1. Introduction
Sustainability, environmental concerns, and technological advancements are the main motivations for the construction industry to employ more designed high-performance materials that are environmentally friendly and affordable than traditional construction materials. Such materials can considerably enhance the service life of buildings while also drastically reducing the need and cost of maintenance [1]. Industrial and agricultural wastes that fulfill the mineral composition criteria of cement have prompted many researchers to investigate their application in construction [[2], [3], [4]]. Such wastes can replace cement or aggregate, enhancing its chemical and physical characteristics, saving costs, and reducing environmental effects. The use of plastic waste materials, building and demolition debris, and bottom ash as aggregates in cement mortars and concrete has been a focus of several studies [[10], [11], [12]]. The use of such recycled aggregate may affect the properties of fresh concrete. It has been found that the use of recycled coarse aggregates reduces the consistency and fluidity of concrete within the first hour after mixing when compared to concrete created with natural aggregates [13,14]. The use of a sufficient amount of bottom ash improves the strength of concrete; the addition of 10 % increases the strength of plain and self-compacting concrete [15]. The abrasion and shrinkage resistance of normally vibrated bottom ash concrete has improved, but it has decreased in self-compacted concrete at various curing ages [16].
Previous investigations have shown that presenting chemical or physical treatments for the waste materials could overcome these issues such as the calcination, grinding and carbon dioxide (CO2) curing process which results in a faster rate of growth in waste-based materials properties than the conventional curing processes [2,[17], [18], [19], [20], [21]]. Since CO2 is the principal greenhouse gas and a contributor to global warming, it is important to understand its role. One of the main sources of CO2 emissions is the cement sector; a contemporary cement plant will create between 0.49 and 0.92 kg of CO2 for every kg of cement produced [22]. On average, it is reported that 0.79 tons of CO2 will be emitted to produce one ton of cement [23]. As a result, responsible companies and researchers have made major efforts to minimize CO2 emissions from industrial and especially cement production by establishing a new manufacturing technique and substituting cement or aggregates with supplemental materials of equal or greater importance [24,25].
Many strategies for reducing CO2 emissions as well as the collection, storage, and sequestration of emitted CO2 have been the subject of research [26]. As a result, there has lately been a lot of interest in using collected CO2 in a process that yields valuable materials. One of the most well-known instances of CO2sequestration is the carbonation of CO2 and the creation of a product with commercial value [24,27]. Numerous studies in this area have shown that CO2curing of cement-based materials (such as mortar, paste, concrete, aggregates, and solid waste from these cement-based products) is a more effective type of CO2 collection [28,29]. Prior research has found that CO2 improves the durability and properties of cement-based building materials [25,30]. Such a process can improve the mechanical properties of concrete and reduce its drying shrinkage [[31], [32], [33]]. CO2-cured concrete is known to provide an extremely rapid strength growth rate and can improve its resistance and durability performance. Introducing the carbonation process early during the cement hydration process will expedite the reaction rate as compared to the conventional hydration processes. The compressive strength of the mortar was nearly the same after 1 h of CO2 curing as it had been after 7 days of wet curing [34].
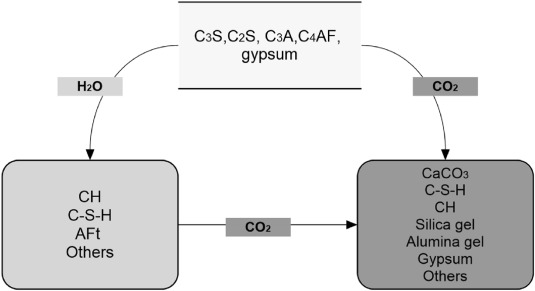
CO2 reacts with cement clinker materials (calcium silicates and their hydrate products) through the carbonation reactions to form calcium carbonate (CaCO3) [18,35]. Such reactions can positively affect the concrete properties by improving their strength [[36], [37], [38], [39]]. Formation of dimensionally stable crystals of CaCO3 and the associated expansions when young plain concrete is exposed to high concentrations of CO2 attributes to considerable early strength [19,40,41]. Fig. 1 depicts a description of the carbonation and hydration of various cement mineral phases.
 Fig. 1. The products of the reaction developed during curing.
Fig. 1. The products of the reaction developed during curing.However, it is important to acknowledge that extended or excessively intense carbonation curing tends to diminish the development of compressive strength during the initial stages [42]. This adverse impact can be attributed to several factors: the retarded hydration of cement due to carbonation curing, the decalcification of Calcium silicates (C–S–H) and the formation of cracks in microstructures caused by carbonation shrinkage [43,44]. For a given concrete mixture, there may exist a threshold of CO2 uptake beyond which prolonged (or excessively intense) CO2 curing during the early stages proves to be detrimental [42]. Consequently, it is crucial to strike a balance in the early-age carbonation of cement-based materials that maximizes CO2 uptake while ensuring the preservation of mechanical and long-term durability performance.
Concrete and other cement-based products are showing promising outcomes in the carbonation revolution, potentially leading to a reduction in the cement industry's CO2 impact. Furthermore, applying a carbonation mechanism in industrial wastes with cohesive characteristics (fly ash, oil palm ash, oil shale ash, etc.) can propel this revolution further [2,45]. The urgency of mitigating greenhouse gas emissions has encouraged researchers to focus on the feasibility of sequestering CO2 in solid waste-based construction materials and developing efficient carbonate cementitious systems. Many studies have been performed in recent years to identify the characteristics of cementitious-based materials cured in CO2 environments, and promising findings have been highlighted by many researchers [18,46,47]. However, a systematic review of waste-based construction materials and new carbonation systems is lacking until now. This paper was developed in a systematic way to provide an overview of the usage of CO2 curing to improve the characteristics of solid waste-based construction materials. The CO2 curing technique and its influence on workability, micro-properties, mechanical performance, and durability are fully reviewed. Furthermore, this work provides a significant overview for future research and elucidates core understandings and current achievements on CO2 curing of solid waste-based construction materials in several areas. This study also highlights the critical issues that must be addressed and remedied in the future.
2. Methodology
The systematic review was performed by using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria [48]. PRISMA is an effective method for being acquainted with the greatest accessible research information on a specific issue. The effectiveness of a systematic review is enhanced by the clarity of each stage of the synthesis process, as well as allowing the reader to concentrate on the advantages of each finding made in collecting information, instead of being attracted to the unnoted difference between studies, as is sometimes the case in other types of reviews [49]. While bibliometrics is a popular approach for building a large picture in a literature evaluation [50].
This work provides a thorough and systematic analysis that seeks to synthesize, identify, and evaluate the literature by detecting curing with CO2 for solid waste-based construction materials in publications. More specifically, all publications during the last six years have been collected and investigated based on the standards for the systematic reviews and our research objectives. The available literature was collected by utilizing search engines from various databases. The same search possibilities were repeated for each database, utilizing the keyword combinations depending on the search strategy accredited in the database.
The following terms were checked in each database by applying the advanced search options and setting the search period to the previously mentioned range. The web of science (WoS) database was searched using the intended search terms as” TS= ((carbon dioxide curing OR CO2 curing OR carbonation) AND (ash OR solid waste) AND (concrete OR aggregate OR cement* OR construction materials))”. Also, the Scopus database was searched by using the “TITLE-ABS-KEY (((carbon dioxide curing OR CO2 curing OR carbonation) AND (ash OR solid waste) AND (concrete OR aggregate OR cement* OR construction materials)))” combination. Publications involving CO2 curing have increased throughout time, which can be ascribed to the expansion of scientific research itself.
The main body of the literature involved 715 studies. About 80 % of the extracted publications were from WOS with the lion's share of the journal papers distantly followed by conference papers. 572 papers were excluded after examining the titles and abstracts and removing duplicates to avoid repetition. After the full-text reading, only 71 papers were connected to the inclusion criterion. Fig. 2 displays the criteria for including and excluding literature (PRISMA statement) at each level. These investigations were thoroughly studied to create the overall foundation for the study map on CO2 curing in waste-based building materials. The study only used original research publications, reviews, and conference papers. To assure the review's quality, every duplicate was carefully checked. To ensure the accuracy and relevance of the data used in the review process, the article titles were selected and analyzed for evaluation. The validity of the approach and outcomes was then verified by conducting a thorough analysis of the study abstracts.
 Fig. 2. PRISMA statement.
Fig. 2. PRISMA statement.3. CO2 curing of waste-based construction materials
During cement hydration, the dry cement mixes with water and forms a solid hydrate phase, leading to volume expansion and reduced porosity, resulting in a strengthened material [51,52]. Carbonation, on the other hand, is a more challenging process in which gaseous CO2 penetrates the concrete, dissolves in the pore solution, and reacts with cementitious phases to form CaCO3 and silica gel [27]. However, due to the fine porosity and water content of hydrated cement, carbonation proceeds slowly under normal conditions [35,53]. Nevertheless, concrete structures exposed to CO2 can act as a CO2 sink over time [54]. Additionally, crushed concrete, demolition debris, and industrial solid wastes can also absorb significant amounts of CO2, although it may occur in an unpredictable manner [51].
This section provides an analysis of the usage of CO2 as a curing method, including the reaction mechanism, implementation procedure, carbonation quality, and mechanical properties of the final product. It also provides details regarding the effect of carbonation curing on waste-based materials, alternative binders, and recycled aggregates. In addition, looking for the conditions of the curing technique in each study was the major key to understanding the differences in the resulting carbonated construction materials, as appeared in Fig. 3. A pre-curing stage is crucial for achieving effective carbonation developments. Pre-curing regulates the removal of water from a mixture after casting and before CO2 exposure. The most important sub-reactions happen in the aqueous state, thus there must be enough water before carbonation can start. High water content can impede the process by obstructing CO2 diffusion to the reactants, while low water content can lead to an insufficient reaction with ineffective outcomes [55]. The CO2 curing stage may be accomplished in two ways, enclosed and flowable reactor systems. The capacity to support increased CO2 partial pressures makes the enclosed system has better reaction efficiencies [56]. Laboratory scale experiments are often carried out in enclosed pressure containers where continuous reaction conditions are carefully regulated and monitored [57]. After carbonation, post-curing allows for additional hydration of the remaining unreacted hydraulic phases. To ensure the best hydraulic reaction, water depletion due to carbonation should be effectively considered at this stage [47,58].
 Fig. 3. Laboratory CO2 curing methodology.3.1 CO2 Curing for Waste-based Mortar.
Fig. 3. Laboratory CO2 curing methodology.3.1 CO2 Curing for Waste-based Mortar.3.1. CO2 curing mechanisms and influencing factors
3.1.1. Early-age carbonation chemical reactions
Unlike weathering carbonation where hydration products are undermined by atmospheric CO2, early-age carbonation engages anhydrous binder minerals directly to form the binding matrix. The CO2 gas acts as a reactant, rather than a catalyst, yielding a binding matrix distinct from that obtained from conventional hydration. To distinguish from deleterious weathering carbonation, the term ‘early-age carbonation curing’ is used. This type of curing is normally finalized within a time frame of a few hours to days.
Carbonation of concrete typically transpires when CO2 dissolves in the water present in the concrete pores and reacts with calcium ions to produce stable CaCO3, which then precipitates within the pore system. As a result of carbonation, the pH of the water in the concrete pores decreases from its usual range of 13–13.5 to approximately 9. While carbonation is a widely recognized natural aging process for concrete, it is a highly intricate phenomenon involving a series of chemical reactions and physical processes that mutually influence one another, these reactions are explained in equations (1)–(4). The stoichiometry of the products (μ) varies with the intensity of the exothermic reactions.(1)(2)(3)(4)
Consequently, providing a comprehensive physicochemical description of all the processes involved is challenging. The primary mechanism of carbonation is the removal of calcium from the hydrate phases, leading to the formation of various polymorphs of CaCO3. C–S–H and their hydrated products comprise a significant proportion of the volume of supplementary cementitious materials (SCMs). These compounds are primarily responsible for the engineering properties of construction materials products. Alite (3CaO·SiO2) and belite (2CaO·SiO2), which are anhydrous minerals of different polymorphs, have been found to react with CO2 according to Equations (3), (4) [27].
The reaction of C–S–H with CO2 leads to the formation of calcium silicate hydrate and CaCO3. During normal hydration, instead of calcium carbonate, calcium hydroxide (Ca(OH)2) is produced. Extensive carbonation of C–S–H results in a decalcification effect and the eventual transformation to silica gel and CaCO3, following Equation (2) [42]. The carbonation of adequately moistened C2S and C3S occurs rapidly within a short timeframe of minutes to hours, as the rapid generation of C–S–H explains the associated increase in strength [59].
Short-term carbonation does not completely react with all material grains; therefore, continued exposure to moist or humid environments after carbonation allows for the subsequent hydration of the unreacted portion that remains [60]. The microstructure of the final paste matrix, which is generated through dual carbonation and hydration curing, exhibits an intermixture of C2S, C3S, C–S–H, CaCO3, and Ca(OH)2.
3.1.2. Changes in microstructure
Earlier investigations demonstrate some differences and a lack of answers in the interpretation of carbonation-induced microstructural alterations. Many researchers believe that proper carbonation curing can contribute to high early-age strength and rapid strength gain of waste-based composites, owing to the microstructure densification caused by the pore filling effect of CaCO3 particles produced during carbonation curing [61]. Ca(OH)2 is organized in a crystalline configuration, which is presumed to be closely intertwined with the C–S–H. The microstructure is distinguished by the formation of calcite in the pores, the decalcified C–S–H gel, and the generation of gypsum from the decomposition of ettringite [18]. Throughout carbonation, the three forms of Ca(OH)2 can potentially be generated. The structure of CaCO3 is distinguished by small, densely packed acicular-shaped crystals [44].
Some microcracking is also evident, which is caused by the expansion of volume during the reaction and the consequence of thermal stresses due to heat produced during carbonation [62]. The C–S–H is not transformed into silica gel through the reaction with CO2. Following carbonation, an abundance of CaCO3 fills the pores of the system when examining waste materials containing heavy metals [63]. Additionally, other metals like Ni and Cr seem to be immobilized in the porosity filled with CaCO3.
3.1.3. Effects of construction waste characteristics on curing with CO2
The characteristics of construction waste play a pivotal role in determining the overall performance of the curing process. These characteristics encompass a range of factors, including particle size (fineness), chemical composition, porosity, and the presence of impurities within the waste materials. Existing research findings demonstrate that these attributes can significantly influence the outcomes of CO2 curing.
For example, finer particle sizes have been shown to enhance carbonation reactions, leading to improved curing efficiency and mechanical properties [64]. Finer powders increase contact area, thereby resulting in a high degree of carbonation. Similarly, the surface area positively correlates with curing efficiency and enhancing reactivity [65]. Porosity impacts the effective diffusivity of gases, and CO2 curing contributes to a reduction in total porosity while affecting nano-meter pore volumes [66].
Likewise, specific chemical compositions within construction waste materials can contribute to more favorable outcomes in terms of strength and durability. The calcium content is a primary reactant during the curing process, contributing to reactivity enhancement. A higher calcium/silicon ratio leads to increased production of CaCO3 crystals within a short curing period [65,67]. The addition of aluminum to C–S–H structure increases the content of bridging silicate tetrahedrons, while the presence of magnesium promotes long-range order in the C–S–H network [68,69]. Moreover, CO2 -cured concrete can serve as a matrix to stabilize heavy metals such as Cadmium (Cd), Chromium (Cr), Nickel (Ni), and Lead (Pb) [70]. Considering the variation in compositions of different cementitious materials, the effects of CO2 curing on cement, fly ash, and lime were studied. A after 2 h of CO2 curing, a concrete prepared with a 50 % slag as a replacement for cement could absorb 8–10 % CO2 by mass of binder and achieved a comparable strength to the concrete subjected to 24 h of normal hydration [71].
Lastly, the free water content is crucial as the liquid phase is necessary for CO2curing. Both excessive and insufficient free water can negatively impact carbonation degree and strength development. This highlighting the need for precise control in the curing process [72].
However, it is important to note that the impact of these characteristics can vary depending on the materials and environmental conditions. Understanding the relationship between construction waste characteristics and CO2 curing performance is vital for optimizing sustainable construction practices. By considering these influences, researchers and practitioners can make informed decisions about the selection and preparation of waste-based construction materials for CO2 curing. This contributing to more efficient and environmentally responsible processes. Despite the progress in this field, there remain research gaps and unexplored aspects. This underscoring the need for further investigation to unlock the full potential of construction waste in CO2curing applications.
3.1.4. Mineral admixtures and other industrial waste with CO2curing
The integration of admixtures and waste materials is crucial to the advancement of the carbonation process, thereby making a significant contribution to the development of construction materials that are more sustainable and long-lasting.
Mineral admixtures, such as Fly Ash (FA), Ground Granulated Blast Slag (GGBS) and Metakaolin (MK), exert a substantial influence on the CO2 curing process. FA, renowned for its high pozzolanic activity, enhances the efficiency of carbonation and, interestingly, demonstrates a lower degree of reaction, resulting in increased resistance to permeation [73,74]. GGBS, through the increase of the water/clinker ratio, facilitates improved exposure to CO2, which in turn promotes the formation of nucleation sites for hydration products [75,76].
Other noteworthy admixtures include Red Mud, which possesses a smooth surface that facilitates a high uptake of CO2 by forming CaCO3 enhancing the alkaline reactants [77]. Limestone Power (LS) promotes the formation of nucleation sites for CaCO3 crystals, although it may impede gas diffusion due to the accelerated early hydration of PC [78]. MK, due to its significant aluminum content, enhances the properties of materials, even at higher levels of replacement of cement after CO2 curing [79]. Furthermore, various other industrial waste materials, such as Quartz powder, Steel Slag, and Sediment, each fulfill a unique role in the CO2 curing process [78,80,81].
These diverse admixtures offer a range of mechanisms to enhance CO2 curing, including the modification of porosity and reactivity, as well as the promotion of the precipitation of beneficial carbonates. Ultimately, they contribute to the sustainability and performance of construction materials.
3.2. CO2 curing for waste-based mortar
This section presents details of the investigations concerning CO2 curing on several types of waste-based mortars like fly ash, waste glass, recycled aggregates, wood wastes, ground granulated blast furnace slag, volcanic ash, etc. Table 1 reports the scientific literature on the CO2 curing method to produce carbonated mortar from industrial and agriculture residues within the three-phases curing strategy. For optimal intake, specimens need to be demolded in order to enhance the surface area for moisture aeration and subsequent CO2exposure in the first phase. For dry mixtures, demolding is done immediately after casting (low w:s) [82,83] or postponed for several hours or up to one day for relatively high w:s ratio mixtures [84,85]. The pre-curing stage can be accomplished in 24 h if ambient temperature and humidity of 20–25 °C, and 40–60 %, respectively are maintained. Carbonated specimens with various pre-curing times show that increased early water loss enhances CO2 uptake.
Table 1. Summary of mix details and laboratory processing of ash-based carbonated mortars.

In the exposure stage, the carbonation reaction rate is mainly regulated by gas flow, which is significantly affected by the selected CO2 pressure and concentration [57,86]. Even with a low CO2 concentration, specimen strength development might reach the desired level with a longer curing period [86]. The determination of gas pressure in the examined studies is a complex decision that is influenced by several critical factors. The examined studies predominantly employ CO2 pressures ranging from 1 to 10 bar, utilizing high purity CO2 gas to enhance its diffusion. The selection of CO2 pressure is made with the intention of regulating the rate at which CO2 diffuses into the target material. Higher pressures have the potential to expedite the diffusion process, resulting in faster and more efficient carbonation reactions. The decision regarding pressure is intricately connected to the characteristics of the construction material under investigation. Materials with low porosity may require higher pressures to ensure deep penetration of CO2 molecules [87]. The composition, porosity, and density of the material can determine the necessary CO2 pressure for effective carbonation [86,88]. In the case of traditional concrete, the typical pressure range for carbonation curing is often between 1 and 10 bar. This range help to achieving effective carbonation, particularly in applications where a greater depth of carbonation is desired. Mortar have more porous than concrete, may necessitate lower pressures to ensure sufficient and uniform penetration of CO2. These chosen pressure values aim to achieve uniform carbonation while also considering practical considerations such as cost and feasibility, as evidenced by the examination conducted by experts.
During the exposure stage, the chamber's temperature and relative humidity might have a range of effects on the reaction rate [89,90]. Temperature increases CO2 diffusion and lowers its solubility. Furthermore, the evaporation of water is increased, and the migration of reactive ions is affected, therefore monitoring is essential at this stage [91,92]. Carbonation reaction is an exothermic reaction, with temperature spikes of up to 70 °C for mortar samples, which may degrade the stability of reaction products and consequently affect the performance of these cured samples. This boosts the relative humidity role, which is generally set at 60–70 % relative humidity (and occasionally higher) to avoid water evaporation in high-temperature scenarios [75,93]. Some studies vacuumed the chamber as a part of preparation before CO2 injection to wipe out the presence of air [83,94]. By running the reaction at vacuum pressure, which lowers water's resistance to absorbing CO2, the goal is to maintain an open pore system to let CO2 enter the specimen [95].
There is a lack of clarity in the studies about the details of post-curing, although researchers recommended the need for undergoing subsequent hydration up to the testing day [82,84,96]. Sharma and Goyal confirm that even spraying water as a post-carbonation curing technique can achieve higher compressive strength at later ages. This increase in strength is due to the late hydration phase, which produces additional C–S–H gel. This late hydration process could not be produced in specimens that had just been carbonated [94]. The carbonation duration may be associated with the compressive strength and binder %, which are also discussed in this work and displayed in Fig. 4 when the individual differences of each kind of examined combination are considered. The compressive strength of concrete with up to 50 % recycled binder content is shown in this figure, which illustrates that increasing the binder ratio will decrease the strength of the mortar produced, whereas it typically increases at 10 %–20 % cement replacement when the carbonation period is up to 24 h or longer.
 Fig. 4. Correlation between curing duration, percentage of replacement, and 28 days compressive strength [38, 55, 75,94, 101, 111].
Fig. 4. Correlation between curing duration, percentage of replacement, and 28 days compressive strength [38, 55, 75,94, 101, 111].Quality control is a key challenge while utilizing these binders in blends to substitute cement or sand and employing carbonation curing, where the ideal content must be maintained to fulfil the required features of cured products. The net CO2 absorption as a mass percentage of the original material before carbonation can be determined to assess the effectiveness of the carbonation cure [47]. In general, many methods for measuring CO2 uptake capacity can be used such as the mass curve technique, mass gain technique, and thermogravimetric analysis technique [[97], [98], [99], [100]]. The mass gain technique, which measures quantities of CO2 uptake by the material by estimating mass variances before and after treatment, is the most used approach for measuring CO2 uptake [47]. Fig. 5 illustrates, taking into account the variations across experiments, the effect of mixed binders on CO2absorption and compressive strength performance of CO2-cured mortar during the curing period. The measured CO2 uptake varied significantly between 6.4 % and 19.2 %, it should be mentioned [55,74]. This variation in CO2 uptake may be directly traced to key factors influencing carbonation treatment such as chemical compositions of binders, particle sizes, pre-curing conditions, CO2concentration, pressure, etc. On the other hand, increasing curing duration directly influences the compressive strength and CO2 uptake; 28 days of CO2curing demonstrated a CO2 uptake of more than 30 % with a compressive strength of 38 MPa [101]. The CO2 uptake capacity of volcanic ash blended mortar was found to generally increase with the amount of ash substitution.
 Fig. 5. Comparison of 28 days compressive strength of waste-based mortar, duration of carbonation curing, and CO2 uptake [55, 75, 104].
Fig. 5. Comparison of 28 days compressive strength of waste-based mortar, duration of carbonation curing, and CO2 uptake [55, 75, 104].3.3. CO2 curing for waste-based paste
The paste is a mix of adhesive materials and liquid, and generally exhibits thixotropic behavior [112]. Several fillers are used in the production of paste such as fly ash, ground granulated blast furnace slag, cement, rice husk ash, metakaolin, fine silica, and limestone powder [113]. Paste strength, on the other hand, is merely a quality control measure, especially for compressive and tensile strength. Paste strengths can be influenced by several parameters, including water-binder ratio, curing conditions, size of the specimen, casting, mixing, loading conditions, and age [114]. Experimental and theoretic investigations on the optimization of paste properties have been performed, especially the promising carbonation method; a brief literature overview is shown below in Table 2 for the main findings for recyclable material-based paste with carbon curing.
Table 2. Summary of mix details, laboratory processing, and main findings of waste-based carbonated pastes.


Carbonation curing is often used after normal curing has been completed. In fact, both the mechanical behavior of the paste and the success of the following CO2 curing may be directly influenced by the length of conventional curing. It was shown that increasing the normal curing time after casting can decrease the carbonation process's capacity and pace for absorbing CO2 [43,[115], [116], [117]]. Song et al. [85] used an electric fan with a wind speed of 1 m/s for 18 h to enhance CO2 penetration and carbonate precipitation by minimizing the sample moisture before exposing it to CO2. Until now, there is no clear correlation between ideal pre-curing duration and carbonation efficiency. Some researchers apply CO2 curing immediately after demolding [[118], [119], [120]], while others delay it up to 90 days [[121], [122], [123]]. Numerous investigations showed that, within the first 24 h of curing, the compressive strength of the CO2-cured paste increased with time, with the rise being most pronounced in the first 2 h [76,85,124], taking into account the details of the testing design for each study.
Another crucial component in the carbonation curing protocol is the period of carbonation. Because carbonation is a diffusion-controlled process, especially at an early age [57,86]. The carbonation duration has a considerable impact on the average pore sizes which are noticeably lower than before carbonization, it can indicate the connectivity between the samples and CO2 gas [18,94]. Because of the thinner thickness of the cement paste compared to the mortar specimens, just roughly 12 h were required to fully carbonate it [85]. It is vital to know that closing and vacuuming the chamber before injecting CO2 is recommended to maintain a pure CO2 environment and pump out the air [75,85,125,126]. Also, CO2 concentration had a direct proportion to the pore size as same as the duration of curing. The specimens cured under a CO2 concentration of 5 % had maximum pore sizes of around 3000 nm, but the specimen cured under a CO2concentration of 10 % had a maximum pore size of less than 2000 nm [127].
The carbonation process does not contradict subsequent hydration, further water curing following the carbonation technique is recommended to provide sufficient moisture [128,129]. When compared to the same characteristics acquired following pure hydration curing, samples exposed to post-curing after carbonation have substantially higher and far more desirable parameters of their hydro physical and mechanical properties [75,126]. After carbonation, samples increase in compressive strength up to 57.64 MPa, and after 28 days of hydration, this value increases to 68.71 MPa [126]. In comparison to the control sample under air curing, the hardened specimen sequestration of CO2 curing contained 30 % fly ash or blast furnace slag had substantially increased rates of 4.13 % and 15.60 %, respectively [121]. Alkalinity content and other operational factors connected to the carbonation process are primarily what define industrial solid wastes' greatest CO2 fixation capacity [130]. If CO2 can react with all of the Ca and Mg in solid waste. Ca-rich fly ash has a higher potential for CO2 sequestration than Ca-poor fly ash because it carbonates quickly and is strong enough to be used in structural construction [51]. Depending on the method used to make the steel, the CaO content in steel slag ranges from 23 to 60 % [81]. It should be made clear that immediate carbonation cannot completely devour particles. As a result, the unreacted component might hydrate by continuing to be exposed to moist or humid conditions after carbonation [34].
Recent articles on fiber-reinforced paste indicated that the mechanisms behind the carbonation of fibered matrix are almost the same [[131], [132], [133], [134]]. Furthermore, because of the higher porosity of the fiber matrix, fiber-reinforced composites are more responsive to carbonation [128]. The previous investigation generally acknowledged the increase in compressive strength of reactive MgO and polyvinyl alcohol fibers-based paste with carbonation, which is attributable to the precipitation of hydrated magnesium carbonates [134]. After 1 day of carbonation, the compressive strength of carbonation-cured specimens with 70 % binder reached 22.55 MPa. When extending the carbonation period to 7 days, the compressive strength increased by 86.2 %.
The CO2 curing of paste appears to have a complicated effect on the microstructure, short CO2 exposure time raises the compressive strength and vice versa [135]. Even though prolonged curing would result in more CO2exposure, an appropriate curing period should be applied. During CO2 curing, some of the bonded water is lost, causing the crystalline structure to diminish without influencing the polymerization rate [72,136]. The increased compressive strength is due to the denser structure. Mechanical resistance begins to decrease after the early curing phase due to a rise in carbonation degree towards the paste's interior regions, as well as carbonation of the inner CH phases and hydrated C–S–H over extended carbonation durations [137].
Many studies investigated the mechanical properties of cement pastes incorporating carbonated slag. The cement pastes incorporating carbonated slag all demonstrated satisfactory volume stability as the slag percentage of replacement rose from 10 % to 50 % [127,136,138]. After 7 days of carbonation and 11.3 % CO2 absorption, CO2 curing for reactive MgO-based binder achieved a compressive strength of 37.5 MPa [125]. Despite the superior mechanical performance, the carbonation period was long, which limited the value of its application in the construction sector, where rapid manufacture is crucial. The development in compressive strength was shown to be increased as CO2 intake increased as well as pore volume and pore size were decreased [139]. According to phase analysis, carbonation had the biggest influence on C–S–H and Ca(OH)2concentrations. In the carbonation technique, CH has an advantage over C–S–H because compressive strength rises more slowly since C–S–H carbonates later in the carbonation process [18,139].
3.4. CO2 curing for waste-based concrete
Carbonation of concrete systems is not a new concept; humans have employed alkaline earth hydroxide cement as a bonding agent to construct buildings that hardened as a result of their reactions with atmospheric CO2 [142,143]. The CO2content in the natural environment is around 0.0418 %; natural carbonation develops at a slow rate because of this low CO2 concentration [144]. The natural carbonation depth of normal concrete may take more than 80 years to reach 15 mm [145]. With the growth of CO2 technology and the advantages of carbon sequestration, further emphasis has been placed on creating an environmentally friendly alternative binder by carbonating solid waste.
Carbonation-based cementing techniques differ greatly from traditional (hydration-based) cementing procedures in that the strength bearing is primarily dependent on the carbonation reaction rather than cement hydration. The very slow diffusion of CO2 into the concrete matrix determines the carbonation rate of early-age concrete. Moreover, the presence of carbonation products, such as CaCO3 particles, can block the pores within the concrete matrix, making CO2 diffusion more difficult [16]. As a result, previous studies on carbonation curing have focused on thin concrete specimens that allow for rapid CO2 diffusion throughout the entire depth. Additionally, the extent of carbonation varies with depth, affecting the properties of the concrete. Excessive carbonation can lead to the degradation of calcium silicate hydrate (C–S–H), the primary hydration product and binding agent in concrete, resulting in reduced strength. Therefore, maintaining concrete strength is crucial to fully utilize its CO2 absorption potential. Furthermore, the carbonation curing method is primarily suitable for precast concrete due to the requirement of a closed curing chamber.
Assessing and focusing on earlier efforts can give a first step forward in a modification that not only decreases the carbon footprint of the concrete but also improves its strength and durability, with the objective of overcoming the restrictions in the present technologies listed above. Table 3 highlights the experimental details and processes of curing waste-based concrete in CO2 on the laboratory scale.
Table 3. Summary of mix details and laboratory processing of waste-based carbonated concrete.

3.4.1. Normal strength concrete
Several research have been carried out to study the effect of carbonation curing on conventional Portland cement and various types of recycled binders. When the mixture design specifics are ignored, the revised experiments show that the water-to-binder ratio has a considerable impact on carbonation; as shown in Table 3, the majority of the reviewed studies have used a water binder ratio that varies between 0.4 and 0.5. It has been determined that the carbonation depth is in direct proportion to the water-to-binder ratio [160,161]. Several procedures, including water curing, environment curing, and direct drying in an oven, were used before CO2 curing [88,146,151]. Because of the larger sample size, the pre-curing period in concrete is slightly longer than that in paste and mortar.
Nisar and Bhat investigated the effects of relative humidity during the pre-curing phase at a constant temperature on compressive strength and carbonation depth; they tested three humidity percentages (30 %, 45 %, and 60 %) at (27 ± 2) °C with different percentages of replacement [155], and their results are given in Fig. 6. At a relative humidity of 30 %, there were no carbonation effects detected. There is a linear association between binder replacement levels and carbonation depths of samples treated to varied relative humidity levels for each binder replacement level. Carbonation depth varies inversely with relative humidity. This is due to the fact that CO2 cannot penetrate into concrete at greater humidity levels because the pores are saturated with water [[162], [163], [164]]. Furthermore, water is emitted during the carbonation reaction, slowing it down. The depth of carbonation decreases as the compressive strength increases from 7 to 14–28 days. This is because as the compressive strength increases, the porous structure gets more refinements [86,165].
 Fig. 6. Effect of pre-conditioning under the temperature of (25 ± 2) °C with (45, and 60)% relative humidity values and different percentages of replacements (0, 5, 10, 15, and 20)% [155].
Fig. 6. Effect of pre-conditioning under the temperature of (25 ± 2) °C with (45, and 60)% relative humidity values and different percentages of replacements (0, 5, 10, 15, and 20)% [155].The strength of concrete is affected by various parameters such as the amount of binder used and the duration of CO2 exposure. Fig. 7 which was drawn from previous investigations indicates that carbonation duration has a direct impact on concrete strength [146,147,156]. It is worth noting that each research has used a different type of binder, but the obtained results are almost the same. After 90 days of curing, tensile strength and compressive strength were increased by 19.4 % and 4 %, respectively, at a 5 % percentage of replacement [147,156]. This increment is restricted at low curing times and high replacement percentages as shown in Fig. 8 [146,149]. The samples treated for 8 h with CO2exhibited a 44 % improvement in compressive strength compared to 2 h of curing and a 40 % increase in tensile strength at a 5 % replacement.
 Fig. 7. The effects of CO2 curing duration and percentage of replacement on (a) Compressive and (b) Tensile concrete strength [146,147,156].
Fig. 7. The effects of CO2 curing duration and percentage of replacement on (a) Compressive and (b) Tensile concrete strength [146,147,156]. Fig. 8. CO2 curing duration and percentage of replacement effects on the compressive and tensile strengths [149]The efficacy of CO2 concentration on concrete strength was assessed by replacing 0, 10, 20, and 30 % of fine aggregate with sawdust particles, the experimental variables included CO2 concentration at 0, 25, 50, and 75 %, 2 h of CO2 exposure, and neglecting the effect of chamber temperature and relative humidity [150]. Fig. 9 provides the conclusive outcome of these experimentations. Based on the same water-to-binder ratio, it was discovered that when the replacement ratio grew, the compressive strength of concrete decreased and increased as the CO2 concentration increased. Tensile strength dropped as the sawdust content in the mix increased, and all samples showed a higher tensile strength following carbonation curing compared to the control mixes with the same replacement ratios. Lower CO2 concentrations result in less CO2 permeating the specimens and the formation of fewer reaction products [86].
Fig. 8. CO2 curing duration and percentage of replacement effects on the compressive and tensile strengths [149]The efficacy of CO2 concentration on concrete strength was assessed by replacing 0, 10, 20, and 30 % of fine aggregate with sawdust particles, the experimental variables included CO2 concentration at 0, 25, 50, and 75 %, 2 h of CO2 exposure, and neglecting the effect of chamber temperature and relative humidity [150]. Fig. 9 provides the conclusive outcome of these experimentations. Based on the same water-to-binder ratio, it was discovered that when the replacement ratio grew, the compressive strength of concrete decreased and increased as the CO2 concentration increased. Tensile strength dropped as the sawdust content in the mix increased, and all samples showed a higher tensile strength following carbonation curing compared to the control mixes with the same replacement ratios. Lower CO2 concentrations result in less CO2 permeating the specimens and the formation of fewer reaction products [86]. Fig. 9. CO2 concentrations and percentages of replacement effects on the compressive and tensile strengths [150].
Fig. 9. CO2 concentrations and percentages of replacement effects on the compressive and tensile strengths [150].Furthermore, earlier study results revealed that chamber temperature during curing with constant concentration has a significant influence on the carbonation depth and compressive strength of concrete, as illustrated in Fig. 10[88]. Temperature and carbonation depth, as well as concrete compressive strength, have a linear relationship. The carbonation depth improved from 6.1 mm at 10 °C up to 21.3 mm at 30 °C. The carbonization products of samples vary with temperature; the primary products are particulate CaCO3, polyhedral spherical vaterite at 10 °C–20 °C, and aragonite at 30 °C.
 Fig. 10. Carbonation chamber temperature and percentages of replacement effects on the compressive strength and depth of carbonation [88].
Fig. 10. Carbonation chamber temperature and percentages of replacement effects on the compressive strength and depth of carbonation [88].3.4.2. Reinforced concrete
Concrete with a low water-to-binder ratio, according to the updated research, can be treated with CO2 curing at an early age for increased strength development. The achieved strength might be equal to or greater than the control mix [98]. However, neither any research has been conducted on the CO2curing of high water to binder ratio concrete. The concern is that humid concrete has significant liquid content, including water and plasticizer, which may block CO2 uptake. Furthermore, steel bars are commonly used to strengthen wet-mix concretes. There are tribulations with the decline of alkalinity in concrete caused by early carbonation, which could cause reinforcing steel corrosion.
The corrosion of concrete subjected to atmospheric carbonation has received a lot of attention. When developed concrete reacts with atmospheric CO2, it undergoes a gradual weathering carbonation. The gradual reaction lowers the pH of the concrete which is harmful to its long-term efficiency [166]. Steel is thought to remain in a passive condition in the alkaline environment offered by the concrete pore solution. Atmospheric carbonation might damage the passivation coating on the steel surface which expedites the corrosion rate [42]. Early CO2 curing differs from weathered carbonation. It is added to fresh concrete within 24 h of casting. As a result, additional hydration will occur following early carbonation [167,168]. For early age CO2 curing, there are two ways; if CO2 exposure is done immediately after molding, a reaction between C3S or C2S and CO2 occurs. Carbonation occurs in both anhydrous and hydrous phases when CO2 is supplied after a while of initial hydration [42,169,170]. In either scenario, the reaction products are a hydrate-carbonate hybrid.
An early-age CO2 curing investigation was applied for 100mm × 100mm × 300 mm rectangular reinforced concrete, samples were cured in a CO2 chamber for three different periods, namely, 7, 14, and 28 days, at a pressure concentration of (20 ± 3) %, and chamber conditions at (20 ± 2) ºC, (70 ± 5) % relative humidity [159]. CO2 sequestration potential was characterized by the depth of carbonation. It was increased by 36.4 % as the curing duration increased from 7 to 28 days a 40 % replacement. This was also associated with an increase in the compressive strength from 10.45 to 13.9 MPa, as shown in Fig. 11.
 Fig. 11. CO2 curing effects on fly ash-based reinforced concrete compressive strength and depth of carbonation [159].
Fig. 11. CO2 curing effects on fly ash-based reinforced concrete compressive strength and depth of carbonation [159].Earlier investigation reveal that CO2 curing seemed to have no impact on the pH of the region surrounding the reinforcement steel [167,168,171]. This revealed that the proposed CO2 curing technique faced no concern of reinforcement steel corrosion and might be used for a variety of reinforced concrete applications. Due to calcium carbonate formation and crystallization inside the matrix, CO2curing seems to have reduced volume and pore size [73,168].
3.5. CO2 curing for waste-based/recycled aggregates
Accelerated urbanization invariably increases the demand for building construction as well as demolition. This has raised the demand for natural aggregates as building materials, and the extraction of these natural resources has caused significant environmental disruption. Alternative raw materials are desperately needed in the construction and building industry [172,173]. Furthermore, huge amounts of waste are generated annually in the process of producing electricity including boiler slag, fly ash, clinker, and bottom ash [[174], [175], [176]]. Fly ash accounts for more than 70 % of total ash wastes, with bottom ash accounting for the remaining 10–20 % [[177], [178], [179]].
Recycling these wastes into fine and coarse aggregate has lately been adopted as the most effective waste disposal option worldwide, and the widespread use of recycled fine and coarse aggregate reduces the need for natural resources, making the building sector more sustainable [40,180,181]. Natural mining resources are depleting, and waste disposal locations become scarce, especially in developed countries. As a result, waste recycling and reuse are unquestionably required in terms of environmental conservation and resource usage. The ways to increase the performance and quality of recycled waste aggregate replacement have received a lot of attention in recent decades to facilitate waste management efficiently. In recent years, several researchers have recommended using a CO2 curing process to increase the quality of recycled waste as a replacement for natural aggregates. Table 4 highlights laboratory scale conditions from the revised studies that attempted to improve the characteristics of reused waste using CO2 curing. According to the literature, the standard carbonation method and pressured carbonation method are the popular procedures for recycled aggregate carbonation. By raising the CO2pressure, pressurized carbonation technology is created. The aggregates were first set in a drying room to reach an acceptable moisture level before being put in a chamber with a temperature of 25 ± 3 °C and humidity of 50 ± 5 %, respectively.
Table 4. Summary of laboratory processing of carbonated aggregates.

Much research has been carried out to investigate the characteristics of recycled concrete aggregates and produced carbonated recycled concrete aggregates. The formation of CaCO3 by a carbonation reaction is primarily responsible for the enhanced properties of recycled concrete aggregate. Pretreatment may result in more thorough carbonation and a noticeable improvement of recycled aggregate properties [40,182]. Various pretreatment techniques were provided in order to assess their impact on the resulting aggregates. The mechanical distinctions between dry and watery carbonation are poorly comprehended. Furthermore, normal curing under room temperature or high relative humidity before CO2 exposure results in pellets that become 60–80 % stronger than those cured under moist curing (28 days). The water absorption values of the pellets are lower due to the formation of a dense layer of calcite (CaCO3)which will result in a lower water absorption value [29,183,184]. Another study looked at the effect of different pre-soaking solutions on the properties of carbonated recycled aggregates, and the results showed that aggregates pre-soaked in calcium hydroxide had the highest content of carbonated compounds, as well as the lowest powder content and water absorption, regardless of calcium hydroxide concentration [185].
The findings of the various studies (summarized in Table 5) revealed that when the particle size is small and crushed material is alternately wetted and dried, the amount of captured CO2 has significantly increased [186,187]. Furthermore, because recycled aggregates have smaller particle sizes, they have a greater specific surface area exposed to CO2 curing, as well as a reduction in water absorption and an increase in bulk density with the decrease in particle sizes of carbonated recycled aggregates [188].
Table 5. Summary of mix details and main findings of carbonated aggregates.

The CO2 concentration is also an essential part of the CO2 curing of recycled aggregates. When the CO2 concentration was greater than 20 %, the growing CO2 concentration had less of an influence on the concrete carbonation percentages, according to the data [185,189]. Furthermore, the carbonation percentage of recycled aggregates with a CO2 concentration of 100 % is virtually the same as that of recycled aggregates with a CO2 concentration of 20 % after 28 days of carbonation. This is due to the fact that carbonation percentages are governed by the rate of hydration product dissolution rather than CO2concentration; also, high CO2 concentrations result in a thicker microstructural matrix, which reduces CO2 diffusion [189,190]. Increasing CO2 concentration to 100 % will also result in the decalcification of C–S–H, which has an undesirable influence on the characteristics of carbonated aggregate [67,191]. However, raising the CO2 pressure did not significantly enhance the carbonation efficiency [57,67]. Water absorption, for example, has clearly reduced when CO2pressure increased from 0.1 bar to 5 bars; however, the crushing strength dropped as CO2 pressure increased [188].
Aggregate carbonation efficiency tends to rise with CO2 curing time until the aggregates are completely carbonated. CO2 curing of treated recycled concrete aggregates has improved physical qualities such as apparent density, water absorption, and porosity when compared to untreated aggregates [[192], [193], [194]]. Changes in the shape and a process of lowering the size of cracks were seen in all studied samples as CO2 curing progressed [183].
The usage of steel slag and miscanthus powders in conjunction with CO2 curing may be able to address the problem of cement and bio-fiber aggregate incompatibility [195]. This approach might be utilized with a variety of biomaterials, including coconut fibers, hemp fibers, and sawdust. Miscanthus powders can increase the number of CO2 transit channels in the aggregate formed, resulting in quicker carbonation of steel slag particles. A high proportion of miscanthus powder, on the other hand, may result in a porous aggregate structure as well as insufficient strength. When compared to traditional carbonated steel slag, the 10 % miscanthus powder sample performed the best in terms of strength. More research is needed into the durability and performance of carbonated aggregates in a variety of conditions, including as hygrothermal qualities in high humidity settings and fire-resistant.
3.6. CO2 curing for ash-based blocks and cement-bonded particleboards
The concrete hollow blocks are made out of a mixture of cementitious materials, sand and aggregates. It has become a key role in the modern building sector by minimizing cement demand in masonry work and lowering construction costs [199]. Previous research has examined the utilize of carbonation as an alternative to traditional hydration for cement-bonded particleboards and bricks made from recycled materials in order to speed up the production process. The basic preparations for laboratory and mix design setup are summarized in Table 6. In general, samples with a low water-to-binder ratio were chosen to avoid water leakage during pressure application during casting and molding [200]. Increasing the CO2 pressure and exposure duration within a specified range is beneficial to boosting the reaction rate [66]. Carbonated cement-bonded particleboards have equal, if not greater, mechanical, durability, and physical characteristics to their air-cured and hydrated counterparts, Fig. 12 depicts the effect of various curing conditions and mixture details on the flexural strength. CO2-cured samples air-cured for 7 days had the same strength as products air-cured for 28 days. As CO2-cured composite alternatives, fly ash and blast furnace slag were considered. Because CO2 gas carbonated both free CaO and MgO to form calcite and magnesium calcite, the combination of blast furnace slag or fly ash with CO2 curing resulted in a synergistic strength increase. The ability of CO2 curing to boost binder-fiber interface intactness, decrease surface microcracks, and populate the capillary with freshly formed carbonate components is ascribed to this increased performance [80,[201], [202], [203]].
Table 6. Summary of mix details and laboratory processing of waste-based carbonated blocks and particleboards.

 Fig. 12. Flexural strength of particle boards with different materials under several curing conditions [201].
Fig. 12. Flexural strength of particle boards with different materials under several curing conditions [201].When the cement was replaced by sludge generated after drinking water treatment, similar trends in mechanical metrics were observed, however, the strength was lower because of the more porous and crystalline microstructure [204]. The blocks' durability was increased by reducing water capillary absorption and enhancing resistance to sulfate assault [59,204]. The primary downside of CO2 curing was the increased leaching of aluminium and copper ions, especially in the beginning. Despite this, the leaching concentrations were within allowable levels, proving that carbonated concrete blocks made from drinking water sludge were ecologically friendly building materials.
A further experiment discovered that CO2 curing enhanced the compressive strength development rate early on, but the increase in strength later on was minor [200]. Metakaolin enhances CO2 absorption in CO2-cured paste samples, with a peak at a substitution rate of 30 %. Early CO2 curing and the usage of metakaolin can lower the environmental effect, according to a carbon footprint study of the environmental impact of the generated samples. CO2 curing was used to increase compressive strength for recycling contaminated sediment blocks by accelerating carbonate and hydrate formation and densifying the porous structure [80,205]. More moistening and air curing allowed for additional chemical modification and improved the physical properties of sediment blocks by increasing CO2 reactivity and diffusion in the hydrated cementitious matrix pore network [206]. Ca2+ dissolution from calcium-containing phases such as (CH), CO2 hydration to generate H2CO3, and solvation of gaseous CO2 to form aqueous CO2 all require water. When there is inadequate water, CO2 and CH do not ionize entirely [72]. However, when enough moisture is present, the carbonation process improves, resulting in the formation of pore-filling solid CaCO3 in the wet cement pore [18]. The addition of magnesium oxide cement significantly improved the metal immobilization and leachability, ensuring that all sediment blocks satisfied the acceptance requirements for on-site reuse [207]. The results of the toxicity characteristic leaching method showed that all of the binders efficiently immobilized the pollutants in sediments.
3.7. Review studies on waste-based construction materials incorporating CO2 curing
As evidenced in Table 7, which presents an overview of review papers resulting from the systematic data extraction method applied to the reviewed articles, a substantial body of research has been published with the primary objective of unraveling the intricate carbonation mechanism and enhancing the reaction process across a diverse spectrum of construction materials and binding agents. These extensive publications collectively aim to contribute to a deeper comprehension of carbonation and its multifaceted applications within the construction industry.
Table 7. List of previous reviews published on CO2 curing for construction materials.

Notably, the concept of CO2 sequestration, storage, and utilization within the construction sector has received considerable attention in prior reviews, some of which provide in-depth analyses of this subject. Moreover, significant research efforts have been directed towards investigating the carbonation processes, conditions, and the resultant physical and mechanical characteristics of cement-based materials. An interesting conclusion drawn from these studies is that the swift development of strength in cement-based materials leads to enhanced efficiency in the manufacturing of carbonation-cured products.
However, there is a noticeable gap in the literature when it comes to review papers addressing waste binder technology. This gap presents a significant opportunity for future research endeavors. In order to streamline research and laboratory work, it is advisable for researchers to publish review articles from a variety of perspectives. These may encompass pre-curing product performance, detailed descriptions of curing conditions, and post-curing product performance. A focus on comparative analysis across various types of construction materials, particularly structural concrete, can significantly enrich our understanding of this technology and its underlying phenomena. VOS viewer software clustering technique was used to validate the keyword co-occurrence since it includes algorithms that surpass other algorithms in terms of performance and time complexity to get a better understanding of the research gaps. Keyword co-occurrence is used in its logical architecture. The keywords are all terms that may be used to describe the core of a research article and stronger relationships can be expected between them. A data file in research information systems (RIS) format was loaded into VOS viewer to assess the co-occurrence of the term in the screened studies, as appeared in Fig. 13. The keywords map was divided into eight clusters, each of which depicts the network of relationships between them, application scenarios, and developing technologies.
 Fig. 13. Keyword's co-occurrence map.
Fig. 13. Keyword's co-occurrence map.To create a clear analysis of the studied components in the screened research. Fig. 14 presents the map created using data reported in the screened studies, the taxonomy highlights the binder used in construction materials cured using CO2 technology. Since it plays an important role in the carbonization process and the final product's efficiency. Obviously, a wide range of laboratory-scale research has been conducted to investigate the possibilities of employing fly ash as a material for CO2 sequestration in full or partial as a replacement of cement or fine aggregates. These materials deserve particular attention to be reused in several types of construction materials; because they are generated in large amounts and have a very low reuse rate (see Fig. 15).
 Fig. 14. Types of binders used in the screened studies.
Fig. 14. Types of binders used in the screened studies. Fig. 15. The circular economy system diagram with reflection of the conceptual framework of the research.
Fig. 15. The circular economy system diagram with reflection of the conceptual framework of the research.In addition to insightful perspectives, there is a pressing need for descriptive statistics to elucidate and comprehend the uses and outcomes of employing different materials and procedures. The dearth of comprehensive comparisons within the existing body of work necessitates a more cohesive framework for researchers to evaluate and select the most suitable carbonation applications according to their specific requirements.
This review distinctly sets itself apart by employing a rigorous systematic review procedure, which not only enhances the credibility and contemporary relevance of the studies but also minimizes the potential for random evaluations. Furthermore, this review undertakes the crucial task of highlighting the significant strides made in recent years while also acknowledging the challenges faced in the field. It makes valuable contributions by proposing potential solutions to facilitate the widespread adoption of carbonation technologies. The overarching aim is to lay the groundwork for future research in this dynamic domain, providing essential recommendations and practical guidance on the effective utilization of CO2 in construction, ultimately paving the way for its broader and more impactful application.
3.8. Advancements in CO2 curing: modeling and technologies
3.8.1. Modeling of phenomena involved in the CO2 curing process
CO2 curing process in SCMs is an intricate and multifaceted occurrence characterized by a series of complex chemical reactions and changes in the microstructure. CO2 from the surrounding environment infiltrates the surface of the concrete or mortar. It initiates a sequence of reactions that ultimately lead to the creation of CaCO3 and the modification of material properties as previously discussed. This section explores the ability to model these phenomena, shedding light on the underlying mechanisms of the carbonation process.
Few attempts have been made to simulate and measure the benefits of the pressurized CO2 curing of concrete waste, despite its significant environmental advantages. Various modeling techniques have been employed to analyze the intricate phenomena intrinsic to the carbonation process in different types of construction materials. This diverse range of modeling methods includes mathematical equations designed to forecast the carbonation front of ordinary concrete [[211], [212], [213]].
Moreover, diffusion models have been utilized to facilitate the simulation of CO2infiltration into the material and its subsequent migration throughout the concrete matrix. These models play a crucial role in simulating the carbonation behavior of mortar samples during CO2 curing, based on the one-dimensional diffusion of CO2 gas. However, these models are not suitable for assessing the amount of CO2 uptake in concrete waste due to their higher specific area [57]. Nevertheless, they contribute to our understanding of how CO2 permeates the material and how this affects both the depth and extent of carbonation.
These models are essential in providing detailed insights into the impact of microstructural changes on the mechanical and transport properties of the material. Thermodynamic modeling has been employed to examine changes in pore structure and the capacity of CO2 binding. The thermodynamic modeling predicts the carbonation of Ca(OH)2, followed by the carbonation of the C–S–H phase [57]. This hybrid modeling approach offers a comprehensive perspective on the chemical foundations of the carbonation process. Additionally, it predicts a two-step decalcification process of the C–S–H phase, where calcium is initially released from the interlayer of the high-Ca C–S–H, resulting in the formation of a decalcified C–S–H phase. In the second step, Ca is released from the decalcified C–S–H, leading to the final decomposition of the C–S–H phase. Thermodynamic modeling has also been used to predict the formation of denser sodium carbonates at CO2 concentrations below 1 % and sodium bicarbonates at higher CO2 concentrations. These predictions align well with experimental observations [214].
By enhancing the CO2 uptake potential and quantifying the environmental impact of CO2-treated construction materials, these models may offer a practical approach for building engineers and industries to capture and utilize CO2. Therefore, to gain a better understanding and measure the environmental benefits of pressurized CO2-cured concrete waste, it is crucial to establish an appropriate model for evaluating the amount of CO2 uptake as well as introducing machine learning in this field.
3.8.2. CO2 curing technologies and innovations
The domain of CO2 curing has observed the emergence of several innovative technologies and advancements that possess the potential to enhance the performance of waste-based materials. Noteworthy among these is the utilization of nanotechnology, which entails the integration of materials at the nanoscale, such as nano-silica and nano-TiO2, to expedite carbonation reactions [65,[215], [216], [217]]. It ultimately augments the mechanical and durability properties of waste-based construction materials. Recent research has demonstrated that the inclusion of nano-TiO2 changes the capture of CO2 in solidified cement pastes. It has been suggested that the use of less than 1 % nano-TiO2 by the total weight of cement promotes the natural carbonation process of mortar samples within 7 days [218]. Furthermore, a study has indicated that the utilization of nano-TiO2 may enhance the uptake of CO2during curing while concurrently enhancing the strength of the material [65,219]. Both effects can potentially be attributed to the enhancement of the microstructure facilitated by the nanoparticles. Also, alterations in the microstructure of material pastes may extend an influence on the self-cleaning activity of the material [220,221].
Digital and artificial intelligence (AI) also assume a pivotal role, facilitating precise control and customization of the carbonation process to accommodate the characteristics of waste-based materials. Prior to the mid-1980s, models for the depth of carbonation in concrete were developed through linear and nonlinear regression, taking into account various factors such as mix details and exposure conditions [211]. With the increasing utilization of AI and ANN in various fields particularly in the realm of carbonation depth [151,212,213,222]. Researchers began incorporating physical-chemical formulations pertaining to the hydration reactions of paste and the dissolution of CO2 within the matrix, enhancing the evaluation of carbonation depth [151,223]. ANN has become common practice in the modelling of carbonation phenomena to mitigate uncertainties in the models [[223], [224], [225], [226]].
Carbonation accelerators represent another promising development, facilitating the rapid carbonation of waste-based materials. These accelerators influence the carbonation of lime pastes and mortars, resulting in shorter setting times and higher mechanical strength [227]. However, in some cases, shrinkage may be considered a drawback. Carbonation accelerators such as ceramic dust, TiO2nanoparticles, and synthesized aluminosilicates have been employed [219,228,229]. The addition of nano-TiO2 to lime mortars led to a higher concentration of CO2 in gaseous form on the surface of the material. This resulted in significant improvements in compressive and flexural strength, as well as resistance to weathering parameters.
In addition, alkali-activated binders, which frequently employ waste materials, can be strategically designed to enhance carbonation, thereby reducing the carbon footprint and simultaneously improving material performance. The impact of CO2 curing is reliant upon several factors, including the type of binder, the water-to-binder ratio, the type and concentration of the activator, and the specific conditions under which curing takes place (e.g., temperature, relative humidity, CO2 concentration, and curing time) [[230], [231], [232]]. Specifically, an enhancement in strength is observed with CO2 curing when a paste sample is activated through a combination of NaOH and Na2SiO3 in a 5:5 ratio [120]. Conversely, the compressive strength of NaOH/Na2SiO3-activated slag mortars exhibit a decline following CO2 curing [233]. Moreover, CO2 curing at a consistent CO2 concentration proves to be more effective than when conducted within a CO2-pressure vessel [234]. Advanced quality control and monitoring systems equipped with sensors have emerged as vital tools in real-time tracking and optimization of the carbonation process for waste-based materials [235].