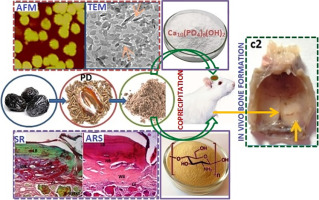
1. Introduction
Bone repair represents a major research focus of bone defects emanating from malformation, injuries, tumor surgery and osteoporosis necessitating clinical intervention to restore the level of tissue function [1]. Past decade has witnessed a staggering array of biomaterials proposed as “ideal” scaffolds for bone regeneration nevertheless few have reached clinical efficacy [2]. High cost, limited availability, immune compatibility, and disease transmission etc., are few downfalls to list that have been considered as the major roadblocks in the commercialization of available bone grafts [3]. Bone tissue engineering is an emerging area aimed to meet these requirements with laboratory-grown constructs supplemented with the allogenic and autogenic grafts [4] that can be surgically implanted to mitigate the existing shortcomings. Notably, there is rising demand for materials with characteristics of renewability, non-toxicity and biodegradability. In line with these requirements, biocompatibility is also an important prerequisite for bone constructs and thus we focused our research on the development of natural biomaterials incorporated nanocomposite [[5], [6], [7], [8]]. Chitosan (CS) is one such natural origin candidate considered for bone tissue engineering [9] due to its resemblance with the structure of the glycosaminoglycans of natural bone along with its comparable biocompatibility, antibacterial and non-antigenic properties [10]. Being a polysaccharidebiopolymer, chitosan consists of reactive amine and hydroxyl groups that are responsible for enhanced osteoblastic growth and in-vivo bone formation and thus find ample use in tissue engineering and drug delivery industry [11]. Over the years, research has focused on conjugates of biopolymers and inorganic nanofillers to accelerate osteogenesis with improved mechanical propertieshaving suitable surface roughness [12] by introducing nanotopography that can mimic the bone nanostructure [13,14]. These inorganic nanofillers contain elements similar to natural bone mineral such as hydroxyapatite that is advocated as a precursor of bone apatite crystals [15]. Thus, in view of suitable osteoconductivity, biocompatibility, non-toxic and bioactive features, inclusion of nano-hydroxyapatite (n-HA) into polymer matrix has ignited interest for bone tissue engineering [16,17].
Phoenix dactylifera, commonly known as the date palm or dates, is a monocotyledonous woody perennial fruit species of Arecaceae family grown in the arid regions of the Arabian Peninsula, North Africa, and the Middle East [18]. It consist of 70% carbohydrates most of which is in the form of sugars. Although date fruit is the primary product but its seed also documented to be effective in treating agues [18]. Furthermore, methanolic extract of date seeds possess anti-inflammatory activity in the rat adjuvant arthritis model [19]. The chemical composition of date seeds (% on an oven-dry weight basis) comprises of: ash content (1.1 ± 0.1); lignin (23 ± 3.1); holocellulose (75 ± 1.5); α-cellulose (20 ± 1.8) and hemicelluloses (55 ± 1.5) [20]. Beside the aforementioned constituents, date seeds are rich source of significant minerals such as potassium, phosphorus, magnesium, calcium etc. [21,22], which are also the key constituents of bones and teeth. Date seeds are also found to contain higher quantity of protein and fat and are excellent dietary fibre source in comparison to pulp [23]. Another distinguishing characteristic of PD seeds is that they are natural source of many active compounds such as polyphenols, flavonoids, Vitamin C and Vitamin E isomers (tocopherols and tocotrenoids), all act as antioxidants that help in bone remodeling [24]. Bone is a dynamic tissue that continuously undergoes renewal and remodeling by the coordinated action of osteoblasts, osteocytes, osteoclasts and multiple molecular agents. High levels of reactive oxygen species (ROS) hamper the osteoblasts activity and differentiation, thereby the mineralization and osteogenesis while the antioxidants promote differentiation of osteoblasts and mineralization [25]. As compared to natural antioxidants sources, the synthetic antioxidants exhibit more toxicity, are less cost-efficient, and also have lower potency and that is why the use of the former has been encouraged and favored.
In general, date seeds are ground and added to the feed of domesticated animals and mostly devoid of any harmful effect. Although date palm is known for its extensive nutritional value, Phoenix dactylifera seeds (PD) or date seeds are still less explored components. Among the leading varieties of dates, Ajwa date cultivated in the Al Madinah region of western Saudi Arabia, is one of the most popular and expensive dates, and ascribed for its abundant medicinal value. Referenced in “Hadith” and Islamic historical literature, Ajwa dates are believed to cure many chronic ailments. With the vision of an analogy of the date pulp (pericarp) surrounding the seed similar to the flesh surrounding the bone, this study was aimed at developing novel biomaterial conjugate employing date seeds thus broadening its application in the field of bone tissue engineering. Hitherto, no such reports have been performed with date seeds and hence we thought it worthwhile to explore the capability of Phoenix dactylifera seeds incorporated CHA nanocomposite scaffold for bone tissue engineering applications. Thus in the present study, we combined Ajwa date seeds with CS and n-HA to functionalize synergistically a ternary nanocomposite scaffold (PD-CHA) which is investigated for its potency to be used as an efficient bone construct. The resulting nanoconjugate was expected to harness desirable properties of each individual component yielding a superior nanocomposite scaffold for bone regeneration. The proposed PD-CHA nanocomposite scaffold has been screened for different physicochemical properties along with various in-vitro investigations. Furthermore, we tested the capacity of PD-CHA nanocomposite scaffold in the repair of critical-size defect in rat calvarium to prove its suitability for bone regeneration application. To evaluate the newly formed bone, reports of radiographical and histological examinations are presented.
2. Materials and methods
2.1. Materials
Ajwa dates at tamr stage or full ripe stage were harvested from local markets of Saudi Arabia. Commercially available bone substitute Cerabone (Botiss Biomaterials, Germany) was used in this work. [Ca(NO3)2·4H2O] (99%), (NH4)2HPO4 (DAHP) (99%), Na2SO4, NaOH (>97%), CH3COOH (99.8%), DMSO, CaCl2, KCl, MgCl2·6H2O, K2HPO4·3H2O, NaHCO3, NaCl, tri-(hydroxylmethyl) aminomethane [TRIS], sodium dodecyl sulfate, p-nitrophenyl phosphate, diethanolamine, EDTA, glutaraldehyde, ethanol, HCl, and ammonia solution (25%) have been obtained from Merck, Mumbai, India. (MTT) 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide), chitosan (CS) (DD > 85%), phosphate buffer saline (PBS) and Dulbecco's modified Eagle's medium (DMEM) were purchased from Sigma-Aldrich (USA) and Invitrogen, USA, respectively. They were all of analytical reagent grade and could be used directly without further purification. All aqueous solutions were prepared with deionized water (DI).
2.2. Experimental
2.2.1. Preparation of n-HA
HA nanoparticles (n-HA) were synthesized by wet chemical method as reported previously [6,7].
2.2.2. Preparation of seed material (PD)
Ajwa date fruits were harvested at the fully ripened form called as “tamr stage” and its seeds were soaked in water followed by repeated washing to remove any adhering date flesh. The seeds were then air-dried followed by oven drying for about 12 h at 50 °C and milled in a heavy-duty grinder to form its powder [20].
2.2.3. Preparation of PD-CHA nanocomposite scaffold
The PD-CHA nanocomposite scaffold was synthesized via co-precipitation method by mixing aqueous solutions of the three components. CS powder (1 g) was dissolved in 100 mL DI water (in 2 wt% aqueous acetic acid) with continuous stirring for 24 h to attain a homogeneous suspension (solution I). The solution II is made by dispersing the as synthesized HA nanoparticles (1 g) in 100 mL DI water after sonication for 30 min. The solution of PD seeds was made with 100 mL DI water after sonication for 55 min (solution III). Then, the reaction is continued by drop-wise mixing the solutions I and II until the contents were thoroughly mixed followed by gradual mixing of the solution III. The reaction mixture thus obtained was kept under constant stirring at 1200 rpm at room temperature. To facilitate the nucleation of n-HA which is expected at high pH, 0.5 M NaOH solution was used to maintain the pH of the resulting mixture in the range of 10–11 followed by stirring at 1200 rpm for 24 h. The slurry was aged for the next 24 h at room temperature and on completion of the reaction; the precipitate was separated out after thorough washing with DI water until the filtrate became neutral. Finally, the prepared precipitate was dried in vacuum at 65 °C. The CHA (chitosan-nano-hydroxyapatite) binary nanocomposite scaffold was prepared adopting the same procedure in 1:1 ratio, to compare its various properties with those of the proposed ternary nanocomposite scaffold.
2.3. Characterizations
The CHA and PD-CHA nanocomposite scaffolds underwent various physicochemical and in-vitro characterizations which have been discussed in Supplementary material.
2.4. Statistical analysis
Quantitative results are presented as the mean ± standard deviation. Statistical significance was evaluated using Student's t-test. A value of p < 0.05 was considered statistically meaningful [26].
3. In-vivo study
3.1. Ethics statement
In compliance with the guidelines for the ethical treatment of animals [27] all the experiments protocol was approved by the Institutional Animal Ethics Committee (IAEC), Central Animal House, J.N. Medical College, Aligarh Muslim University. Surgeries were performed under general anesthesia and utmost care was taken to reduce the animal suffering.
3.2. Cerabone: commercial formulation
Cerabone (Botiss biomaterials, Zossen, Germany) is a commercially available natural bovine bone grafting material [28].
3.3. Nanocomposite scaffold implantation: animal surgery
For the animal experiments, eighteen male adult Albino rats weighing between 250 and 300 g were employed. The body weights were closely monitored to confirm feeding and expected growth rates. The surgeries were attempted as described previously under semi-sterile conditions with animals under ether general anesthesia [27,29]. Briefly, a critical cranial defect of 8.0 mm diameter was made at the calvarium of each rat using flat fissure stainless steel dental bur (no.712) under controlled speed. The operation had to be proceeded carefully to avoid damage to the dura mater and brain with copious saline irrigation for lowering the temperature. The gamma radiation sterilized samples were then implanted into the defects, and the incision was stitched. The animals were monitored following standard post-operative animal care protocols by giving routine antibiotic and analgesic. Animals were assigned into three groups (n = 6 per group) in which the defect was; 1) left blank as sham control group; 2) filled with Cerabone (CB); 3) filled with PD-CHA nanocomposite scaffold. At 4 and 8 weeks post-surgery, animals were euthanized by overdose of general anesthesia.
3.4. Radiovisiography [RVG] analysis
The specimens were subjected to radiovisiography before decalcification for all groups as a supplement to the histological analysis using radiographic sensor (Suni Plus-0543, USA). Since radiopacity or X-ray attenuation is directly linked with the increase in bone thickness or bone density, the formation of new bone or gain in bone density (GBD) has been quantified using radiovisiographs [30,31] shown in Fig. 6 in the manuscript. The gain in bone density (GBD) has been quantified through Image Analysis toolbox of the commercial software package (MATLAB 6.1, The MathWorks Inc., Natick, MA, 2000). This parameter refers to the percentage of bone growth in terms of ratio of gray values of the defect region and the original bone region within a particular image. It is understandable that the pixel values are dependent on several scanning parameters. However, the comparison of gray values within a given image has the potential to furnish significantly accurate degree of bone growth. GBD has been computed using Eq. (1).(1)where,
-
DAGV represents the average gray value of defect region.
-
BAGV represents the average gray value of natural bone region.
A profile of gray values has also been drawn to facilitate the visual comparison between bone and defect regions within images taken after 8 weeks of Control, Cerabone, and PD-CHA in Fig. 7. These profiles exhibit the variation of gray values along a line passing through natural bone and defect region simultaneously. The Average Gray Value Line (AGVL) shows the average gray values of natural bone while defect region shows both the range of defect region along the profile line as well as the average gray values of defect region.
3.5. Tissue preparation for histological assessment
For histological examination, the defect and surrounding tissues were fixed as described in our previous report [27] and embedded in paraffin. Coronal 5-μm thickness, paraffin sections were cut and stained with Hematoxylin-Eosin (HE), Sirius Red (SR), Alizarin (ARS), Alcian Blue and Nuclear Fast Red (ACB) using standard methods and observed under trinocular light microscope (Olympus, BX4 Japan). The findings were recorded in sample photomicrographs captured under different magnifications.
4. Results and discussion
4.1. TEM and AFM
TEM was used to observe the comparative morphological analysis of the PD-CHA and CHA nanocomposite scaffolds (Fig. 1). Needle-like particles having average particle size < 20 nm are homogeneously distributed contrary to agglomerated distribution of particles in case of CHA with average particle size of ~25–34 nm. This is very likely due to the interactions between cellulosic component present in PD with CS and n-HA that resulted in the consistent dispersion and appears to control the size of particles [32,33].
 Fig. 1. Representative TEM Micrographs of (i) CHA (ii) PD-CHA nanocomposite scaffolds and topographies of AFM images of (a) CHA (b) PD-CHA nanocomposite scaffolds and their (c) RMS and Z range.
Fig. 1. Representative TEM Micrographs of (i) CHA (ii) PD-CHA nanocomposite scaffolds and topographies of AFM images of (a) CHA (b) PD-CHA nanocomposite scaffolds and their (c) RMS and Z range.AFM was used to evaluate the surface roughness of the CHA and PD-CHA nanocomposite scaffolds (Fig. 1) that revealed that the surface of PD-CHA nanocomposite scaffold was rougher than the surface of CHA nanocomposite scaffold with RMS value 14.83 ± 3.2 nm and 3.95 ± 0.3 nm, respectively. The comparative Rz analysis of PD-CHA and CHA nanocomposite scaffolds displayed significant rise in the Rz values [86.95 ± 3.60 nm and 73.71 ± 5.38 nm, respectively] [34]. Hence AFM results clearly verified the effect of PD incorporation into chitosan and hydroxyapatite matrix that significantly imparted a rough surface thereby promoting key factors such as protein adsorption, alkaline phosphatase activity, osseointegration etc.
4.2. SEM, EDS and in-vitro biomineralization assay
SEM, EDS and biomineralization assay have been discussed in Supplementary material (Section S.2.1).
4.3. FTIR
Fig. 2a displays comparative FTIR spectra of CS, CHA, PD-CHA and PD-CHA(SBF). The FTIR spectra of binary CHA and ternary PD-CHA and PD-CHA(SBF) exhibited the bands characteristic of chitosan and nano-hydroxyapatite [13]. The FTIR spectrum of PD-CHA showed the presence of HA displaying the phosphate bending modes of vibration in the range of 520–610 cm−1 [35]. The bending vibrations of N H (amide II band) of CS get overlap with the stretching vibration of C
H (amide II band) of CS get overlap with the stretching vibration of C O in hemicellulose present in date seed discerning a broad peak in the region of 1670 cm−1–1700 cm−1 [36]. However, the band in the region of 1035 cm−1–1061 cm−1 is ascribed to the overlapping between stretching mode of vibration of phosphate group in n-HA with the C
O in hemicellulose present in date seed discerning a broad peak in the region of 1670 cm−1–1700 cm−1 [36]. However, the band in the region of 1035 cm−1–1061 cm−1 is ascribed to the overlapping between stretching mode of vibration of phosphate group in n-HA with the C O
O C stretching vibration of CS and the C
C stretching vibration of CS and the C O stretching vibration of cellulose and hemicellulose present in PD seeds [27,37]. The OH stretching bands of CS and n-HA get shifted slightly and appeared in the region of3367–3450 cm−1 due to overlapping with the O
O stretching vibration of cellulose and hemicellulose present in PD seeds [27,37]. The OH stretching bands of CS and n-HA get shifted slightly and appeared in the region of3367–3450 cm−1 due to overlapping with the O H stretching vibrations of PD signifying the possibility of hydrogen bondingamong the three components present in PD-CHA nanocomposite scaffold [32,33]. The C
H stretching vibrations of PD signifying the possibility of hydrogen bondingamong the three components present in PD-CHA nanocomposite scaffold [32,33]. The C H stretching band of CS get overlapped with the asymmetric C
H stretching band of CS get overlapped with the asymmetric C H bands in methyl and methylene groups present in PD and appears as a broad band in the range of 2872–2927 cm−1 [38]. The FTIR spectra of PD-CHA after incubation in SBF for 8 weeks showed bands assignable to hydroxyl group along with a new weak intensity bands at 874 cm−1 (v2), 1416 and 1485 cm−1(v3) characteristic of CO32− indicative of the formation of some amount of CO32−moiety in n-HA in presence of PD [39]. The presence of carbonated apatite is advantageous for bone mineral (expected to be 4–8 wt% in the human body), as it enhances the mechanical consistency and bioactivity of the apatite leading to more osteoconduction and tissue in-growth expected on implantation [40,41].
H bands in methyl and methylene groups present in PD and appears as a broad band in the range of 2872–2927 cm−1 [38]. The FTIR spectra of PD-CHA after incubation in SBF for 8 weeks showed bands assignable to hydroxyl group along with a new weak intensity bands at 874 cm−1 (v2), 1416 and 1485 cm−1(v3) characteristic of CO32− indicative of the formation of some amount of CO32−moiety in n-HA in presence of PD [39]. The presence of carbonated apatite is advantageous for bone mineral (expected to be 4–8 wt% in the human body), as it enhances the mechanical consistency and bioactivity of the apatite leading to more osteoconduction and tissue in-growth expected on implantation [40,41].
 Fig. 2. (a) FTIR Spectra of CS, CHA, PD-CHA and PD-CHA(SBF) scaffolds (b) XRDdiffractograms of human bone, CHA, PD-CHA, PD-CHA(SBF) and CS scaffolds. Mechanical strength analysis: (c) Compressive modulus (d) compressive strength (e) compressive stress-strain curves of CS, CHA and PD-CHA scaffolds. *p < 0.05 showing statistical difference compared to CHA nanocomposite scaffold.
Fig. 2. (a) FTIR Spectra of CS, CHA, PD-CHA and PD-CHA(SBF) scaffolds (b) XRDdiffractograms of human bone, CHA, PD-CHA, PD-CHA(SBF) and CS scaffolds. Mechanical strength analysis: (c) Compressive modulus (d) compressive strength (e) compressive stress-strain curves of CS, CHA and PD-CHA scaffolds. *p < 0.05 showing statistical difference compared to CHA nanocomposite scaffold.4.4. XRD
The XRD patterns of original human bone, CHA, PD-CHA, PD-CHA(SBF) and CS are shown in Fig. 2b. The CS spectrum (inset Fig. 2b) exhibits the characteristic XRD diffraction peaks at (2Ɵ) 10.5°, 19.8° and 22.2° which have been suppressed in case of CHA and PD-CHA. In case of CHA, not all hydroxyapatite peaks were clearly identified, however, in case of PD-CHA, the characteristic peaks of n-HA crystallites [JCPDS 09-0432 standard] [42] were present which matches well with the XRD peaks of apatite present in the original human bone displayed in Fig. 2b. This warrants that the interfacial binding between n-HA particles and PD-CS matrix may possibly responsible for the n-HA crystallization [43,44]. A new peak obtained at (2Ɵ) 20.2° in case of PD-CHA may be attributed to native cellulose and hemicellulose present in PD [38]. The XRD patterns of PD-CHA post 8 weeks incubation in SBF (Fig. 2b) indicated the obvious presence of hydroxyapatite peaks due to formation of apatite upon nucleation with the appearance of new peak at (2Ɵ) 27° that marked the presence of carbonated apatite [41] with the slight broadening of peaks indicating poor crystallinity [15,45].
4.5. Mechanical strength
The compressive strength of the CHA and PD-CHA nanocomposite scaffolds were tested using universal testing machine. The cylindrical pellets of the samples have been prepared in agreement with the guidelines [ASTM F 451-95] for compression mechanical test [46]. The results of average compressive modulus (Fig. 2c) revealed that PD-CHA exhibited significantly higher compressive modulus [1312 ± 3.44 MPa] comparable to the values observed for human compact bone relative to the CS and CHA. Similarly, compressive strengths increases in the order CS < CHA < PD-CHA with considerably higher value of 91.98 ± 1.64 MPa for PD-CHA as depicted from Fig. 2d, suggesting that addition of PD in CHA matrix has considerably improved the modulus and strength at par with that of cortical bone [47,48]. The compressive stress-strain curve of the CS, CHA and PD-CHA were shown in Fig. 2e, where CHA and PD-CHA followed a linear behavior during the initial stage of compression with the stress increased sharply and then subsequently reduced until final fracture point was reached. The increase in mechanical strength parameters of PD-CHA may be explained possibly in terms of the enhanced intermolecular hydrogen bonding between n-HA, cellulose present in PD seeds and CS moieties thereby imparting rigidity to the resulting system [32,48].
4.6. Ex-vivo cell viability
The human osteoblast like MG-63 cells were cultured on PD-CHA, CHA and CS scaffolds with various concentrations (0–128 μg/mL) for 24 h revealed substantially enhanced metabolic activity of the cells in case of PD-CHA compared to CHA and CS (Fig. 3a). Notably, over 70% cells viability has been observed in case of PD-CHA at all investigated concentrations compared to CHA and CS samples in view of the cellulosic components and antioxidants present in the PD which is found to play an inductive role for cell attachment and proliferation [49].
 Fig. 3. Biocompatibility studies: (a) Cell viability assay (b) protein adsorption assay (c) alkaline phosphatase activity, platelet adhesion study of PD-CHA (d) CHA (e). *p < 0.05 showing statistical difference compared to CHA nanocomposite scaffold (scale bar = 5 μm).
Fig. 3. Biocompatibility studies: (a) Cell viability assay (b) protein adsorption assay (c) alkaline phosphatase activity, platelet adhesion study of PD-CHA (d) CHA (e). *p < 0.05 showing statistical difference compared to CHA nanocomposite scaffold (scale bar = 5 μm).4.7. Protein adsorption assay
Fig. 3b displayed the amount of protein adsorbed on the surface of different scaffolds viz., CS, CHA, PD-CHA and tissue culture plate (TCP) after the incubation of samples with FBS. It was observed that the PD-CHA nanocomposite scaffold showed significantly higher protein adsorption with average values of 108 ± 8.49 μg/cm2 compared to CHA and CS that may be understood by virtue of enhanced RMS roughness with higher surface to volume ratio imparted by PD that provide a conduit for more protein binding sites [48].
4.8. ALP activity
Fig. 3c illustrates the in-vitro ALP activity of osteoblasts cultivated with PD-CHA and CHA nanocomposite scaffolds got increased with time. No significant difference in ALP activity was noticed up to day 4, however, a higher ALP activity was observed for PD-CHA nanocomposite scaffold after culturing for 8 days compared to CHA on account of its relatively more rougher surface denoting first check-point for osteogenic differentiation. ALP activity was observed to get reduced on the commencement of day12 possibly indicating that the cells tend to show an accelerated differentiation and an early onset of maturation phase [50]. Therefore, PD-CHA nanocomposite scaffold is expected to provide an effective biocompatible substrate stimulating cellular differentiation.
4.9. Platelet adhesion study
Fig. 3d–e shows SEM images, after in-vitro platelet adhesion tests for PD-CHA and CHA nanocomposite scaffolds. In case of PD-CHA, PD appeared to restrict platelet activation with relatively less platelets adhered on its surface which appeared to be nearly round. However numerous platelets aggregates were observed in case of CHA indicating the platelet adhesion and activation nature of chitosan [51].
5. Ex-vivo radiological and histological analysis
5.1. Clinical results
All animals showed wound healing within 7 days with no sign of infection during the whole post-implantation follow-up periods.
5.2. Radiographic and histological assessment
The in-vitro studies were augmented by in-vivo studies using the albino rat model to investigate the suitability of PD-CHA nanocomposite as a biomaterial in-vivo [52,53]. Calvaria samples were collected post-mortem and fixed in formalin for 7 days and subjected to radiovisiographic (RVG) evaluations. Subsequently calvaria were decalcified in EDTA for 4 weeks [54], dehydrated through graded series of ethanol and embedded in paraffin [55]. Paraffin sections of 7 μm thickness were stained with Hematoxylin and Eosin (H & E, Sigma), Sirius Red [56,57] (SR, Sigma), Alizarin (ARS, Sigma) and Alcian Blue-Nuclear Fast Red (ACB, Sigma) separately [58]. The surgical procedure has been shown in Fig. 4P. The findings have been discussed as the following:
-
(a)
Sham control group: The histological examination of H&E, SR and ARS stained specimen from blank control group revealed complete lack of healing in the defects in all the albino rats for over a time period of 4 weeks post-surgery (Fig. 4Q, panel A). In fact, the blank group showed no signs of ossification even 8 weeks post-implantation [59] and was observed to be mainly filled with fibrotic connective tissues with just increased vascularization as visible in Fig. 4Q (panel B). These results are consistent with the RVG findings shown in Fig. 6a–a1, that revealed only 18.20% and 35.8% gain in bone density (GBD) after 4 weeks and 8 weeks, respectively, consistent with the previous reports [60,61]. This one also gets additional support from its gray value profile after 8 weeks which quantifies bone mineral density as shown in Fig. 7. The gray value at the parietal bone (host bone) region was found to be significantly higher than at defect site indicating nearly no bone formation in the sham control group.
-
(b)
Cerabone group: In case of commercial Cerabone material (CB group), there was also no bone formation after 4 weeks. The defect region was occupied with irregular collagen fibres consisting of multiple aggregates of Cerabone material as visible from its radiograph (Fig. 6b) each surrounded with connective tissue suggestive of only fractional assimilation (Fig. 4 panel C (i–iii)). However, there is no obvious presence of new bone as inferred from the histological findings. The GBD was found to be only 40.5% [Supplementary material (Fig. S2)], that is most likely due to non-dispersed CB itself and not because of the actual bone formation. Absence of calcification is also confirmed by ARS staining. 8 weeks post implanted CB group showed the presence of new bone in addition to loose connective tissue (Fig. 4 panel D (i–iii)) which is in conformity with the GBD being 86.7%. The gray value profile after 8 weeks for CB group (Fig. 7) indicated that bone density is increased in the defected region confirming the new bone formation which supports the histological findings.
-
(c)
PD-CHA group: An interesting disparity was observed in case of PD-CHA nanocomposite scaffold that revealed progressive repair response as early as 4 weeks post-implantation and a more pronounced bone healing was observed in comparison to both the control and CB groups (Fig. 5a–d) which is also in agreement with the RVG analysis (Fig. 6c). The histological feature was remarkable distinguishing the regions of neogenerated bone, woven bone, fibrous tissue and host bone could be separately. Thus PD-CHA nanocomposite appears to have very strong osteoregenerative capacity even in absence of prior cell seeding or co-administration of any growth factors. These findings are also in full agreement with GBD of 68.6% [Supplementary material (Fig. S2)].
 Fig. 5. Histology of bone at 4 (a–d) and 8 weeks (e–h) post-surgery of PD-CHA group. Stain H&E, SR, ARS and ACB. Initial magnification ×200 [LB: lamellar bone, NB: new bone, WB: woven bone, CF: collagen fibre, OB: osteoblasts]. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.)
Fig. 5. Histology of bone at 4 (a–d) and 8 weeks (e–h) post-surgery of PD-CHA group. Stain H&E, SR, ARS and ACB. Initial magnification ×200 [LB: lamellar bone, NB: new bone, WB: woven bone, CF: collagen fibre, OB: osteoblasts]. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.) Fig. 6. Radiovisiographs 4 weeks: (a) sham control, (b) Cerabone, (c) PD-CHA group, and 8 weeks post-surgery: (a1) sham control, (b1) Cerabone, (c1) PD-CHA respectively. Harvested calvaria viewed from inside: (a2–a2′) sham control, (b2–b2′) Cerabone, (c2–c2′) PD-CHA after 8 weeks post-surgery with arrows indicating defect site.
Fig. 6. Radiovisiographs 4 weeks: (a) sham control, (b) Cerabone, (c) PD-CHA group, and 8 weeks post-surgery: (a1) sham control, (b1) Cerabone, (c1) PD-CHA respectively. Harvested calvaria viewed from inside: (a2–a2′) sham control, (b2–b2′) Cerabone, (c2–c2′) PD-CHA after 8 weeks post-surgery with arrows indicating defect site.
 Fig. 4. (P) Steps of surgical procedures: (a) Exposed rat calvarium (b) selected area on parietal bone (c) round critical size defect (8 mm). Defect filled with (d) PD-CHA nanocomposite scaffold (e) skin stitched with silk. (Q) Histology of bone: Representative photomicrographs of the sections of sham control group at 4 weeks [panel A (i–iii)] and 8 weeks panel B (i–iii); Cerabone group at 4 weeks [panel C (i–iii)] and 8 weeks [panel D (i–iii)] post-implantation. Stains H&E, SR and ARS; initial magnification ×200 [OB: osteoblasts, CF: collagen fibre, CB: Cerabone, MB: mature bone, FT: fibrous tissue].
Fig. 4. (P) Steps of surgical procedures: (a) Exposed rat calvarium (b) selected area on parietal bone (c) round critical size defect (8 mm). Defect filled with (d) PD-CHA nanocomposite scaffold (e) skin stitched with silk. (Q) Histology of bone: Representative photomicrographs of the sections of sham control group at 4 weeks [panel A (i–iii)] and 8 weeks panel B (i–iii); Cerabone group at 4 weeks [panel C (i–iii)] and 8 weeks [panel D (i–iii)] post-implantation. Stains H&E, SR and ARS; initial magnification ×200 [OB: osteoblasts, CF: collagen fibre, CB: Cerabone, MB: mature bone, FT: fibrous tissue].