, top view; (b) orthogonal pattern, cross-section view; (c) displaced double layer pattern, top view; (d) displaced double layer pattern, cross-section view. (From Ref. [169].)