1. Introduction
The transport phenomenon in process engineering is concerned with the exchange of mass, energy, momentum and or charge between observed and studied systems. The fundamental analysis of these phenomena is based on the principle of conservation between the system and the environment, hence the different phenomena responsible for the transport of the quantity are considered independently having in mind that their effects sum up to zero. The concept of transport phenomena is grounded on two basic principles: the conservative principle and the constitutive principle. The conservative principles are described by the continuity equations, while the constitutive principle deals with the response of the system to changes in stimuli. and the encompasses all aspects of physical change, however, the scope is limited to artificially engineered systems.
Mass transfer is an aspect of transport phenomena that deals with reaction, separation, heat transfer and electrochemical systems [1]. Mass transfer systems in cases involving diffusion are generally governed by Fick's law which states that; the diffusion flux from a region of higher concentration to a region of lower concentration is proportional to the concentration gradient and the diffusivity of the quantity within the medium. There are four ways by which transfer can occur: pressure gradient, forced diffusion, temperature gradient, and chemical potential [2]. The general mass balance for a quantity with a system is given by the relation:(1)
The pulp consists of fibres, usually acquired from wood. The pulping processaims at separating the fibres fixed in the wood or plant matrix, this can be achieved either mechanically or chemically. Mechanical methods require so much electric power, but on the other hand, they make use of practically the whole wood material; i.e there is a high yield of the process. In chemical pulping, only about half of the wood is converted to the pulp, the other half is dissolved in a modern chemical pulp mill, however, there is no demand for external energy. For a chemical process to be economically feasible, it has to consist of an efficient recovery system. Spent cooking chemicals and the energy in the dissolved organic material is recovered. The pulp obtained is coloured, the degree of colouring is dependent on the pulping process. For certain paper grades, the dark pulp is expected to be bleached, bleaching leads to whiter paper, this gives better contrast between the print and the paper. Also, bleaching leads to cleanliness, as beaching removes impurities that otherwise turn up in the paper as dots, and age preservation, as bleaching can remove chemical structures in the pulp material that otherwise in time make the paper yellow [[3], [4], [5], [6]]. Paper technology on the other hand involves the knowledge required in the unification of the fibres to form the paper web. The pulp can be converted into several products. Paper is one of the major products of pulp, it is required for conveying information in magazines, newspapers, and books. In the case of hygienic products, the property of the pulp to absorb fluids is of significant importance whereas for other paper products such as those used for printing, the strength property of the paper is of significant importance as it gives the surface ability to accommodate layers of paints [7]. Furthermore, paper strength is a major feature in paper products aimed to keep and protect other commodities, dry or liquid [[8], [9], [10]]. Paper has lots of advantages compared to other materials competing with paper for renewable raw material(wood or other plants), the pulping and papermaking processes have low effluents to the recipient and the paper packages are easy to recycle [[3], [4], [5], [6]].
This study examined the utilization of mass transfer in the pulp and paper industry, specifically focusing on the challenges, advantages, operations, and future prospects. Mass transfer plays a crucial role in various processes involving reactions, separation, and heat transfer. In the context of producing pulp and paper from raw materials, these processes are essential. The application of mass transfer in these processes is significant as it helps establish target yields, specifications, and enhances efficiency. The key processes where mass transfer principles are employed include drying, chemical washing, pulp digestion, and pulp bleaching. The scope of this study specifically centers on Kraft pulping, which is the dominant pulping method worldwide. The Kraft process is highly relevant due to its ability to produce strong pulp, conserve energy, recover chemicals, reduce wood demand, and improve wood quality [11,12].
2. Methodology
The method adopted for the study involved an extensive literature review on the subject matter (Mass Transfer in the Pulp and Paper Industry − Challenges, Benefits, Processing and Prospects). A brief overview of the pulp and paper industry is presented in section 3 of this article, with subsections that describe the state-of-art of the pulping process and critically review the mass transfer operations occurring in each major equipment/unit (preprocessing, drying, chemical washing, pulp digestion and pulp bleaching unit). Also, a case study analyzing the mass transfer mechanism in the Anthraquinone (AQ) pulping process was presented in section 3. In section 4 of this article, the challenges associated with transport processes in pulping, environmental considerations and prescribed solutions were highlighted. Section 5 gives deep insights into the futuristic technological advancements in the mass transfer and prospects of the pulping process. A summary/conclusion of the findings of this review article is then provided in section 6. The sources used included the internet, previous reports and publications of notable researchers (including the present authors) on the application of mass transfer in the pulp and paper industry, principally the challenges, benefits and prospects. The peer-reviewed publications were searched from scincedirect.com, Google Scholar, and Stanford Search works, and references from relevant articles using the search phrases (sources of environmental pollution, forms of environmental pollution, adverse effects due to environmental pollution etc.). Only articles published in the English language were included.
3. Overview of pulp and paper industry
Speculations indicated that at the dawn of the digital age, there would be drastic growth in the use of computers hence, a resultant decline in the use of papers on a global scale [13], however, in the early 2000s, this was not the case because there was increased demand for printing, publicity, record management, communication and writing papers [14]. But in recent years the graphic papers (printing and writing, newsprint) and fibres have been experiencing a steady decline in demand, but this decline has been supplemented by increased demand for packaged
material goods requiring the use of paper and hygiene products [15]. Although the factors that affect the demand for paper associated products vary from one country to another, where some regions are experiencing more growth in demand than others and vice versa, reports still show prospects of growth potential in the pulp and paper industry today in future. The pulping industry has not been able to completely overhaul the conventional pulping processes with new techniques, however, the industry has improved the traditional processes to enhance yields and quality of the pulp.
Kraft pulping process makes up the largest share of the global production of virgin pulp for papermaking [16]. This is due to the better chemical recovery, its ability to work effectively for both hard and softwood feedstock, and also the production of high-strength pulps [17]. Kraft pulping usually produces better pulp strength and paper quality compared to the mechanical processes, reusability of chemicals used and higher energy efficiency and the products can be used to make viscose [18]. The pulping methods that involve the use of chemical agents as implemented in the Kraft process are known to produce a paper of higher quality than mechanical pulping processes [19].
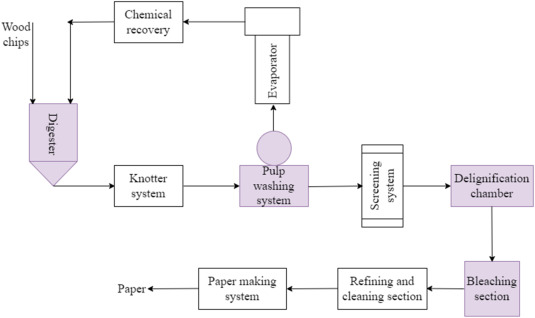
Pulp and paper are made from cellulosic fibres and plant materials such as wood fibres, rags, cotton litres, sugarcane bagasse and flax. Wood, a major feedstock for pulp and paper processing consists of 50 % cellulose, which is the target feed component for paper and pulp processing [20]. Lignin is a chemical component that binds the cellulosic fibres in wood together and the process of paper processing is done in such a way as to remove as much lignin as possible without compromising fibre integrity so also eliminating impurities that may affect the quality of the paper. The manufacture of the paper consists of 2 major steps consisting of various processes within them. Fig. 1 shows the first step that involves the process of converting the feed to the pulp, and the second step involves the transformation of the pulp to paper. The operations and the processes involved are as follows [21]:
-
1)
Material Preparation: This involves debarking and chipping of the wood, screening, and storage.
-
2)
Pulping: This involves the removal of lignin from the cellulosic fibres. This can either be carried out with the aid of chemicals or by mechanical means at high temperatures.
-
3)
Pulp washing: This involves the process of obtaining unbleached pulp free of unwanted compounds by countercurrent washing with water and secondary condensates.
-
4)
Pulp screening, cleaning and fractionation: This involves the removal of oversized particles called shives from the fibres.
-
5)
Bleaching: This involves the reduction of the remnant lignin content to increase the brightness of the pulp by using various bleaching chemicals.
-
6)
Chemical recovery: This involves the reclamation of chemicals used within the pulping process and combustion of remnant woody materials separated from pulp to generate steam and electricity for the process.
-
7)
Stock preparation and paper making; This involves all of the operations put in place to ensure pulp properties meet product specifications. The operations practised in the paper mills are Dispersion; Refining Metering and blending of fibre and additive.
 Fig. 1. The paper manufacturing process [22].
Fig. 1. The paper manufacturing process [22].3.1. The state-of-art in pulp and paper
The digester in the manufacturing process is a region of the most chemical process associated with the mass transfer because lignin and lignocellulosic materials are removed to free the fibre of the wood without compromising quality. This is where most of the pulping occur and the most dominant pulping process is the Kraft process [23].
Wood species composition cannot be generalized because their composition varies with the weather, geographical location, soil and region within the tree in which the wood was derived, although the composition of wood typically ranges from is made up of 40–50 % cellulose, 20–30 % hemicellulose, 20–30 % lignin and 10 % other substances [23].
The Kraft process uses an alkaline cooking liquor with a pH value close to 14. The composition of the cooking liquor is; sodium sulfide, sodium sulfate, sodium hydroxide and sodium carbonate.
The amount of extractives in wood varies from 5 to 20 % by weight including a variety of organic chemical compounds. The Kappa number is a dimensionless number used to measure the lignin content of wood pulp defined as a mass fraction of lignin * 100/0.15.
As we are approaching a more accountable and responsible production process, the importance of ozone bleaching for paper production has gained a lot of attention in recent years [24] most especially as a replacement for the conventional chlorine bleaching process [25]. The mass transfer process is quite complex consisting of solid-chemical absorption, and gas-liquid mass transfer, followed by a self-decomposition and oxidation process [26,27].
The issues associated with climate change and conservation of forests have ignited interest in other non-wood sources of pulp such as straw, plant fibres(bamboo), bagasse and fibre crops (kenaf) as alternatives to wood fibres for sources of raw materials used in papermaking [[28], [29], [30]]. Generally, non-wood plant fibres are costly to collect and process compared to wood fibres, especially in regions where wood supplies are abundant. Non-wood fibres are easier to cook compared to wood fibres and require fewer chemicals [31]. In such cases, the Kraft process would be best replaced with exclusively soda cooking.
As a result of the facility cost, and environmental and health issues associated with chlorine bleaching agents, oxygen delignification process is now being utilized as a pre-bleaching agent [32]. The two-film theory is popularly used to describe the mass transfer kinetics occurring, which considers two film layers; liquid film and gas film [33] (Fig. 2). A simplification of the process as a dilute-solution-chemical-absorption-diffusion model was used to describe the kinetics [34].(2) is the rate of change of ozone numbers,
 Fig. 2. The two-film theory model.
Fig. 2. The two-film theory model.KG is the overall mass transfer coefficient for gas film,
KL is the overall mass transfer coefficient for the liquid film,
PAG is the ozone pressure in the gas phase,
PAi is the ozone pressure in the gas-liquid equilibrium interface,
CAi is the ozone concentration in the gas-liquid equilibrium interface,
CAL is the ozone concentration in the liquid phase,
a is the interfacial area,
V is the volume of the system.
The film theory takes its background from three hypotheses [33]:
-
i)
That a stagnant interface exists between the liquid and the gas phase and diffusion takes place from the gas to the liquid phase.
-
ii)
The equilibrium composition of the gas and liquid phase exists at the interface
-
iii)
That the uniformity of the composition exists in the gas or liquid phase outside the interface at the turbulent regimen.
The amount of ozone that has transferred into the pulp can be represented by the equation:(3)(4)
N is the mass of the ozone transferred,
H is the solubility coefficient,
t is the diffusion time,
KL is the overall mass transfer coefficient for the liquid film,
kL is the liquid mass transfer coefficient,
kG is the gas film mass transfer coefficient,
CAL is the ozone concentration in liquid film,
CA* is the ozone concentration in equilibrium between the liquid and gas phase,
X is the distance to the fibre surface,
C is the ozone concentration,
a is the interface per unit of volume,
V is the solution volume.
Since the solubility of ozone in water is nearly zero, it can be said that the film layer would be of major relevance to the resistance of mass transfer [33], Hence, to improve the efficiency of mass transfer, it would therefore be necessary to reduce the film resistance.
The diffusion of ozone into the cell wall is guided by Fick's second law as;(5)
The significance of kinetics studies in the ozone bleaching process helps in determining and improving the efficiency of mass transfer and also determining and improving the delignification efficiency. However, this study is not the only factor for achieving the efficiency and delignification targets, the importance of pH, chemical additives, and other factors are usually considered as these would also impact the model and equipment design for the process.
Ozone, being a strong oxidizing agent is known to reduce the viscosity of bleached pulp and is reported to reduce chemical requirements by 20–30 % relative to the Kraft process. Ozone is known to decompose rapidly and has raised issues of low efficiency and selectivity for lignin oxidation, hence causing a depletion in fibre strength for designs that require further alkali bleaching [[33], [34], [35]] (see Fig. 3).
 Fig. 3. Diagrammatic representation of high concentration ozone bleaching process.
Fig. 3. Diagrammatic representation of high concentration ozone bleaching process.(a. Cellulose conc. change, b. ozone conc. change, c. Lignin conc. change)
Reports have indicated that ozone oxidation can generate highly oxidizing HO−and HOO− radicals [36]. These radicals operate such that they oxidize cellulosic reducing terminals and alcohol-hydroxyl groups into carboxyl and carbonyl groups respectively.
Research had shown that Hydroxylamine has a high diffusion rate during the bleaching process and can access the pulp fibre and react with the cellulose and hemicellulose content of the pulp before the ozone reaction takes place. Hydroxylamine occupies the active cellulosic sites leaving lignin for the ozone which is preferable to ozone; all of this occurs under the influence of the accelerative diffusion characteristics of hydroxylamine leading to a lower decomposition of ozone before it reacts with lignin [33,37].
Paper is typically considered a porous medium and the drying process involves multiphase regions of liquid-solid and gas. The moisture content ranges from 45 to 65 % on a wet basis after passing through the presser, to reach the target moisture content so that the paper would have the desired strength, the dryer is supposed to drop the moisture content value to about 8 %. To show the importance of the drying section in paper manufacture, about 34 % of all industrial facilities' investment in the process is dedicated to the drying process [38].
In the Kraft process of digestion, it has been known that within a range of temperature and pressure, cellulosic contents of low molecular mass dissolve well in lye [39]. However, for cellulosic contents of higher molecular masses, pretreatment is usually needed to get the desired solubility such as enzymatic pretreatment, steam explosion and hydrothermal pretreatment help to dissolve cellulose to a pulp consistency of about 8 % [[40], [41], [42], [43]].
The delignification of the Kraft process occurs in three major phases. The first phase involves the extraction of about 20 % of both lignin and carbohydrate into the lye solution, this phase has been known to affect the kinetics to a great extent with high lignin selectivity until after about 90 % lignin content has been removed (second phase) [43]. The third phase involves the removal of the remaining lignin at the expense of a large loss of carbohydrate and is interrupted at a transition point to retain pulp quality. As shown in Fig. 4.
 Fig. 4. Selectivity relation in the removal of carbohydrate and lignin on Kraft process of softwood [43].
Fig. 4. Selectivity relation in the removal of carbohydrate and lignin on Kraft process of softwood [43].The chemistry guiding the delignification process has been derived from lignin model compounds, simulation of substructures in lignin and analyses of polymeric lignin. These studies have helped to conclude that the fragmentation of polymeric lignin and the presence of hydrophilic groups are requirements for the removal of lignin from pulp [43,44]. The hydrophilic group such as hydrosulphide ions help in the fragmentation of the cleavage connecting the phenylpropane units by the β-O-4 linkage [[43], [44], [45]]. The alkaline conditions the benzyl alcohol structure equilibrates with the quinone methide. In the presence of hydrosulphide ions, a further equilibrium condition leads to the formation of a benzyl thioalcohol structure. The formation of benzyl thioalcohol structure in a nucleophilic reaction attacks the β-carbon atom leading to the formation of episulphide and a new phenolic end group [46]. The episulphide structure being unstable hence releases sulphur to form polysulphide in the liquor. Furthermore, under alkaline conditions, polysulphide forms hydrosulphide and thiosulphate ions.
The kinetics of cleavage of β-O-4 is that it is independent of hydroxide and hydrosulphide ion concentration, however, the non-phenolic β-O-4 are influenced by hydroxide ions [45]. As shown in Fig. 5. The reaction of carbohydrates in the pulp during the Kraft process usually results in large yield of diffusion, especially during the initial stage of the cooking process [47,48]. The chemistry of the process involves a peeling reaction where successive de-polymerization of polysaccharides occurs, of which the process initiates from the reducing end group. At temperatures greater than 170 °C, more random alkaline hydrolysis most especially of the glucosidic bonds takes place.
 Fig. 5. Reaction progression for the cleavage of phenolic β-O-4 in lignin in the Kraft process [43].
Fig. 5. Reaction progression for the cleavage of phenolic β-O-4 in lignin in the Kraft process [43].The peeling reaction occurs with polysaccharides containing glucomannans, xylans and cellulose, the process occurs such that the reducing end of the polysaccharide forms and equilibrium between the hemiacetal and the open aldehyde from, in the presence of alkali, further equilibrium is formed between the aldehyde and keto form [49] See Fig. 6. For the first equilibrium case, the reaction would result in a stable polysaccharide chain. An example of a stable polysaccharide is the glucoisosaccharinic acid in softwood Kraft pulping liquors which is known to contain about 1 kg per 10 kg of pulp.
 Fig. 6. Peeling reaction mechanism [43].
Fig. 6. Peeling reaction mechanism [43].Although Anthraquinone's main use has been for the stabilization of carbohydrates against the peeling process in which it serves as an additive, however, Anthraquinone pulping is known to act as the catalyst that boosts the delignification rate as well as its yield [50].
3.2. Mass transfer in pulp and paper industry
Mass transfer in the pulp and paper industry has been of utmost importance and applied in the major process involved in the conversion of raw materials to finished products. The mass transfer is used to determine feed ratios, set target yields, set desired specifications and improve the efficiency of the process. Some of the areas where the mass transfer is applied are discussed.
3.2.1. Preprocessing (chipping and debarking)
The chipping process after debarking is very important in the production of paper, such that the chipping process which involves the reduction of log sizes into small bits is highly dependent on the homogeneity of the chips [51]. This homogeneity of the chips is important because if there isn't that way, there would be more raw material consumption, and the energy requirement of the system would increase as a result of heat localization within the system [51]. The screening process with follows the chipping process is also important because this process involves the removal of chips that are not properly chipped leading to long sizes, as well as sawdust [52]. The sawdust can be burnt to produce energy or sold and the rejected long-sized chips are sent back for re-chipping [52]. If these processes are gotten right, they can go a long way in the production of high-quality chips, reduce waste from wood raw materials and improve the environmental impact associated with energy and chemical waste. Although this process is not integral to mass transfer processes, however, it is of importance because properly sized and configured allow for homogeneity, hence allowing reactions to occur all around as against only localized portions in the chips feed. This could have a significant impact on the yield and performance of the process.
3.2.2. Drying as mass transfer
The major consumer of energy in the pulp and paper industry is steam generation, which is mostly used in pulping and drying paper [53]. Drying is one of the major processes used in the pulp and paper industry before the desired quality structure of the paper can be obtained. It is a process of moisture content removal from the paper. Contact drying with steam-heated cylinders is the predominant method of drying in the industry [54]. It has been observed that asides from direct contact heat transfer for serving as an agent for inducing mass transfer, the role of air serving as the drying medium or as a moisture carrier cannot be overemphasized. The air medium around the paper surface constitutes the mass transfer aspect of the process. As regards the mass transfer aspect of drying, the factors that most influence paper drying operation are [54]:
-
1)
steam pressure;
-
2)
the humidity of the carrier air medium;
-
3)
mass transfer coefficient; and
-
4)
velocity.
Mass transfer occurs when a mass of water from the paper is moved to the air medium during drying. The driving force is usually concentration (partial pressure). It usually occurs in different modes; convection-diffusion, molecular diffusion and bulk movement. This process is shown in Fig. 7.
 Fig. 7. Mass transfer at interface [54].
Fig. 7. Mass transfer at interface [54].The ease of removal of moisture from the cellulosic fibre is fundamental for papermaking as this affects the rate of production as well as the consumption rate of energy. Drying is the most demanding part of the paper manufacturing process [55]. Two phases are involved; the first phase is the impingement of low-solids fibrous suspension into one or between a pair of fabrics that is highly permeable, here some of the water would escape just by gravity and inertia to flow out of the cellulosic mixture [56]. The next phase involves a subtle agitation of the developing paper web using hydrofoil, hydrofoil tends to jostle to wet web and frees up channels for drainage and also tends to make the paper uniformly distributed within the sheet plane [56]. The third phase involves the application of vacuum, usually utilizing vacuum flat-boxes and perforated rolls. When drying takes place, very low fibre content is initially used (∼2–3 %) of fibre water suspension is used, this is done to achieve a non-uniform fibre orientation. Thereafter, water content is removed by drying with the aid of gravity, suction and pressing. The final process of removal of water content is usually done by drying cylinders, which form the largest and most expensive part of the length of the pulp dryer.
The rate of molecular diffusion within the paper towards the air-paper interface is described by Fick's law;(6)
MA is the mass transfer of A per unit are, kg/hr.m2
Dv is the molecular diffusivity (area/time)
CA is the concentration of A
x is thickness.
For the vapour diffusing from the paper into the air carrier medium, the concentration gradient is expressed as a partial pressure difference;(7)
kg is the mass transfer coefficient.
Po partial pressure of water at the paper surface.
P1 is the partial pressure of water in the surrounding air.
-
a)
Properties of carrier air medium
Atmospheric air as the carrier medium is usually not dry and contains varying amounts of water vapour depending on the temperature and the humidity of the environment. Observations have indicated that under other similar conditions warm air has absorbed more moisture than cold air, thus making the importance of the condition of air in the drying process.
-
i)
The partial vapour of moisture in paper relative to air
Initially, during the drying process, the partial vapour pressure at the paper surface is the same as for a free water surface at a given temperature and the condition prevails as long as transport can bring new water to the surface replacing the water that is evaporated [54]. At the end of the process, the partial vapour pressure on the web surface is lower than that of the free water surface. The partial vapour pressure of the free water can be calculated using Antoine's equation;(8)
P is the partial pressure in kPa.
T is absolute temperature in Kelvin.
A, B, C, constants: A = 8.007131; B = 1730.630, C = 233.426.
-
ii)
Relative humidity
This is the ratio of the amount of water contained in the air and the total amount it could accommodate at the same temperature. When the relative humidity is 100 %, the air is said to be saturated, at this stage, the air can only accommodate more moisture if the vapour pressure of the water is greater than at the drying surface exceeds that of the moisture in the air at a particular temperature, however, under these conditions, a mist would be formed hence making the mass transfer medium an inefficient mass transporter. The best air carrier would be the one with the least moisture contained within it. The relative humidity can be calculated using the formulae;(9)
ps is the saturated vapour pressure of water at temperature, T
p is the partial pressure of the water vapour.
RH is the relative humidity.
- iii)
Absolute humidity is the moisture content of the dry air. Psychometric chartscould be used to obtain the humidity provided wet bulb and dry bulb temperatures are known. Alternatively, it can be calculated thus;(10)
H is the absolute humidity, H2O/kg dry air.
P is the partial vapour pressure
p is the barometric pressure.
Mw is the molecular weight of water.
Ma is the molecular weight of air (28.97)
-
b)
Sorption isotherm
As a result of the hygroscopic nature of paper, the partial vapour pressure at the web surface is known to be a function of local temperature and moisture content. A correlation between equilibrium moisture content and relative humidity at a particular temperature is a sorption isotherm. The sorption isotherm of paper depends on the paper fibres. The model that has been adopted to be best suitable for mechanical pulp [55].(11)
ρ is the relative humidity of the air inside the chamber/room/store.
C1 = 47.58, C2 = 1.877, C3 = 0.10085, C = 1.0585.
Z is the equilibrium moisture ratio.
T is the temperature.
3.2.3. Washing of used chemicals
The importance of washing in paper manufacture cannot be overemphasized due to its cost-saving and chemical recovering function. In the pulp and paper industry, there is usually a need for chemical recovery which is recycled into the process. This is also carried out to obtain satisfactory quality for subsequent processing. Countercurrent washing is generally used to obtain good recoveries and relatively low dilution ratios. Rotary vacuum filters are usually used and re-slurrying of the stock between stages. This idea here is to separate sodium and lignin from the pulp.
The purpose of washing is to remove unwanted solutes such as metals and solvents. The benefits include; the minimization of the losses associated with chemical usage in cooking the process, maximizing the recovery of organic substances for further processing or in the case of black liquor, for incinerationand obtaining a clean pulp product [57]. The idea behind washing works such that, the process is optimized to require the minimum amount of water possible. This is the conserve freshwater resources and reduces the cost of water treatment. The brown stock washing is to help in removing the maximum amount of liquor dissolved solids while using the least amount of water. If these dissolved solids are left behind, it would require more bleaching agents, and less energy recovery [58]. Excess washing water is costly because more work would be required by evaporators and dryers, hence increasing steam cost [57].
-
a.
Operational mechanism
The theory behind the washing process is similar to the one associated with the displacement of a liquid with another miscible liquid through a porous medium of a certain thickness which in this case is the pulp pad. The liquid phase is partly bound to the pulp fibre representing a stationary liquid, so also the liquid phase is partly moving. The flowing bulk fluid causes a displacement by acting on the free part of the fluid causing a substitution of that region due to channeling, variation in porosity and or fibre size distribution [59] as shown in Fig. 8. The displacing fluid operates such that it creates a concentration gradient driving force between the bulk flowing liquid and the stationary part of the fibre, which leads to the diffusion from the stationary part into the bulk flowing region. The efficiency and kinetics of this process are heavily dependent on the diffusion distance and the porosity of the fibre network [60].
 Fig. 8. A visualization of the displacement process [43].
Fig. 8. A visualization of the displacement process [43].The process is usually mathematically described by differential material balance over differential layer height Δz for the solute and hence the resulting equation is integrated over the total height z.
The rotary vacuum drum filter is used for the procedure of stripping the pulp of chemicals and particles present in the mixture. The pulp cake is continuously formed which is dewatered and washed before it proceeds further [61]. In the wash zone, mass transfer is induced as a result of spatially varying concentration gradients [61], where there is molecular diffusion of the molecules according to Fick's Law. In the wash zone of the rotary filter as indicated in Fig. 9, the pulp usually goes in the transverse direction for the flow of wash liquor. Sodium sorption on the pulp has been discovered to be pH-dependent due to the effects of ion exchange. Sorption can be neglected up to the 99 % recovery level. The concentration of each solute in the wash solution can be entered as an element in the column vector, (X), and the corresponding concentrations in the pulp mat can be entered as an element in the column vector, (C). At steady-state, if the sorption effects are neglected, the variation in solute concentration in the wash liquor in the direction normal to the cake surface can be represented by Ref. [61];(12)
 Fig. 9. Schematic diagram of the wash zone [61].
Fig. 9. Schematic diagram of the wash zone [61].The multicomponent mass transfer coefficient matrix [k], accounts for the diffusional interactions among the solutes and is governed in structure by the corresponding matrix of diffusion coefficients. The dispersion coefficient, D, is taken to be a scalar quantity, independent of solute identity, but dependent on mat geometry and wash liquor flow [61].
W is the volumetric flow rate.
is the column vector whose elements are the solute concentrations in the wash liquor within the wash zone
Xn is the column vector whose elements are the solute concentrations in the wash liquor leaving any point in the wash zone of the Nth stage.
Q is the volumetric rate of liquor held in a filter cake.
Cn is the column vector with elements, Ci
bn column vector whose elements are the solute concentrations in the slurried feed to the Nth filter.
3.2.4. Pulp digestion
When the wood chips feed are undergoing digestion in the pulping unit, it is desired to achieve consistent pulp quality despite unstable feedstock conditions. Furthermore, if the required level of delignification is not achieved during this stage, more chemical consumption would occur during the other unit operations and more toxic wastes would be released into the environment (Fig. 11). Here, the chips are allowed to be impregnated with the alkali so that the pulp can be formed for further processing. Extended impregnation time has also been used as a method of reducing reject chips to increase the overall yield of the pulp. The extended period allows for better penetration of the cooking liquor into the chips allowing for a more homogenous mixture.
As a result of the variation of effective alkaline composition (12–14 % for softwoods, 8–10 % for hardwoods), the supply of the appropriate concentration of alkali plays a major role in determining how well the pulp has been cooked and the rate of the cooking process [62]. The most effective way of achieving this is by utilizing the least required amount of alkali concentration needed to achieve the goal and varying the concentration of the cooking to achieve the desired cooking rate. This is necessary because higher alkali concentration could alter the composition of the retained cellulose, increased the cost of production and environmental impact [[63], [64]].
During the process of Kraft pulping, it is usually of utmost importance to ensure that the alkali is distributed homogeneously all around the wood chips [65]. The essence of the impregnation process is to give the process the residence time to allow for optimal penetration of the alkali into the wood to ensure that the pulp is uniform throughout, this adds to what makes pulp of higher quality. The penetration process involves the movement of the chemicals and other associated liquids into the air-filled cavities of the wood chips as a result of hydrostatic pressure until the fibres become saturated [[66], [67]]. This process is usually carried out at elevated tempreature such that hot steam is usually infused into the system (digester) or the liquor is first heated before being used on the wood chips. Chemical charge, cooking time, the temperature of cooking, and solid to liquid ratio are the major variable that is considered during the cooking [51], such that the yield and kappa number are controlled at the end of the process by optimization methods.
In Fig. 10, an aqueous mixture of sodium hydroxide and sodium sulphide is used to break the lignin away from the cellulose fibre producing pulp of the desired Kappa number (the measure of residual lignin content in the pulp). For a particular feedstock that has been characterized, mass balance calculations help to determine the concentration and the volume of sodium hydroxide/sodium sulphide mixture needed to get the target kappa number. So also, the properties of feedstock (such as composition) may vary from batch to batch of the wood feedstock which serves as a disturbance to a system for continuous processes. The control objective is to monitor the Kappa number to ensure that it is stable and one way of doing that for mass transfer is to alter flow rates (in the case, lye). This serves as a basis for the formation of models that are used to adjust process conditions to meet feed requirements [63].
 Fig. 10. Batch pulp digester [68].
Fig. 10. Batch pulp digester [68]. Fig. 11. Effect of H-factor on Kappa number and yield [66].
Fig. 11. Effect of H-factor on Kappa number and yield [66].The H-factor is the cooking time temperature needed to achieve a certain kappa number [65]. The H-factor can is shown as an area under the Kappa number curve as indicated in Fig. 12. This figure helps in predicting the kappa number [66]. Although the cooking process cannot remove all of the lignin present, without having a damaging effect on the pulp, this is why the bleaching process is necessitated after cooking in other to prevent rejecting pulp [69]. The connection between H-factor and kappa number is shown in Fig. 11.
 Fig. 12. Mass transfer involving oxygen as a bleaching agent [70].
Fig. 12. Mass transfer involving oxygen as a bleaching agent [70].To ensure that the alkali penetrates the wood chips properly, the right liquor to wood ratio must be employed. A sufficient amount of the white liquor would need to be added into the digester such the liquor to wood ratio ranges from 3:1 to 5:1, at ratios greater than this, there is the tendency for dilution of the mixture hence, decreasing the concentration of the active chemical, which resultantly reduces that rate of the reaction involed in the cooking process [68].
The delignification selectivity in terms of yield against kappa number, and viscosity against kappa number, is a major limitation to consider [71]. The selectivity of the process is highly dependent on the concentration of sulphide such that high selectivity targets would require high sulphide [72] concentrations, most importantly during the transition phase from initial to bulk delignification. The selectivity is usually lowered when a carbon-carbon bond cleavage of the b-c-linkage results in the formation of formaldehyde and styryl aryl structures [73], the sulphide ions help by inhibiting the fromation of these by-products. The effect of sulphide ions such as those from Na2S leading to higher yield and stronger pulp are significant until it has 15 % sulphide, beyond this concentration, the effects are less significant making work to be done on optimizing the yield with the cost of increasing sulphidity [63].
3.2.4.1. Mechanism of mass transfer in anthraquinone (AQ) pulping – a case study
Model systems have been developed to explore the mass transfer properties of AQ. Studies have indicated that AQ must be reduced to Anthrahydroquinone (AHQ) before any permeation of the membrane of the wood chips feedstock can occur. However, if the reduction permeation is known to only occur at high concentrations.
AQ itself cannot diffuse because of its insoluble characteristics but when it is reduced via redox reaction, to AHQ, it becomes soluble in pulping liquor, hence allowing diffusion to take place. However, there is also oxidation during the cooking in the digester, this makes AQ deposit out of the solution. In pulping, the reaction from AQ and sorption of the diffusion species on the chip surface must be taken note of. This leads to the modification of the Fick's second law of diffusion including both reaction and sorption terms:(13)
ε i is the void fraction inside the chip.
C is the concentration of AQ
ρ is the density of the chip
c is the concentration.
X chemical consumed by sorption
t is the time.
ECCSA is the effective capillary cross-sectional area
w is the thickness dimension of the chip.
To understand the kinetics of the process to know the residence time of the pulp in the digester that would favour the target kappa number, the well-established model of the soda-AQ delignification process can be expressed thus;(14) is the delignification rate
L is the Lignin content.
is the rate of constant for the delignification reaction[OH−] and [HS−] are the concentration of hydroxide and hydrosulphide ions
AQ is the weight percentage of AQ charge based on oven dry weight.
3.2.4.2. Pulp analysis
In the analysis of the pulp produced, 3 main parameters are considered; kappa number, alpha-cellulose and viscosity [74,75]. The essence of the Kappa number is to determine the degree of delignification and the amount of chemical requirement for bleaching, used in the mill control work. Measurement of the viscosity of the cellulose solution of known concentration is a good indicator of the cellulose degree of polymerization. Alpha cellulose, being a long-chain molecular faction of holocellulose is the portion that provides resistance to the solubilization of shorter-chain cellulose and hemicellulose in strongly basic solutions [[76], [77], [78]]. The soluble portion is measured by subtracting the soluble portion which is usually determined by oxidizing the moisture with potassium dichromate, from the insoluble alpha cellulose fraction [63.