1. Introduction
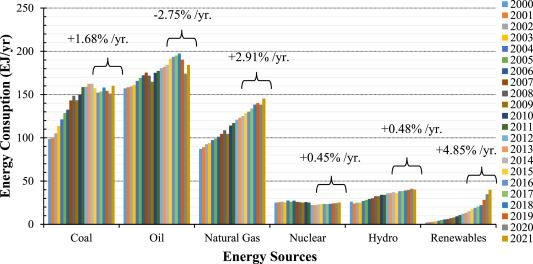
Energy crisis and air pollution scenario of the world getting severe in recent years due to the increasing fuel consumption rate and rapid industrialization [[1], [2], [3]]. The U.S. Energy Information Administration (EIA) has predicted that by 2050, global energy consumption may increase by 50% [4]. Around 80% of the global energy demand is still fulfilled by using fossil fuels [5,6]. The key factor contributing to global warming and increasing greenhouse gases is the excessive burning of fossil fuels [7,8]. According to the United States Environmental Protection Agency (USEPA), CO2 emission contributes roughly 80% of all greenhouse gas emissions, and 65% of those emissions come from burning fossil fuels [9]. The world is gradually shifting to renewable energy sources for energy consumption considering the steady increase of CO2emission from fossil fuels combustion. Between 2017 and 2021, oil consumption declined by 2.75% each year, respectively, while renewable energy consumption increased by 4.85% per year (Fig. 1).
 Fig. 1. Global energy consumption from various sources from 2000 to 2021 [10].
Fig. 1. Global energy consumption from various sources from 2000 to 2021 [10].Solar energy is the most prevalent of the various types of renewable energy sources. Research and development strategies are going on to produce hydrocarbon fuels from CO2 using solar energy. Photocatalytic production of hydrocarbon fuels from CO2 is a viable technique for tackling global environmental issues while also ensuring future energy security [11]. Different semiconductor materials used as a photocatalyst in CO2 photoreduction process. They are TiO2, Cu2O, ZnO, ZnS, CdTe CdS, CdSe, WO3 and Bi2WO6 [[12], [13], [14], [15]]. TiO2 is the most used photocatalyst for its abailability, stability, photoactivity and low toxic material properties [16,17]. Along with fuel generation TiO2 has diverse applications, as shown in Fig. 2. Since the discovery of TiO2 as a photocatalyst by Fujishima and Honda, it has percieved a lot of attention as a possibility for CO2 photoreduction into hydrocarbon fuels. Fig. 3depicts the growing rate of publications dealing with TiO2 photocatalysts and CO2 photoreduction. There is a huge increase in number of studies considering photocatalytic CO2 reduction in the last three years (Fig. 3b).
 Fig. 2. Application of TiO2-based photocatalysts.
Fig. 2. Application of TiO2-based photocatalysts. Fig. 3. Number of publications searching the terms: a) TiO2 photocataly* or titanium dioxide photocataly*; and b) photocatalytic CO2 reduction or photocatalytic CO2 conversion or photocatalytic conversion of CO2 or photocatalytic carbon dioxide reduction or photocatalytic carbon dioxide conversion or photocatalytic conversion of carbon dioxide. [Only journal articles (original and review) and conference papers included using Scopus database searched on November 15, 2022].
Fig. 3. Number of publications searching the terms: a) TiO2 photocataly* or titanium dioxide photocataly*; and b) photocatalytic CO2 reduction or photocatalytic CO2 conversion or photocatalytic conversion of CO2 or photocatalytic carbon dioxide reduction or photocatalytic carbon dioxide conversion or photocatalytic conversion of carbon dioxide. [Only journal articles (original and review) and conference papers included using Scopus database searched on November 15, 2022].TiO2 is the most affordable, readily useable, and well-characterized UV light active semiconductor photocatalyst. However, practical applications of TiO2 are greatly hindered by its wide inherent band gap (Eg = 3.2 eV for anatase), quick recombination of photogenerated charges, and low solar light utilization (about 5%) [18,19]. Therefore, considering practical standpoint, it is crucial to increase the capacity of TiO2 for light absorption and electron-hole separation efficiency. Nevertheless, CO2 is a highly stable molecule (ΔG° = −400 kJ mol−1) with linear symmetrical configuration, fully oxidized carbon, and an average carbon-oxygen double bond energy of up to 804.4 kJ mol−1 (at 298 K) [20]. Thus, strong photocatalyst is required to convert it into value-added chemicals. TiO2produces electron and holes when exposed to UV light, which aids in the dissociation of CO2 bonds. The rate at which CO2 breakdown will depend on how quickly electron-holes are generated. Improvement of charge separation efficiency and enhancement of visible light absorption can be attributed TiO2 as strong photocatalyst to convert CO2 [21]. Along with morphological modification, most widely used approaches to produce highly effective TiO2 for CO2 photoreduction is the modification of TiO2 surface by using metal deposition, metal doping, non-metal doping, dispersion on supports, carbon-based materials doping, surface sensitization and heterojunction methods [22,23].
With increasing research investigating to improve CO2 photoreduction performance of TiO2-based photocatalysts, summarizing those findings systematically is deemed crucial. Some descriptive review works on TiO2-based CO2 photoreduction have been done [7,22,24], as far as our knowledge there is no systematic review performed. Systematic review offers a structured overview on a particular topic that aids academics and researchers in keeping updated with the literature [25]. The purpose of this paper is to systematically summarize the recent advances with future challenges of TiO2-based photocatalysts for conversion of CO2 to hydrocarbon fuels. Particularly, various modification techniques of TiO2 to enhance CO2 photoreduction are extensively discussed. Moreover, great importance is also given to providing deeper understanding on factors affecting the CO2 photoreduction performance. Finally, future challenges and way forwards for practical applications of TiO2photocatalysts for CO2 photoreduction are described.
2. Methods
This work was driven by investigating recent progress and future challenges of TiO2-based photocatalysts for photoreduction of CO2 to hydrocarbon fuels. In order to fully understand the current progress and impending challenges we explored the recent literature as the primary source. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), a well-known published protocol to perform a systematic literature review was used in this study. This method uses very clear and systematic approaches that are specifically chosen to avoid bias, resulting in accurate information gathering which leads to create trustworthy conclusions.
2.1. Literature search process
The present study was carried out utilizing two key databases, Scopus and Web of Science (WoS) along with other sources, because both databases are reliable and cover more than 256 areas of study, including environmental fields [26]. We formulated a search string based on our understanding and knowledge in relevant field. The search string used in this study tabulated in Table 1. The literature was searched using advanced search options in both databases. First, we queried both database in early June 2022 and second time on the November 15, 2022, for getting maximum publications in the year 2022. Fig. 4 depicts the general screening methods and the flow of identifying relevant publications for reviewing. In the identification stage 337 articles from Scopus, 707 articles from WoS database and 171 articles from other sources were retrieved. When researchers retrieve publications from two or more databases using the same library format, merging the articles from databases to undertake unique analyses might be difficult, particularly when the databases are large. In this study unique technique suggested by Echchakoui [27] to merge publications of all databases was used. In the screening process, total of 201, articles were excluded due to duplication in both databases. Inclusion and exclusion criteria were set to further refine the 1014 results obtained after removing replicate and merging both articles of both databases.
Table 1. Searching string used in this study and the total number of publications from two important databases.
| Database | Searching String | Total | Combined |
|---|---|---|---|
| Scopus | TITLE-ABS-KEY ((“TiO2″ OR “Titanium Dioxide” OR “TiO2Photocataly*" OR “TiO2 Semiconductor”) AND (“CO2″ OR “CO2 Reduction” OR “Carbon Dioxide Reduction” OR “Reduction of Carbon Dioxide” OR “Conversion of CO2”) AND (“Photocatalytic CO2 Reduction” OR “Photocatalytic Carbon Dioxide Reduction” OR “Photo-electrocatalytic Carbon Dioxide Reduction” OR “Photocatalytic Production of Solar Fuels” OR “Photocatalytic Production of Methane” OR “Carbon Dioxide to Methane” OR “CO2 to CH4″ OR “CO2 to Hydrocarbon Fuels")) | 337 | 853 |
| Web of Science (WoS) core collection | TS=((“TiO2″ OR “Titanium Dioxide” OR “TiO2 Photocataly*" OR “TiO2 Semiconductor”) AND (“CO2″ OR “CO2 Reduction” OR “Carbon Dioxide Reduction” OR “Reduction of Carbon Dioxide” OR “Conversion of CO2”) AND (“Photocatalytic CO2Reduction” OR “Photocatalytic Carbon Dioxide Reduction” OR “Photo-electrocatalytic Carbon Dioxide Reduction” OR “Photocatalytic Production of Solar Fuels” OR “Photocatalytic Production of Methane” OR “Carbon Dioxide to Methane” OR “CO2 to CH4″ OR “CO2 to Hydrocarbon Fuels")) | 707 |
Note: The data here includes reviewed and original articles of all languages.
 Fig. 4. Flowchart of study based on the PRISMA recommended protocol.
Fig. 4. Flowchart of study based on the PRISMA recommended protocol.2.2. Eligibility criteria
We refined our results by only considering exclusion criteria: (i) review articles; (ii) book series & book chapter; (iii) conference proceedings; (iv) non-English language; (v) published before year 2018; (vi) meta-analysis & articles in press; and (vii) articles not open access & access by UMP library. After considering the articles based on inclusion and exclusion criteria, 102 studies remained in eligibility stage. A database with the 102 articles containing titles, abstracts and full texts was created in Mendeley reference management software. More importantly, at this step, all of the papers' titles, abstracts, and contents were carefully scrutinized to make sure they met the inclusion requirements and were appropriate for use in the current study in order to fulfil the objectives. Consequently, a total of 40 articles were excluded because they are not based on empirical data and focused on TiO2-based photoactivity. Finally, total 62 remaining articles were selected to analyze.
3. Results
3.1. Overview of the selected studies
Research on photocatalytic CO2 reduction to produce hydrocarbon fuels using TiO2-based photocatalysts has been increasing for getting alternative options to reduce pressure on fossil fuels. In this review, 62 articles from 17 different countries of the world were selected based on the criteria depicted in Fig. 4. The largest number of studies were carried out in Asian region (Fig. 5a). The highest around 42% (n = 26) of studies reviewed were conducted in China (Fig. 5b). All articles selected for this study were published between 2018 and 2022. Fig. 5c exhibits year wise selected publications number. About 60% (n = 37) of studies reviewed were published between 2020 and 2021. The articles reviewed were obtained from 36 different journals including nine (09) publishers. Most of these journals were published in the subject areas of chemical engineering, materials science, environmental science, chemistry, and energy.
 Fig. 5. Number of studies reviewed from specific countries: a) projected in the world map, b) projected in a bar chart and c) year-wise selected studies.
Fig. 5. Number of studies reviewed from specific countries: a) projected in the world map, b) projected in a bar chart and c) year-wise selected studies.3.2. Recent progress of TiO2-based photocatalysis
Titanium dioxide (TiO2) is the most studied semiconductor photocatalysts, and it has been utilized remarkably in the field of environmental purification and energy generation. It is worth mentioning that almost two third of the recent publications on photocatalysis utilizing TiO2 as a photocatalyst which shown in Fig. 6. When exposed to a photon with an energy equivalent to or more than its bandgap, TiO2 can produce electron–hole pairs. Based on the specific electronic pathway maintained by a certain species, the disposition of photogenerated electrons (e–CB) and holes (h +VB) may be identified [28]. Typical reaction mechanism involved in photocatalytic processes and approach in CO2photoreduction is illustrated in Fig. 7a, b. The discharge of heat occurs when the input energy is depleted.
 Fig. 6. Recent publications on photocatalysis utilizing different semiconductors from 2018 to 2022. (Publications search by using Scopus database).
Fig. 6. Recent publications on photocatalysis utilizing different semiconductors from 2018 to 2022. (Publications search by using Scopus database). Fig. 7. (a) Mechanism involved in photocatalytic process (b) Typical approach in CO2 photoreduction and (c) Band positions of certain semiconductors in relation to CO2 reduction energy levels (Reproduced from Refs. [28,29,30] with permission).
Fig. 7. (a) Mechanism involved in photocatalytic process (b) Typical approach in CO2 photoreduction and (c) Band positions of certain semiconductors in relation to CO2 reduction energy levels (Reproduced from Refs. [28,29,30] with permission).Generally, appropriate semiconductor is classified by three key characteristics: firstly, the bandgap energy (Eg), secondly, the locations of conduction band (CB) and valence band (VB), and thirdly, the behaviors of photogenerated electrons and holes [28,31]. The rates of interface charge recombination and interfacial charge carrier are the important behaviors of photogenerated electrons and holes [32]. One of the fundamental barriers to the practical application of TiO2photocatalysts is charge recombination at deep level defects, which reduces TiO2's photocatalytic performance [[33], [34], [35], [36]]. Yu et al. [36] proposes a method that employs shallow-level defects thermally stimulating the migration of trapped electrons above the deep-level defects via solution plasma processing (SPP) technology. Semiconductor photocatalysts with Eg > 3.0 eV, for example, are solely active in UV light, while those with Eg < 3.0 eV are more effective in visible light. The band positions of mostly used semiconductors (such as TiO2, Cu2O, CdSe, ZnO, CdS, WO3 and Bi2WO6) and redox potentials versus (vs.) Normal Hydrogen Electrode (NHE) of CO2 reduction at pH = 7 are shown in Fig. 7c. The association between band energy and the redox potential of the product selectivity determines the types of compounds that can be created from the process [37]. The redox potential and band gap relationship also applies to CO2 reduction to various hydrocarbon fuels, such as CH3OH, HCOOH, and CH4. Both TiO2 and ZnO, which are widely used photocatalysts, have advantages and disadvantages. Use of TiO2 instead of ZnO can be favorable in terms of bandgap, cost, stability, and many other characteristics. Since its discovery in 1972, researchers have been attempting to extract the photocatalytic benefits of TiO2 as well as solutions to its drawbacks.
3.3. Factors affecting the photocatalytic activity of TiO2-based photocatalysts
3.3.1. Particle size and shape of the photocatalysts
The photocatalytic performance of a catalyst is comprehensively affected by the particle size and shape of the catalyst because a catalyst with small particle size and desired shape holds sufficient surface-active sites and there is a more possibility to promote the interfacial charge separation [31]. The synthesis methods of photocatalysts have significant influences on their morphology [38]. Sol-gel, template assisted synthesis, hydrothermal treatment and electrochemical anodization are frequently used methods for the synthesis of TiO2 nanostructures. Among these methods electrochemical anodization allows for the development of self-organized TiO2 nanostructures with low cost and simplicity of geometry control (length, diameter, and wall thickness) by the use of appropriate anodization parameters [39]. It has been revealed that TiO2nanoparticles showed better photocatalytic performance than that of TiO2microparticles, which can be attributed to their smaller diameter [40]. The travel distance required for photogenerated charge carriers are shortened when particle size decreases, hence, lowering charge recombination. Li et al. [41] investigated the consequences of particle size on the morphology and photocatalytic activity by alkali treated TiO2. By varying the alkali-hydrothermal duration (0–48 h), TiO2 nanoparticles with various crystalline sizes were produced by them. Among the prepared samples, pore volume of TiO2-24 was found maximum with minimum pore diameter. The XPS spectrum of TiO2-X showed TiO2-24 had lower intensity versus binding energy curve. TiO2-24 nanoparticles exhibited better photocatalytic performance under visible light was due to the collective impacts of surfaces defects and crystalline size.
The morphology (shape) of nanoparticles has also influenced on the improvement of photocatalytic performance. The electron-hole recombination in nanotube (NT) shaped photocatalyst is greatly retarded because photogenerated electrons need to travel vectorially along the NT wall. For instance, Huang et al. [42] constructed one dimensional TiO2 nanostructures through a single-step hydrothermal method. Results revealed that one dimensional (1D) TiO2 nanostructures catalysts had a high specific surface area, improved photocatalytic CO2 reduction. Methane was produced at highest yields of 19.16 and 12.71 μmol/g over the TiO2 nanotubes (TNT) and nanorods(TNR), respectively, which were approximately 2.33 and 1.48 times higher than TiO2 nanoparticles (TNP). The effect of facet on TiO2 nanostructures and their photocatalytic performance was investigated by Kowalkińska et al. [43]. They noted that TiO2 with octahedra exposing {101} facets had the maximum photoactivity and mineralization efficiency when exposed to UV–vis light, with these properties decreasing as subsequent facets develop and are exposed more.
3.3.2. Surface area of the photocatalysts
Besides, morphology (shape, size) control of TiO2, the increase of specific surface area is another promising approach to achieve photocatalytic activity improvement. The surface area of nanoparticles depends on their porosity and particle size [44]. One dimensional TiO2 nanostructures, especially, TNT has a high specific surface area and higher rate of photocatalytic CO2 reduction ability than other nanostructures [42]. Due to the tubular shape and length, TNT intrinsically offer a high surface area [45]. Das et al. [46] explored a hierarchical ZnO–TiO2 heterojunction strategy-driven technique to enhance photocatalytic performance by combining enhanced surface area and interfacial charge carrier. They reported that combining structural tuning with heterojunction leads to enhanced surface area and useful charge carrier separation, which improves photocatalytic activity. Wang et al. [47] investigated S-Scheme TiO2@ZnIn2S4photocatalyst for successful CO2 photoreduction as shown in Fig. 8. The results indicated that improvement of photocatalytic CO2 reduction is ascribed to the enlarge specific surface areas with sufficient active sites. When the TiO2@ZnIn2S4 heterojunction is subjected to light, electrons are excited and easily move to the VB of ZnIn2S4 and recombine with the photogenerated holes of ZnIn2S4 due to the interface band bending and Coulomb interaction (Fig. 8a). As illustrated in Fig. 8b, the main photoreduction products of samples are CO, CH3OH, and CH4. In particular, when compared to pristine ZnIn2S4 and TiO2, the overall CO2 photoreduction conversion rates of the examined S-Scheme TiO2@ZnIn2S4 photocatalyst enhanced by 2.75 and 4.43 times, respectively, reaching 18.32 μmol h−1 g−1.
 Fig. 8. Influence of specific surface area on enhancement of photocatalytic CO2reduction (a) illustration of the S-scheme transfer process between TiO2 and ZnIn2S4 and (b) Yield of products in photocatalytic CO2 reduction (PCR) reaction of different samples with stability tests (Adopted from Ref. [47] with prior permission from Wiley Publisher).
Fig. 8. Influence of specific surface area on enhancement of photocatalytic CO2reduction (a) illustration of the S-scheme transfer process between TiO2 and ZnIn2S4 and (b) Yield of products in photocatalytic CO2 reduction (PCR) reaction of different samples with stability tests (Adopted from Ref. [47] with prior permission from Wiley Publisher).3.3.3. Calcination temperature
Calcination refers to the process of heating a material to a high temperature that is lower than the melting point. It significantly affects the produced photocatalysts' morphology, crystallinity, surface area, pore-volume, and phase structure, which influences the photocatalytic performance as well. Wu et al. [48] decorated Pd-calcined black TiO2 nanoparticles in an argon environment using the sol-gel method. At various calcination temperatures, the synthesized photocatalyst was examined, and the maximum behavior was recorded photocatalyst calcined at 400 °C. In another study, In another study, Phromma et al. [49] investigated particle size, crystallite size, and phase separation of TiO2nanoparticles to determine the impact of calcination temperature on photocatalytic activity. The results showed that anatase had a significant impact on photocatalytic activity between 300 and 600 °C, however the particle size of TiO2 was shown to have a dominant impact between 600 and 700 °C. Morawski et al. [50] examined the effect of calcination temperature on TiO2/rGO for the CO2 photoreduction. Above 500 °C calcined temperature substantial reduction of band gap energy and BET surface area was observed (Fig. 9a). This is due to the aggregation of TiO2 particles during calcination and incorporation of rGO in composite. The highest yield for CH4 and CO production was observed for TiO2/rGO-10 without calcination, followed by H2 calcined at 1000 °C (Fig. 9b).
 Fig. 9. Effect of calcination temperature (a) properties of photocatalysts and (b) products yield of tested photocatalysts during CO2 photoreduction with H2O (Reproduced from Ref. [50] with due permission from Elsevier).
Fig. 9. Effect of calcination temperature (a) properties of photocatalysts and (b) products yield of tested photocatalysts during CO2 photoreduction with H2O (Reproduced from Ref. [50] with due permission from Elsevier).3.3.4. Photocatalytic reactors
The efficiency of photocatalytic CO2 reduction is substantially influenced by the photocatalytic reactors utilized [51]. In most cases, CO2 photoreduction is performed in either batch or continuous-flow mode reactor systems. For the case of batch type reactors, product accumulation and re-adsorption, as well as additional reverse or side reactions, are always a possibility [11]. Overall, batch type reactors are not a suitable choice for long-term or large-scale applications. Alternatively, continuous-flow type reactor systems can improve the aforementioned issues in batch type reactors [52]. Dilla et al. [53] constructed a tubular continuous flow reactor to improve the interaction between gaseous CO2 reactants and the TiO2 photocatalyst surface, allowing the approach to be used on a larger scale and in industrial application.
It is indispensable to ensure that the photocatalyst is illuminated to its maximum extent and comprehensive interaction of reactants occurs with the photocatalyst surface. In general, this entails two approaches: increasing the catalyst surface area and increasing the quantity of light incident on the reactor. A monolith photoreactor was constructed on a continuous photocatalytic reactor by Tahir [54] after loading the TiO2 photocatalyst over structured 3D MAX Ti3AlC2. The higher yield rate of CO, H2 and C2H6 with good stability was observed using the monolith photoreactor in comparison with the fixed bed photoreactor (Fig. 10). Higher photocatalytic conversion rate was reported due to the lighted surface area to volume of the monolith photoreactor, high flow rates, decreased pressure drop, increased catalyst loading, and efficient exploitation of photon energy. Giusi et al. [55] developed a unique gas progression photocatalytic reactor relying on copper-functionalized nanomembranes for CO2 photoreduction. They used a concept that was substantially distinct from traditional photocatalytic methods. It was possible to demonstrate for the first time the extremely selective CO2 conversion to C1–C2 carboxylic acids without production of H2, CO, CH4, or other hydrocarbons due to the unique properties and conditions of the photoreactor.
 Fig. 10. Evaluation of CO2 photoreduction activity of fixed bed and monolith type photoreactor: (a) production of CO; (b) production of H2; (c) production of C2H6; (d) process of fixed bed type reactor and (e) process of monolith type photoreactor system (Adopted from Ref. [54] with permission from Elsevier).
Fig. 10. Evaluation of CO2 photoreduction activity of fixed bed and monolith type photoreactor: (a) production of CO; (b) production of H2; (c) production of C2H6; (d) process of fixed bed type reactor and (e) process of monolith type photoreactor system (Adopted from Ref. [54] with permission from Elsevier).3.4. Modification techniques to enhance the photocatalytic CO2reduction
TiO2 photocatalysts received noteworthy attention in the field of CO2 reduction reactions as they are readily available, affordable, stable, and environmentally safe. However, the two key problems preventing practical use are the restricted ability to absorb in the visible light spectrum and the quick recombination of photogenerated electrons and holes [56,57]. Through surface modifications, the properties of photocatalytic materials can be changed significantly, which provides the possibility to improve photocatalytic activity and even induces new photocatalytic reaction paths. In this section, the latest research progress of surface modifications techniques to improve the CO2 photoreduction performance will be discussed. Several surface modification strategies as shown in Fig. 11 have been used overcome the drawbacks of TiO2 and enhancing the CO2 photoreduction.
 Fig. 11. Modification techniques to improve the CO2 photoreduction performance.
Fig. 11. Modification techniques to improve the CO2 photoreduction performance.3.4.1. Metal deposition
Metal deposition is an appealing approach to enhance the photocatalytic activity of TiO2 nanocomposites. Although metal deposition is an expensive modification method, it significantly enhances electron–hole separation in semiconductor materials [22]. The ability of metals to accept photogenerated electrons improves as the working functionality of the metal increases. Consequently, it improves the electron–hole separation, hence enhances the overall photocatalytic performance of TiO2. The absorption edge of TiO2 is reduced when low bandgap energy metals are deposited, adjusting the visible light response [7]. Platinum (Pt), palladium (Pd), copper (Cu), nickel (Ni), gold (Au), and silver (Ag) are some of the most typically deposited metals. Devi et al. [58] used Pt-coated GO wrapped TNTs to increase CH4 production by CO2photoreduction at a moderate temperature and pressure. They have successfully produced CH4 at a high rate of 3.42 mmol g−1 h−1. Reñones et al. [59] performed a study on Au–Ag deposited TiO2 photocatalysts for CO2 reduction. Compared to only producing syngas over pure TiO2, the results showed that bimetallic catalysts can alter the reaction selectivity into CH4 under UV light irradiation. Sanz-Marco et al. [60] examined Ni deposited LED-driven TiO2 for visible-light expanded conversion of CO2. The photocatalytic conversion of CO2 into CO, CH4and C2H6 alkanes was examined under LED irradiation at different wavelengths (Fig. 12). Results showed that when Ni/TiO2 catalysts were illuminated at 365 nm, maximal productivity of CH4 was 450 mmol g−1 h−1 and CO was 250 mmol g−1 h−1, with C2H6 production being nearly constant throughout the process, reaching roughly 2 mmol g−1 h−1. Under the visible region of the solar spectrum the productivity of CO increased significantly compared to CH4 and C2H6 (Fig. 12c and d). Liu et al. [61] studied the performance of CO2photoreduction by implying Cu deposition on different TiO2 substrates. It was revealed that both TiO2@Cu and H–TiO2@Cu showed exceptional improvement for CO and CH4 production. Another important finding was also found from the study that not only synthesis technique significantly enhances the CO2reduction activity after Cu deposition, however the substrate utilized for loading and the chemical state of inserted material will also affect the following catalytic activity. Recently, Zhan et al. [62] examined Pd nanoparticles on surface of TiO2 for enhancing photocatalytic reduction of CO2 to CH4. The Pd–HN–TiO2 composite, which contains Pd, was synthesized by coordinating Pd(OAc)2 with N-heterocyclic carbene in the skeleton, while HN serving as a platform to link TiO2 with extensively dispersed Pd nanoparticles and increase CO2 adsorption. Results showed that with a progress rate of 237.4 mol g−1 h−1and selectivity of more than 99.9%, this composite with a surface area of 373 m2 g−1 improves CO2 photoreduction efficiency to CH4. Pure TiO2, HN had a low CO2 reduction efficiency for CH4 and CO production, however the evolution rate of CH4 increased somewhat after loading with Pd, while the rate of CO remained almost constant.
 Fig. 12. Productivity of different products for Ni/TiO2 catalyst under LED light irradiation (a) at 365 nm untreated, (b) at 365 nm activated, (c) at 460 nm and (d) white light (Adopted from Ref. [60] with permission from Royal Society of Chemistry).
Fig. 12. Productivity of different products for Ni/TiO2 catalyst under LED light irradiation (a) at 365 nm untreated, (b) at 365 nm activated, (c) at 460 nm and (d) white light (Adopted from Ref. [60] with permission from Royal Society of Chemistry).The visible-light activity of various semiconductor materials has been reported to be improved by Au and Ag nanoparticles deposition due to the effects of localized surface plasmon resonance (LSPR) and Schottky barrier development. With regard to the LSPR, an electromagnetic field is developed, and the photoreaction is enhanced through scattered photons, plasmonic energy transfer and electron excitation [63]. However, the development of a Schottky barrier increases photoactivity by trapping and extending the lifetime of the electrons. Khatun et al. [64] deposited Au nanoparticles in the TiO2 nanotube by simple electrochemical deposition method to improve CO2 photoreduction to CH4. Light harvesting properties of prepared Au-TNTs catalysts showed improvement under visible light owing to its LSPR behavior. The photocatalytic performance of TNTs and Au-TNTs increased considerably, yielding of 8.26% and 14.67% higher CH4 production than bare TiO2, respectively. In another study, Feng et al. [63] demonstrated the photo-deposited Ag nanoparticles at two orders: MgO deposition followed by Ag (Ag/MgO/TiO2) and Ag deposition followed by MgO (MgO/Ag/TiO2) to improve the performance of CO2photoreduction. They revealed that the Ag/MgO/TiO2 catalyst with seven deposited atomic layers of MgO and 5% Ag was 14-fold more active compared to pure TiO2 for CO and CH4 production. Li et al. [65] studied the photothermal catalytic performance of TiO2-x/CoOx photocatalysts for enhancing CO2photoreduction. The demonstrated 175 times yield of CH4 than bare TiO2 under UV irradiation at elevated temperature.
3.4.2. Metal doping
Metal doping is the most extensively used surface modification approach for preventing photogenerated electron-hole pairs from recombining on TiO2surface [22,66]. Pure TiO2 is not able to absorb high photon energy due to its high band gap energy (3.2 eV), however doping reduces the band gap energy (Fig. 13). The addition of metal nanoparticles in the TiO2 matrix causes structural defects and decreases the TiO2 bandgap, lowering the absorption threshold to visible levels [22]. Nematollahi et al. [67] reported that incorporation of Ni and Bi gradually decreased band gap energy compared to pristine TiO2 from 3.1 to 2.84 eV. Ni–Bi doped TiO2 exhibited 6.5-fold more yield of CH4 compared to pristine TiO2. In diverse investigations, Pt, Au, Ag, Fe, Cu, Ni, Pd, and Ru have been doped in TiO2 matrix and shown to enhance its photocatalytic performance (Table 2).