1. Introduction
Along with air, without which man cannot survive for more than a few minutes, water is one of our most essential natural resources (Aulenbach, 1968). Since the available amount of water is limited and not spatially distributed, proper management of this precious resource is essential to maintain its sustainability.
Globally, the volume of wastewater generated and contaminant loads are increasing due to population increment, economic development, and expansion of urbanization. Moreover, according to the United Nations World Water Development Report (WWAP (United Nations World Water Assessment Programme), 2017), more than 80% of the world's wastewater is discharged into the natural environment without treatment. Likewise, in developing countries, a large volume of wastewater generated is directly connected to the nearest drainage line with very little or without treatment (WWAP (United Nations World Water Assessment Programme), 2017).
Thus, to achieve the sustainable development goal of 2030, any drop of water generated from any source has to be carefully managed during every part of the water cycle. Also, to minimize the negative impacts on the aquatic environment, every wastewater generated from each source has to be efficiently treated before being released into receiving bodies.
To remove the pollutants from the wastewater, there are many treatment techniques such as biological, Physicochemical, and advanced oxidation processes. Physicochemical treatments have good removal efficiency. However, their use is limited because of the large volume of chemicals required during the process and the generation of a large volume of sludge at the end of the treatment (Kurniawan et al., 2006). Again, biological systems are not always suitable due to their requirement of large physical space, some microorganisms are sensitive to some chemical complexes, and long treatment processes (Núñez et al., 2019). Moreover, biological methods are difficult to employ for textile effluent, as the textile effluent is recalcitrant to biodegradation. The advanced oxidation process is an alternative method to meet their requirements for chemical and biological methods (Wang and Xu, 2012).
Recently, due to restricting environmental regulations and laws, researchers have shown that electrochemical treatment systems are promising technologies to prevent or reduce pollution problems. Aitbara et al. (2016) conducted a laboratory-scale continuous electrocoagulation treatment of dairy wastewater collected from factories in Algeria using Al electrodes. As a result, 90% of COD and 98% of Turbidity were able to eliminate at the optimal conditions (current density 15 mA/cm2, electrodes gap 1 cm, supporting electrolyte KCl 2 × 10−2 M, temperature 20 °C, and pH of 7.03) with practical energy consumption between 2 and 3.5 kWh/m3. On the other hand, Amour et al. (2016) investigated the performance of continuous electrochemical reactors and the effect of operating parameters (initial dye concentration, initial pH, current density, inlet flow rate, and residence time) on the removal of color and turbidity. Accordingly, 97% of color and 90% of turbidity were obtained at optimal operating parameters of initial concentration less than 300 mg/L, initial pH between 2.3 and 8.8, current density 300 A/m2, inlet flow rate 15 L/h, and residence time of 35 min. At the same time, the electrical energy consumption per kilogram of dye removed was 19.5 kWh. Furthermore, Demir Delil and Gören (2019a) also studied to investigate the performance of combined electrocoagulation and electrooxidation electrochemical methods for real textile wastewater. Besides, the effect of operating conditions (different electrode arrangements, electrode types, initial pH, applied voltage, and electrolysis time) of the process was studied on the removal efficiency of COD and dye color. Finally, at the end of the electrocoagulation experiment the color and COD was able to reduce from 395 Pt–Co to 28 Pt–Co and 1040 mg/L to 115 mg/L, respectively. In this study, the maximum removal of COD and color was achieved at 6 V of applied voltage and pH of 3 with Fe–Fe electrode combination. Similarly, the performance of EO on color and COD removal was performed and 93% of COD removal was achieved at 6 V with Pt–Fe electrode combination. According to this study, EC shows best removal efficiency of than EO. However, the energy consumption of electrooxidation is more economical than electrocoagulation on COD removal.
In the last two decades, electrochemical methods like electrodeposition (ED), electrocoagulation (EC), electroflotation (EF), electrooxidation (EO), and a hybrid of electrocoagulation-electrooxidation (EC-EO) processes, have got a great attraction as efficient technology by researchers (Can, 2014). Indeed, to improve the performance of electrocoagulation, it has been integrated with many other treatment methods such as biological, electro-Fenton, and photo-catalytic (Suárez-Escobar et al., 2016). Very recently, scholars (Al-Qodah et al., 2019) reviewed different research articles focused on the combination of electrocoagulation with various biological wastewater treatment methods as it can enhance its performance and removal efficiency. In this review article, the authors noted that compared to the separate EC process, the combined process of EC with biological treatment method increases the removal efficiency of water pollutants by 20%. Another study by Al-Qodah et al. (2018) reviews research on the performance of electrocoagulation methods assisted by free-radical, ozone, advanced oxidation, and ultrasound energy. In this review article the mechanism, kinetics, and cost of the electrocoagulation process assisted by free radical were well discussed. The authors observed that the effectiveness of the combined processes was enhanced, and better removal efficiency (>95%) was obtained with the electrocoagulation process assisted by ozone. Furthermore, the review articles indicate that the use of ultrasound energy reduces the electrode passivation problem of electrocoagulation and improves the performance of the processes.
Similarly, electrooxidation has been integrated with other treatment processes such as biological (Katsoni et al., 2014), coagulation (Abdessamad et al., 2015), and adsorption (Akrout et al., 2015). Moreover, the electrochemical method, especially electrooxidation using the boron-doped diamond (BDD) electrode has got great attention due to its capacity of removing numerous organic and inorganic pollutants (Ghazouani et al., 2017). However, the power consumption is very high and as a result, it increases the overall cost of the process. On the other hand, Anglada et al. (2010) studied the technical and economic feasibility of EO using BDD based on COD and ammonia (NH4+) concentration reduction, and energy consumption, respectively from landfill leachate at a pilot scale. The results were examined and compared with other methods of advanced oxidation processes. Under the optimum operating conditions, the concentrations of COD and NH4+ were removed up to 160 mg/L and 30 mg/L, respectively with maximum energy consumption of 54 kWh/m3. The pollutants were removed below the level of discharge limit and low formations of by-products.
In this particular review, special emphasis was given to electrocoagulation (EC), electrooxidation (EO), and the hybrid process of electrocoagulation and electrooxidation (EC-EO) system. Even though several studies have covered EC, EO, and a hybrid process of EC-EO systems separately, to our best knowledge, no review work has been done on the hybrid process of EC-EO applied for various pollutant removals from wastewater. Therefore, this paper aims to review the potential of a hybrid of the EC-EO process for the treatment of wastewater generated from various sources. The review paper tried to include the most recent publications of 2020 and describes, discussing, and compares the potential of each electrochemical method.
2. Electrocoagulation (EC) method
Electrocoagulation can be defined as a process to remove contaminants from wastewater by using electricity and to neutralize the negative particles by the formation of hydroxide complexes in water to gather the suspended solid, help bridge, bind and strengthen the floc for sedimentation due to gravity force (Fagnekar Nilesh A., 2015). This process agglomerate the suspended solid in water without chemical coagulant, and the coagulation happened when the direct current (DC) is applied to the aqueous solution. Besides, Electrocoagulation involves the generation of coagulants in situ by electrical dissolution from the respective metal electrodes (Al, Fe, Cu, or Stainless steel). The dissolved metal ion and hydrogen gas are generated at the anode and cathode, respectively (Linares-Hernández et al., 2010a).
Several factors influence the electrocoagulation process such as the type of electrode, the surface area of the anode electrode, the gap between electrodes, the number of electrodes, the size of electrodes, current density, charge loading, pH of the sample, operational time, and addition of supporting electrolyte (Can et al., 2006). The electrodes that are usually used are iron, aluminum, stainless steel, and copper. Among these, due to the high coagulation efficiency of Al3+, aluminum plates are more preferable to use for wastewater treatment in combination with iron plates (Shen et al., 2003). If the solution has a significant amount of Ca2+ or Mg2+ ions, it is recommended to use stainless steel material as a cathode (Chen, 2004). During the EC process cathode is oxidized (loss of electrons) and the water is reduced (gain electrons) resulted in treated water and easy to settle floc (Fagnekar Nilesh A., 2015).
Electrocoagulation Process is an electrolysis process for wastewater treatment using a pair of electrodes (anode and cathode). Electrodes used in the EC process can be arranged in mono-polar or bipolar (Demirci et al., 2015). In the mono-polar electrodes in parallel connection (MP-P), the sacrificial electrodes are connected to the source of power (Fig. 1). Moreover, in the bipolar electrodes in parallel connections (BP–P), the sacrificial electrodes are switched between two parallel electrodes without connection to the power source. That is, there is no interconnection between the sacrificial anodes but the two outer electrodes are connected to the power source (Demirci et al., 2015). Likewise, in the mono-polar electrodes in series connection (MP-S), each pair of sacrificial electrodes are internally connected. Besides, in the bipolar electrodes in series connection (BP–S), the outer electrodes are connected to the power source without an internal connection between the inner electrodes (Song et al., 2017).
 Fig. 1
Fig. 1Despite the majority of EC researches (Dubey and Kumar Prajapati, 2019) which is conducted using a conventional vertical electrode connection, some studies have been done using a horizontally arranged electrode connection (Fouad et al., 2009). For instance, Fouad et al. (2009) obtained 99.8% (from 500 to 6 ppm) of reduction in oil concentration from the aqueous solution within 30 min using a horizontally arranged electrode connection.
A positively charged are attracted to the negatively charged hydroxides in the solution (Fig. 2) and they produce ionic hydroxides that make a strong attraction towards dispersed particles as the counterions cause coagulation.
 Fig. 2
Fig. 2According to many reports and Al Aji et al. (2012), the following three successive stages are the main steps in the EC process:
-
i.
Formation of coagulants by electrolytic oxidation of the electrode. The main reaction occurring at the metal anode is dissolution:
Additionally, water electrolysis occurs at the cathode and anode:
-
ii.
Destabilization of the contaminants, particulate suspension, and breaking of emulsions. A direct electrochemical reduction of metal cations (Mn+) may occur at the cathode surface:
Furthermore, the hydroxide ions formed at the cathode increase the pH of the wastewater thereby inducing precipitation of metal ions as corresponding hydroxides and co-precipitation with hydroxides:
-
iii.
Aggregation of the destabilized phases to form flocs. Anodic metal ions and hydroxide ions generated at the electrode surfaces react in the bulk wastewater to form various hydroxides and built-up polymers. Furthermore, the following physicochemical reactions may also take place in the EC cell (Al Aji et al., 2012): cathodic reduction of impurities present in wastewater, discharge and coagulation of colloidal particles, electrophoretic migration of the ions in solution, electro-flotation of the coagulated particles by oxygen and hydrogen bubbles produced at the electrodes, reduction of metal ions at the cathode, other electrochemical and chemical processes.
The electrocoagulation method has attracted great attention for treating various wastewaters because of its wide application and environmental compatible (Azarian et al., 2018). Also, the EC process has many advantages such as simple equipment requirements, easy operation, a shortened retention time, no or small chemical additions, and less formation of sludge. Moreover, the application and advantages of EC are well discussed and reviewed by (Zaied et al., 2020).
In the EC process different monomeric and polymeric metal hydroxide species are formed through the oxidation of sacrificial electrodes. The metal hydroxides (coagulant) aggregate the colloidal particles in the solution which forms bigger flocs that are removed by sedimentation (Chen, 2004). In those cases, EC was not able to reduce the chemical oxygen demand (COD) level to below the allowable discharge limits. Therefore, to enhance the performance of EC, it is essential to integrate with other methods like electrooxidation, Fenton, photo-Fenton, electro-Fenton, ozonation, photocatalysis, and other degradation methods (Nidheesh et al., 2020).
Another research by Can (2014) conducted a study on COD removal from fruit juice plant wastewater. And, the objective of this research was to investigate the removal efficiency of COD from the fruit juice plant wastewater using three different electrochemical processes; namely electrocoagulation (EC), electrooxidation (EOx), and electro-Fenton Processes (EF). Accordingly, The performance of each process was evaluated by comparing it with each technology (Table 4). The sampled wastewater from fruit juice production had high COD content (20,713 mg/L). During the EC process, Al and Fe electrode materials were used to remove COD from the solution. Again, to remove COD from the wastewater by the EF process, simply H2O2 was added to the EC reactor by changing the anode and cathode electrodes with iron and titanium, respectively. As a result, 52.4 and 64.7% maximum COD removal were achieved by EOx at 60 min and 360 min, respectively. From the EC process, 59.1% and 61.3% of COD were removed using aluminum and an iron anode, respectively. Besides, 84.4% maximum removal of COD by EF was achieved by adding 10 mL of H2O2 at the end of 25 min electrolysis time. Thus, the results showed that electrocoagulation performs less to remove COD. Moreover, this shows even less performance than an air bubble column bioreactor, 92.2% COD removal (Kosseva, 2017). According to the study by Kazem et al. (2012), the highest removal of COD (95.6%) was achieved using the Al electrode at acidic pH conditions between 2 and 3. Therefore, EC is recommended as a suitable technology for poultry slaughterhouse wastewater treatment. From this result, it has been observed that electrocoagulation as a separate technology can remove COD to the maximum level from poultry slaughterhouse wastewater. Chen (2004) reviewed more than 300 research outputs focused on the design, development, and application of electrochemical technologies namely electrocoagulation, electrodeposition (ED), electroflotation (EF), and electrooxidation methods for water and wastewater treatments. Accordingly, ED was found most effective for heavy metal recovery from wastewater. Comparatively, EF is effective in removing organic pollutants, colloidal particles as well as oil and grease. Moreover, EF is effective to separate flocculated sludge from the treated water. Furthermore, Shen et al. (2003) presented the removal of fluoride concentration from synthetic solution using a combined electrocoagulation and electroflotation method. As a result, at optimum operating condition of pH 6, charge loading 4.97 F/m3, and electrolysis time of 20 min the concentrations of fluoride were reduced from 15 to 2 mg/L.
3. Electrooxidation (EO) method
The EO process is an emerging process where contaminants are removed by oxidizing directly at the surface of the electrode or indirectly by generating oxidants in the solution (Özyurt and Camcıoğlu, 2018). Moreover, direct electrooxidation occurs through the generation of physically adsorbed oxygen species (hydroxyl radicals, •OH) or chemisorbed oxygen species (MOx+1) (Comninellis, 1994). Besides, the performance of the anodic oxidation process mainly depends on the selection of an anodic electrode. The types of anodic materials and their performance are clearly described by (Chen, 2004). Moreover, indirect electrooxidation happens by generating chlorine or hypochlorite during anodic oxidation. This process is effective to remove organic and inorganic contaminants at a high concentration of chloride, the best greater than 3 gm/L (Szpyrkowicz et al., 1994).
According to Özyurt and Camcıoğlu (2018), the following equations are the main anodic oxidation of organic pollutants and generating indirect oxidizing agents during the EO process.
-
i.
Direct oxidation:
-
ii.
indirect oxidation:
Furthermore, the formation of chloride anions and hydrogen gas at the cathode both at the acidic and alkaline conditions are stated below (Sirés et al., 2014):Where, S, R is the active spot of anodic surface, and the organic pollutant, respectivelyscholars (Valero et al., 2014) showed that electrooxidation is an effective technique to destroy various persistent and recalcitrant organic pollutants using different potential electrodes (Table 1).
Table 1. Performance of different EO electrodes (Chen, 2004).
| Anode | Pollutant | Current density (A/m2) | CE (%) | Removal efficiency (%) | Comment | Ref. |
|---|---|---|---|---|---|---|
| Granular graphite | Phenol | 0.03–0.32 | 70 | 70, 50 of mineralization | 5-month stable operation | Awad and Abuzaid (1997) |
| Planar graphite | Phenol | 10–100 | 24.6–63.5 | 6-17 of COD | NaOH as electrolyte | Kanna et al. (1995) |
| Pt and Ti/Pt | Phenol | 300 | 30 of TOC | pH 12, initial concentration 1000 mg/L, in 0.25 M Na2SO4 | Polcaro et al. (1999) | |
| Ammonia | 8.5 | 53 | 95 | pH 8.2 using phosphate buffer, poor performance for organics | Marinčić and Leitz (1978) | |
| Glucose | 100–900 | 15–20 | 30 | 1 M H2SO4 | Bonfatti et al. (2000) | |
| 15 organics | 5 | Stucki et al. (1991) | ||||
| PbO2 | Aniline | I = 2 A | 15–40 | >90 (in 1 h) | initial concentration 2/7 mM, pH 2, packed bed of PbO2 | Pulgarin et al. (1994) |
| Phenol | I = 1, 2,3 A | 46–80 | initial concentration 14–56 mM, | Chen et al. (1999) | ||
| Ti/PbO2 | Phenol | 300 | 40 of TOC | pH 12, initial concentration 1000 mg/L, in 0.25 M Na2SO4 | Polcaro et al. (1999) | |
| Landfill leachate | 50–150 | 30 for COD 10% for NH4+-N | 90 for COD, 100 for NH4+-N | Grimm et al. (1998) | ||
| Glucose | 100–900 | 30–40 | 100 | 1 M H2SO4 | Bonfatti et al. (2000) | |
| 2-Chlorophenol | 80–160 | 35–40 | 80-95 of COD | Pb2+ formation, initial COD was 1000 mg/L, 25 °C | Kirk et al. (1985) | |
| IrO2 | Organic | 17 | (Guo, Li, Chen, 2010) | |||
| 1, 4-Benzoquinone | Rupture of rings only | Rodgers et al. (1999) | ||||
| Ti/SnO2–Sb2O5 | 2- hlorophenol | 80–160 | 35–40 | 80-95 of COD | Oxalic acid as intermediates | Bonfatti et al. (2000) |
| Glucose | 100–900 | <20 | 30 | 1 M H2SO4 | Polcaro et al. (1999) | |
| Phenol | 300 | 100 | pH 12, initial concentration 1000 mg/L, in 0.25 M Na2SO4 | (Guo, Li, Chen, 2010) | ||
| 500 | 58 | 70 °C, 10 mM, similar to PbO2 | Kötz et al. (1991) | |||
| Landfill leachate | similar to PbO2 | Grimm et al. (1998) |
According to Chaplin (2014), Tin dioxide (SnO2) has low conductivity, and to improve its conductivity it must be doped with antimony (Sb) which has high conductivity and potential for oxygen progression. Carbon electrodes and titanium sheets coated with active oxides like ruthenium dioxide (RuO2), iridium dioxide (IrO2), or SnO2 have been successively used to oxidize both phenol and chlorophenols (Cossu et al., 1998).
Furthermore, the major drawback of the EO method is that it performs less for treating water and wastewater with a high concentration of suspended solids. Thus, to use the EO treatment system the wastewater containing suspended solids has to be removed first by using other techniques. This can be achieved by integrating with other methods like the hybrid process of EC and EO (Chakchouk et al., 2017).
4. Hybrid of electrocoagulation and electrooxidation (EC and EO) method
Among the electrochemical treatment systems, EC and EO are the most promising techniques due to no or small chemical requirements and the easiness of operation (Yılmaz Nayır and Kara, 2018). As it has been clearly stated by Bhagawan et al. (2016), electrocoagulation is a fast but ineffective process. Relatively, electrooxidation is an effective but slow method, and a hybrid of the two processes gives a practical and promising result (Özyurt et al., 2017).
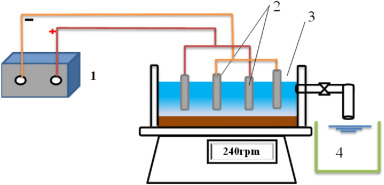
In recent years, a hybrid process of EC and EO on sequential or simultaneous arrangement has been used for the treatment of wastewater sampled from container washing (Yılmaz Nayır and Kara, 2018), soft drink industry (Linares Hernández et al., 2017), fruit juice production plant (Can, 2014), Agro-food industry (Ghazouani et al., 2019a), gelatin production plant (Lakshmi Kruthika et al., 2013), textile synthetic (Raju et al., 2008), textile real (Demir Delil and Gören, 2019b), soluble coffee (Ibarra-Taquez et al., 2017a), petroleum refinery (Bhagawan et al., 2016), landfill leachate (Ghanbari et al., 2020), canola-oil refinery (Sharma and Simsek, 2019), decolorization and removal of an indigo carmine textile dye (Stergiopoulos et al., 2014), pulp and paper mill (Özyurt et al., 2017), dairy production (Chakchouk et al., 2017), COD removal and recovery of tannery industry wastewater (Ghasem et al., 2017), mitigation of virus in drinking water (Heffron et al., 2019), ultrafiltration membrane fouling (Sun et al., 2018a), pre-treatment on the fungal treatment of pistachio processing (Isik et al., 2020), and industrial wastewater (Linares-Hernández et al., 2010b). The treatment flow diagram of the electrocoagulation-electrooxidation process applied for dairy wastewater treatment is shown in (Fig. 3).
 Fig. 3
Fig. 3Indeed, the different pollutants produced from various sources have been removed using batch and a continuous system of EC and EO hybrid processes (Heffron et al., 2019). Batch reactors are more suitable for laboratory and pilot-scale studies with less working volume of the reactor, while the continuous modes of reactors are suitable for industrial wastewater treatment studies with a large volume of flow. Besides, because it is easy to control and manage the operations, the continuous mode/or system is preferred to the batch mode/or system. During the hybrid process of EC and EO processes encompassing both batch and continuous mode, most of the time, a batch system is used to investigate the optimum operating parameters of the treatment method, and then only the effect of the initial concentration of the targeted pollutant and flow rate will be evaluated through the continuous mode of operation (Naje et al., 2017).
Even though several types of research have been done using the batch-type reactor (Yılmaz Nayır and Kara, 2018), however, the research work on the comparison of both Batch- and continuous-type reactor especially on the hybrid method of electrocoagulation and electrooxidation processes are few. This includes the performance evaluation of a continuous flow reactor and batch reactor for electrocoagulation and electrooxidation processes (Ardhan et al., 2015). Lakshmi Kruthika et al. (2013) performed a hybrid process of EC and EO systems with continuous and batch systems of a reactor, respectively. So, the maximum removal was achieved in the range of 38–54%, and 80% of total organic carbon (TOC) using continuous and batch systems of a reactor, respectively. On the other hand, Linares Hernández et al. (2017) researched soft drink wastewater treatment by a hybrid of consecutive electrocoagulation and electrooxidation processes in a batch-type reactor. Accordingly, only 27 and 85% of TOC could be removed using EC and EO, respectively at pH 8.
The physicochemical characteristics of diary effluent are mainly composed of total solids and dissolved solids resulting in a high concentration of COD and BOD5 (Chakchouk et al., 2017). Thus, to remove these pollutants, EC, EO, and hybrid of EC and EO were performed. The results showed that EC was very effective and quick (6 min) to remove colloidal and suspended particles, but ineffective to remove COD. Similarly, EO alone was able to reduce COD by about 40% at 30 min. Therefore, to increase the removal of COD, a hybrid of EC and EO was implemented which reduced the COD level by about 60% at 21 min. The removal efficiency of the three electrochemical treatment technologies applied to reduce pollutants from dairy industry effluent is summarized in Table 2.
Table 2. Physicochemical characteristics of hybrid EC and EO treatment for dairy wastewater (Chakchouk et al., 2017).
| Parameters | Raw sample | EC | EO | Hybrid process of EC-EO |
|---|---|---|---|---|
| COD (mg/L) | 3850 | 2079 | 2503 | 1390 |
| BOD5 (mg/L) | 2800 | 1100 | 1900 | 1000 |
| Suspended matter (mg/L) | 831 | 140 | 180 | 30 |
| Fatty Matter (mg/L) | 230 | 16 | 20 | 6.6 |
| pH | 6.62 | 6.94 | 7.03 | 7.04 |
| Conductivity (mS/cm) | 4.08 | 3.98 | 4 | 3.69 |
| PT | 21 | 2.6 | 20.4 | 0.7 |
| NTK (mg/L) | 140 | 100 | 133 | 79 |
| Cl− (mg/L) | 460 | 426 | 358 | 341 |
| Na+ (mg/L) | 352 | 340 | 345 | 322 |
| K+ (mg/L) | 56 | 17 | 19 | 12.6 |
Also, Yılmaz Nayır and Kara (2018) demonstrated the treatment of container washing wastewater (CWW) using a hybrid of EC-EO. They were focused on the removal efficiency of soluble chemical oxygen demand (sCOD) and color. The experiment was performed first by EC using Al and Fe electrodes. Thus, at the optimum operating conditions (current density of 25 mA/cm2, pH of 5, and electrolysis time of 120 min) maximum sCOD removal of 82% was attained. Similarly, color removal of 95, 95, and 98% were achieved using the Fe electrode at 436, 525, and 620 nm, respectively. However, EC shows less performance to remove sCOD. Therefore, a hybrid process of EC and EO was implemented in which EO was processed as a post-treatment of EC using a boron-doped diamond electrode (BDD). Accordingly, the removal of sCOD was improved to 89% at 420 min, but the removal efficiency of color was decreased to 72, 64, 71% using the same optimum current density and wavelength, respectively. From this, it is possible to conclude that, due to the colloidal particles found in the aqueous solution, EO alone requires a relatively long electrolysis time (not practical) which is also in line with (Chakchouk et al., 2017). On the other hand, Linares-Hernández et al. (2010b) also conducted a study on a hybrid of EC-EO treatment for industrial wastewater. This study aims to investigate the performance of EC and EO separately and in a hybrid process using the real wastewater collected from the outlet of the biological wastewater treatment plant. As a result, EC was very effective in removing colloidal and suspended particles at an operation time of 30 min (a very quick process). However, it was less effective to remove COD, in which almost only half of it was able to reduce from the wastewater. Relatively, EO alone was very effective in removing organic compounds such as COD and BOD5 but it requires a long operation time which is not practical. During the application of the hybrid process, the colloidal and suspended solid particles, as well as many charged species were removed quickly by EC and then EO oxidizes the remaining pollutants. Therefore, the hybrid system is very effective to remove all color, turbidity, and coliforms, BOD5, and COD to the allowable limit within the practical electrolysis time.
Furthermore, Linares Hernández et al. (2017) conducted a study on a monopolar hybrid process of EC-EO for soft drink wastewater treatment. Copper (Cu) was used as an anode and cathode material for the EC method. To achieve this objective the current density was varied with 17, 51, and 68 mA/cm2. During the EC process, only 37.67% of COD and 27% of TOC were able to remove at 20 min operating time and pH of 8. To enhance the removal efficiency of the system EO was performed as a post-treatment of EC. The EO process was performed using BDD as anode and Cu as a cathode at an optimum current density of 30 A/m2. As a result, the removal efficiency was enhanced to 75 and 85% for COD and TOC, respectively. Accordingly, the hybrid system of EC-EO was effective to remove pollutants from soft drink wastewater and it also reduced the operation time and operating cost which contributes to the sustainable reuse of industrial liquid effluent. Similarly, Ghazouani et al. (2019a) conducted a comparative study of electrochemical hybrid systems for the treatment of real wastewaters from agri-food activities. This study aimed to investigate the efficiencies of two electrochemical treatment processes (electrochemical oxidation/reduction (EOR) and electrocoagulation) set through two scenarios (first combination: 3 h of EOR followed by 1 h of EC, and second combination: 1 h of EC followed by 3 h of EOR) for the treatment of two different industrial effluents; namely poultry slaughterhouse wastewater (SHWW) and dairy wastewater (DWW). Besides, the removal efficiency of these systems was evaluated in terms of COD, nitrates, ammonium/ammonia, and phosphates as well as electric energy consumption. Also, the performance of EOR was evaluated on a bipolar electrode arrangement with boron-doped diamond supported on silicon electrodes. The EC treatment system was performed with mild steel electrodes connected in parallel. Accordingly, the results for both scenarios (3 h of EOR followed by 1 h of EC and 1 h of EC followed by 3 h of EOR) are summarized in Table 3.
Table 3. Removal efficiency of EOR and EC for pollutants from SHWW and DWW.