1. Introduction
The looming threat of irreversible climate change (IPCC, 2021) is pressing humankind to develop and adopt sustainable and carbon-negative manufacturing technologies for the production of energy, fuels, and chemicals. Notably, the world formalized such ambitions to tackle climate change and become more sustainable with the Paris Agreement in 2015 (UNFCCC, 2015). While there is a range of mature and increasingly deployed technologies (e.g., solar, wind, fuel cells, among others) for shifting energy production away from fossil fuels to be renewable and carbon-free (He et al., 2020; Luderer et al., 2019), we will for the near term still require carbon-based liquid transportation fuels (e.g., aviation, marine) and most of our everyday products and commodities are carbon-based and almost exclusively derived petrochemically today. Hence, technologies for recycling carbon are required to accelerate the transition towards a circular, low-carbon economy. Another major challenge for maintaining global sustainability is the increasing accumulation of solid wastes (e.g., plastic and municipal solid waste [MSW], agricultural and forest waste) driven by population growth and rising consumption within the linear take-make-waste economy with a global recycling rate of less than 15% (Ellen MacArthur, 2021). Instead of landfilling or burning waste and losing 95% of the value (US$80–120 billion), we need to rapidly develop and deploy both mechanical and (bio-)chemical recycling technologies capable of efficiently recovering carbon from heterogeneous solid waste streams to avert the deterioration of ecosystems (Golwala et al., 2021; Wen et al., 2021).
A promising route to address both challenges of sustainable production of fuels and chemicals and solid waste recycling is through capture and utilization of gaseous one-carbon (C1) waste feedstocks using carbon oxide (CO and/or CO2) fixing microorganisms in a process called gas fermentation (Claassens et al., 2016; Liew et al., 2016; Fackler et al. (2021a). The key feature of gas fermentation is feedstock flexibility as the same biochemical fermentation process can accept various waste gas sources (e.g., industrial waste gases [CO2, CO]) and gasified solid waste (e.g., MSW, biomass syngas [CO, CO2, H2]) as input (Liew et al., 2016; Richter et al. (2016). Advantages of gas fermentation for biofuel and biochemical production include using non-food biomass compared to sugar fermentation (i.e., no impact on food security) and complete carbon utilization in woody biomass (i.e., including lignin) compared to lignocellulosic fermentation. Furthermore, upgrading waste-derived syngas using gas fermentation is preferred over thermocatalytic Fischer-Tropsch synthesis as the former is more selective, robust, flexible, and has lower capital and operational costs (Daniell et al., 2012; Köpke and Simpson, 2020).
This review aims to comprehensively summarize the quantitative and systems-level understanding of the metabolism of key chemolithoautotrophic microorganisms—the biocatalysts—for fermenting carbon oxides. In particular, we focus on biochemical identification of pathways, components, and activities; redox, energy, carbon, and electron balancing; thermodynamics and fluxes; regulatory mechanisms; and genotype-phenotype links in anaerobic acetogens and aerobic hydrogenotrophs. We also briefly cover aspects of genetic engineering of these biocatalysts and provide an overview of the status and future of industrial gas fermentation. We refer the reader to excellent reviews covering other aspects of gas fermentation (Liew et al. (2016); Fackler et al. (2021a); Daniell et al. (2012); ; Bender et al. (2011); Sipma et al. (2006); Schuchmann and Müller (2014) Dürre and Eikmanns (2015); Molitor et al. (2017); Vees et al. (2020); Diender et al. (2021); Panich et al. (2021).
2. Biocatalysts for gas fermentation
A wide range of known autotrophic microbes are considered as biocatalysts for gas fermentation (Dürre and Eikmanns, 2015). For this review, we exclude photoautotrophs, which require light as source of electrons, and only cover chemolithoautotrophs, as this allows for a much simpler process regarding cost and control. We further focus on carbon oxide (CO and/or CO2) fixing microbes only, but some microbes can activate methane (CH4), which is another attractive feedstock as covered in detail in other reviews (Kalyuzhnaya et al., 2015; Strong et al., 2016; Clomburg et al., 2017; Enzmann et al., 2018).
There are seven known natural CO2 fixation pathways (Thauer, 2007; Huber et al., 2008; Sánchez-Andrea et al., 2020). Of these, the Wood-Ljungdahl (or reductive acetyl-CoA) pathway (WLP) found in anaerobic acetogens is considered to be the most efficient around its unique key enzyme CO dehydrogenase/acetyl-CoA synthase (CODH/ACS) (Cotton et al., 2018; Drake et al., 2006; Fuchs, 2011; Fast and Papoutsakis, 2012). The Calvin (-Benson-Bassham; CBB) cycle on the other hand is the most widespread in nature, found in plants, algae but also in aerobic hydrogenotrophic bacteria. Its key enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) is the most abundant enzyme on Earth with an estimated ≈0.7 Gt (Bar-On and Milo, 2019). It has recently also been installed in model systems such as E. coli and yeast (Gleizer et al., 2019; Flamholz et al., 2020; Gassler et al., 2019). In addition, a range of synthetic CO2 fixation pathways have been proposed and developed over the past years and are described in detail elsewhere (Fackler et al., 2021a; Erb, 2019; Bar-Even et al., 2012a; Erb et al., 2017). While these offer significant future potential (Erb et al., 2019), currently the most biotechnologically relevant platforms are anaerobic acetogens and aerobic hydrogenotrophs that rely on the WLP and CBB cycle (Fig. 1). Table 1 provides an overview and compares the key features of native and synthetic CO2 fixation pathways.
Table 1. Key features (Energetic requirements, Number of Steps and other features) of carbon fixation to acetyl-CoA in natural pathways known in chemolithotrophs and key synthetic pathways.
| Pathway | Requirements to produce Acetyl-CoA | Other features | |||||
|---|---|---|---|---|---|---|---|
| Reducing Equivalents | ATP | Steps | |||||
| Fdred | NAD(P)H | Total | |||||
| Native pathways | |||||||
| Calvin Benson Bassham Cycle | – | 4 | 4 | 7 | 10 | Aerobic | Cyclic |
| 3-Hydroxypropionate/malyl-CoA cycle | – | 4 | 4 | 7 | 20 | Microaerophilic | Cyclic |
| 3-Hydroxypropionate/4-Hydroxybutyrate Cycle | – | 4 | 4 | 4 | 15 |
Microaerophilic, Anaerobic |
Cyclic |
| Dicarboxylate/4-Hydroxybutyrate Cycle | 1 or 2 | 2 or 3 | 4 | 4 | 14 | Aerobic | Cyclic |
| Reductive Tricarboxylic Acid Cycle | 1 | 3 | 4 | 2 | 8 |
Microaerophilic, Anaerobic |
Cyclic |
| Reductive Glycine Pathwaya | – | 4 | 4 | 1 | 8 | Anaerobic | Linear |
| Wood-Ljungdahl Pathway | 1 | 3 | 4 | <1 | 8 | Anaerobic | Linear |
| Synthetic pathways | |||||||
| Crotonyl–CoA/ethylmalonyl-CoA/hydroxybutyryl-CoA (CETCH) Cycle b | – | 4 | 4 | 1 | 17 | Aerobic | Cyclic |
| Malonyl-CoA-oxaloacetate-glyoxylate (MOG) Pathway b | – | 4 | 4 | 4 | 9 | Aerobic | Cyclic |
| Synthetic Acetyl-CoA (SACA) Pathway b | – | 4 | 4 | 0 | 5 | Aerobic | Linear |
- a
-
Understood not to function independently during autotrophic growth in native organisms (Song et al., 2020).
- b
-
Form glyoxylate as the fixed carbon product.
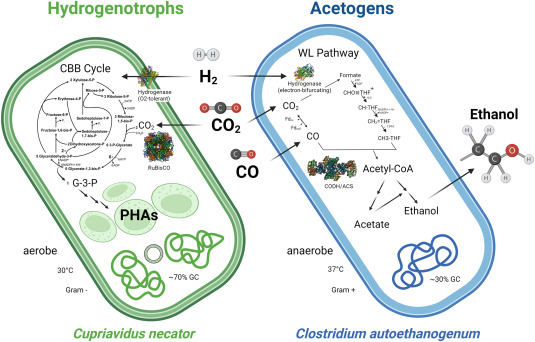
Anaerobic acetogens (specifically the model acetogen Clostridiumautoethanogenum) are already used at a commercial scale today, building on their ability to readily use existing waste streams (e.g. still mill emissions) and natively produce a valuable product (ethanol) with high selectivity. In addition, they are capable of producing other products of value such as butanediol, lactate, butanol or hexanol (Drake et al., 2006) and as such are an ideal platform for fuel and chemical production from a wide range of feedstocks due to their metabolic flexibility (Fackler et al., 2021a; Köpke and Simpson, 2020). Hydrogenotrophs, as aerobic organisms, offer fast growth, high selectivity towards biomass and ATP demanding products, and an easier path for geneticengineering, making them an attractive platform for CO2 conversion with the recent rise of Green hydrogen (Nybo et al., 2015). Model hydrogenotroph Cupriavidus necator (formerly known as Ralstonia eutropha) is capable of naturally producing biodegradable bioplastic polyhydroxyalkanoates (PHAs) such as (polyhydroxybutyrate) PHB (Fig. 1).
2.1. Anaerobic acetogens
The ideal biocatalysts for producing fuels and chemicals from carbon oxides using gas fermentation are anaerobic acetogens (Fig. 1). This is because acetogens can fix CO2 or CO (as well as other C1 substrates such as formate or methanol) using the most energy-efficient WLP pathway (Figs. 1 and 2) (Cotton et al., 2018; Drake et al., 2006; Fuchs, 2011; Fast and Papoutsakis, 2012). While many organisms possess (parts of) the WLP (Ragsdale and Pierce, 2008; Wood, 1991), only acetogens utilize it as a terminal electron-accepting and energy-conserving process for fixing CO2 into cell biomass via the key enzyme CODH/ACS (Drake et al., 2006; Fuchs, 2011). The flexibility to use both CO2 and CO as the carbon source is important as it allows the recycling of waste feedstocks with various compositions and origins. Secondly, gas fermentation using the anaerobic acetogen C. autoethanogenum has been deployed at an industrial scale (Fackler et al., 2021a; Köpke and Simpson, 2020). Thirdly, anaerobic conditions in a sugar-free culture further lower the risk of contamination and prevent flammability concerns with combustible feed gases.
 Fig. 1. Overview of metabolism and key enzymes (ribulose-1,5-bisphosphate carboxylase/oxygenase, RuBisCO; CO dehydrogenase/acetyl-CoA synthase, CODH/ACS) and metabolic and genetic features of hydrogenotrophs and acetogens based on model systems C. necator and C. autoethanogenum. Figure was generated using Biorender.com.
Fig. 1. Overview of metabolism and key enzymes (ribulose-1,5-bisphosphate carboxylase/oxygenase, RuBisCO; CO dehydrogenase/acetyl-CoA synthase, CODH/ACS) and metabolic and genetic features of hydrogenotrophs and acetogens based on model systems C. necator and C. autoethanogenum. Figure was generated using Biorender.com. Fig. 2. Detailed overview of central metabolism in anaerobic acetogens. Methyl and carbonyl branches of the WLP are indicated with green and blue arrows, respectively. Asterisk denotes that the electron-bifurcating hydrogenase HytA-E can both independently oxidize H2 to generate Fdred and NADPH and use H2directly to reduce CO2 to formate in the methyl branch of the WLP using the formate-H2 lyase activity of the HytA-E/FDH enzyme complex. Note that CO2also freely diffuses across the cell membrane. Refer to text for enzyme and metabolite abbreviations. Growth by-products that are unique or the sole product of acetate are shown for most-studied acetogens. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2. Detailed overview of central metabolism in anaerobic acetogens. Methyl and carbonyl branches of the WLP are indicated with green and blue arrows, respectively. Asterisk denotes that the electron-bifurcating hydrogenase HytA-E can both independently oxidize H2 to generate Fdred and NADPH and use H2directly to reduce CO2 to formate in the methyl branch of the WLP using the formate-H2 lyase activity of the HytA-E/FDH enzyme complex. Note that CO2also freely diffuses across the cell membrane. Refer to text for enzyme and metabolite abbreviations. Growth by-products that are unique or the sole product of acetate are shown for most-studied acetogens. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)In addition to the current use of C. autoethanogenum as a cell factory for industrial-scale ethanol production (Fackler et al., 2021a; Köpke and Simpson, 2020), the most-studied acetogens Clostridium ljungdahlii, Clostridium ragsdalei, Clostridium carboxidivorans, Acetobacterium woodii, Moorella thermoacetica, and Eubacterium limosum can natively ferment CO2 or CO to acids (e.g., acetic acid, lactic acid, butyric acid, hexanoic acid), alcohols (e.g., ethanol, butanol, hexanol), or the specialty chemical 2,3-butanediol (2,3-BDO) (Drake et al., 2006, Liew et al., 2016, Phillips, 2015). It is remarkable that carbon oxides are converted into these products as the WLP operates at the edge of thermodynamic feasibility and ATP is scarce (Schuchmann and Müller, 2014; Greene et al., 2019; Mahamkali et al., 2020). Production of these chemicals is only possible because acetogens use a third mode of energy conservation/coupling, electron bifurcation, discovered recently (Herrmann et al., 2008; Li et al., 2008; Buckel and Thauer, 2018). Acetogens are not just attractive biocatalysts for gas fermentation but could also shed light on the origin of life as the WLP is potentially the first biochemical pathway on Earth (Fuchs, 2011; Russell and Martin, 2004; Varma et al., 2018; Weiss et al., 2016). Furthermore, ∼20% of CO2 on Earth is fixed and converted to billions of tons of acetic acid per year by acetogens, making them important players in the global carbon cycle (Drake et al., 2006; Ljungdahl, 2009).
2.2. Aerobic hydrogenotrophs
In addition to anaerobic acetogens, aerobic H2-oxidizing organisms have also emerged as promising gas fermentation biocatalysts (Fig. 1). This group utilizes H2 as reducing power, and the oxidation of H2 in these bacteria is catalyzed by [NiFe] enzymes called hydrogenases (Friedrich and Schwartz, 1993; Schwartz et al., 2006). The Gram-negative beta-proteobacterium C. necator H16 is one of the most widely studied and industrially relevant hydrogenotrophs. This facultative chemolithotroph can grow at high rates on mixtures of H2 and CO2 (Panich et al., 2021) and accumulate biomass to high levels. It utilizes the CBB cycle (photosynthetic dark reactions) for fixing CO2 (Fig. 1, Fig. 3) and is naturally capable of producing and accumulating high levels of bioplastic PHAs as a way to store carbon (Panich et al., 2021; Nybo et al., 2015; Nangle et al., 2020).
 Fig. 3. Detailed overview of central metabolism in aerobic hydrogenotrophs. The membrane-bound hydrogenase (MBH) complex transfers electrons from H2oxidation to the electron transport chain, then reducing molecular O2 to H2O. Protons re-enter the cell by the ATPase to produce ATP. The soluble hydrogenase (SH) catalyzes H2 oxidation and transfers electron potential to the production of NADH. NADP+ is reduced to NADPH via NADH oxidation. The ATP and NADPH generated through hydrogen oxidation by MBH and SH are utilized in the Calvin-Benson-Bassham cycle for carbon fixation. The Regulatory Hydrogenase (RH), a hydrogen-like protein, regulates MBH and SH transcriptions. In the presence of molecular H2 and/or growth on energy-limiting substrates the transcription factor HoxA is activated: the subunit HoxJ phosphorylates the HoxA transcription factor, suppressing MBH and SH transcription; otherwise, the complex HoxBC releases the inhibitory effect HoxJ. Little is known about Hy4 and both RH and Hy4 are not considered as metabolically active hydrogenases. Carbon assimilated either auto or heterotrophically is used to produce biomass and synthesize PHB molecules for carbon storage.
Fig. 3. Detailed overview of central metabolism in aerobic hydrogenotrophs. The membrane-bound hydrogenase (MBH) complex transfers electrons from H2oxidation to the electron transport chain, then reducing molecular O2 to H2O. Protons re-enter the cell by the ATPase to produce ATP. The soluble hydrogenase (SH) catalyzes H2 oxidation and transfers electron potential to the production of NADH. NADP+ is reduced to NADPH via NADH oxidation. The ATP and NADPH generated through hydrogen oxidation by MBH and SH are utilized in the Calvin-Benson-Bassham cycle for carbon fixation. The Regulatory Hydrogenase (RH), a hydrogen-like protein, regulates MBH and SH transcriptions. In the presence of molecular H2 and/or growth on energy-limiting substrates the transcription factor HoxA is activated: the subunit HoxJ phosphorylates the HoxA transcription factor, suppressing MBH and SH transcription; otherwise, the complex HoxBC releases the inhibitory effect HoxJ. Little is known about Hy4 and both RH and Hy4 are not considered as metabolically active hydrogenases. Carbon assimilated either auto or heterotrophically is used to produce biomass and synthesize PHB molecules for carbon storage.In addition, the hydrogenases of hydrogenotrophs are unique as they are tolerant to oxygen (since oxygen is the final electron acceptor in this organism) and not inhibited by low levels of CO, which makes it possible to cultivate the microorganism using industrial off-gases as feedstock. Moreover, the microorganism can grow under mixotrophic conditions (both organic compounds and H2 can be used as a source of energy), in the interface of anaerobic and aerobic environments, easily adapt between heterotrophic and autotrophic lifestyles, and it is not limited to ATP production (Müller et al., 2013; Alagesan et al., 2017; Aragno and Schlegel, 1981). This metabolic versatility makes C. necator an important candidate for producing fuels, chemicals, and single-cell protein (SCP) for food and feed, utilizing inexpensive CO2 streams as feedstock. Not surprisingly, the potential of C. necator as a biocatalyst to produce a number of bioproducts has been demonstrated (Nybo et al., 2015), and the commercial production of edible protein from CO2fermentation is already a reality (Kiverdi, 2021, NovoNutrients, 2021, Solar Foods, 2021).
Though C. necator is the most utilized hydrogen oxidizing bacteria, advancements have been made to understand the metabolism of other hydrogenotrophs with interesting properties to produce biochemicals via gas fermentation. This group includes Hydrogenophaga pseudoflava, also a Gram-negative β-proteobacterium with a carboxydotrophic lifestyle, which genome has been recently annotated and a genetic engineering toolbox has been developed to produce bisabolene from syngas (Grenz et al., 2019). Another chemolithoautotrophic bacterium capable of utilizing CO and H2 and fixing CO2is called Oligotropha carboxidovorans. Its genome sequence was first published in 2008 and comparative genome analysis were conducted to unveil specific aspects of its metabolism (Paul et al., 2008, 2010). More recently, global gene expression analysis and new genetic manipulation tools led to a more detailed understanding of the metabolism of O. carboxidovorans under autotrophic conditions (Siebert et al., 2020). Hydrogenovibrio marinus is a mesophilic, obligately chemolithoautotrophic hydrogenotroph that produces carboxysomesat low CO2 concentrations (equal or lower than 0.15%) (Arai and Ishii, 2019; Toyoda et al., 2018; Yoshizawa et al., 2004). Its complete genome sequence was published in 2019 (Arai and Ishii, 2019) showing that this microorganism uses three different RubisCO enzymes (CbbLS-1, CbbLS-2, and CbbM), and the transcription of each of the three RubisCO genes varies according to the CO2concentration (Toyoda et al., 2018; Yoshizawa et al., 2004). Finally, the continuous development of genetic tools to manipulate these non-model organisms brings the potential to create improved new hosts for biosynthesis of important materials from carbon oxides.
3. Metabolism of anaerobic acetogens
3.1. Key pathways, components, and activities for autotrophic growth
The WLP is what makes acetogens so unique (Fig. 2). The WLP, and the recently confirmed reverse glycine reduction pathway (Sánchez-Andrea et al., 2020), are the only known linear pathways for autotrophic growth. The WLP emerged several billion years ago, at a time when oxygen was not present in the atmosphere. It comprises a collection of unique metalloenzymes which use tetrahydrofolate (THF) as the sole cofactor. Six enzymes comprise the methyl branch of the WLP, including CO dehydrogenase (CODH), formate dehydrogenase (FDH), formyl-THF synthetase, methenyl-THF cyclohydrolase/dehydrogenase, methylene-THF reductase (MTHFR), and a methyltransferase. The methyl branch of the WLP produces methyl-THF, which then donates a methyl group to be condensed with Coenzyme A and a molecule of CO from the carbonyl branch of the WLP. This condensation reaction is mediated by the CODH/ACS complex and results in the formation of acetyl-CoA.
This fascinating pathway is supported by an incredible redox machinery, which can interconvert redox on demand. Firstly, the iron-sulfur flavoprotein Nfn (NADH-dependent reduced ferredoxin:NADP + oxidoreductase) complex that acts as transhydrogenase to balance the cellular redox needs, coupling the exergonic reduction of NADP+ with reduced ferredoxin (Fdred) and the endergonic reduction of NADP+ with NADH in a reversible reaction (Wang et al., 2010). Secondly, the Rnf (Rhodobacter nitrogen fixation) complex, a proton or sodium ion-translocating ferredoxin:NAD + -oxidoreductase (first discovered in context of the reverse reaction during nitrogen fixation in the purple non-sulfur bacterium Rhodobacter capsulatus) that acts as an energy-coupled transhydrogenase to balance ferredoxin needs with NADH and generate a transmembrane ion gradient that in turn drives ATP synthesis (Hess et al., 2013, 2016; Tremblay et al., 2012; Müller et al., 2008). Acetogens harvest chemiosmotic ATP by transferring protons or ions across the membrane using an electrochemical gradient (Fig. 2). The gradient is used to drive ATP synthesis via an ATP synthase. Some acetogens utilize an energy-converting hydrogenase(Ech), which generates a membrane potential to drive the ATP synthase (Das and Ljungdahl, 2003). M. thermoacetica is able to generate membrane potential using cytochromes and menaquinone. Alternatively, using proton or sodium ion translocation, the Rnf complex contributes to ATP synthesis in the absence of cytochromes and menaquinone. Both rely on electron bifurcation, the third form of energy conservation/coupling in biology, to maximize energy conservation. This mode of energy generation is crucial as no net ATP is produced in the WLP. If cells produce acetate, the ATP from its synthesis is consumed by the formyl-THF synthetase in the methyl branch of the WLP (Fig. 2). Energy can also be obtained from H2 through a number of hydrogenases, which capture energy in the form of ferredoxin to drive the Rnf complex. Additionally, H2 can be used for CO2 reduction to formate using the electron-bifurcating HytA-E/FDH complex (Wang et al., 2013). These unique features open enormous possibilities to supply energy and support carbon fixation for acetogen fermentation from renewable hydrogen sources.
3.2. Thermodynamics and electron bifurcation
As the most energy-efficient pathway for fixing CO2 into acetyl-CoA, many aspects of thermodynamic energetics in acetogens have captivated scientists for decades. Unlike other microbes, where metabolism is controlled at the transcriptional or translational level, energy metabolism in acetogens is highly influenced by the redox level and is controlled by thermodynamics (Richter et al., 2016; Mahamkali et al., 2020). Acetogens regulate their metabolism by carefully balancing the intracellular concentration of metabolites to ensure cellular homeostasis to thrive in low-energy substrates.
CO2 reduction into CO in the carbonyl branch is especially thermodynamically challenging since the pair has a very low standard redox potential (E0ʹ) of −520 mV (Thauer et al., 1977), thus only Fdred (E0ʹ [Fdox/Fdred] ∼ 420–500 mV) (Buckel and Thauer, 2018) from the known WLP electron carriers can be used for CO2reduction. However, even with H2 (E0ʹ [H+/H2] = −414 mV) (Thauer et al., 1977) as the source of electrons for reducing Fd for subsequent CO2 reduction to CO using the CODH/ACS complex, the reaction sequence would still be endergonic (standard Gibbs free energy change (ΔG0ʹ) ∼ +20 kJ/mol) (Schuchmann and Müller, 2014). Nature has found a clever solution to overcome such challenges by using a third mode of energy conservation/coupling – flavin-based electron bifurcation (Herrmann et al., 2008; Li et al., 2008; Buckel and Thauer, 2018; Wang et al., 2013; Schut and Adams, 2009).
In its simplest term, electron bifurcation couples endergonic oxidation-reduction reactions with exergonic ones to overcome thermodynamic barriers and minimize free energy waste (Peters et al., 2016). In the case of CO2reduction to CO in acetogens, exergonic reduction of NAD+ with H2 (E0ʹ [NAD+/NADH] = −320 mV) (Buckel and Thauer, 2018) drives the coupled endergonic reduction of Fd with H2, yielding a near-equilibrium state (ΔG0ʹ ∼ +2 kJ/mol) for the combined bifurcation and CO2 reduction reactions (Schuchmann and Müller, 2014). The activity of an electron-bifurcating hydrogenase is thus essential in acetogens for realizing CO2 reduction to CO in the carbonyl branch of the WLP (Schuchmann and Müller, 2014; Müller et al., 2018). Notably, deletion of the electron-bifurcating hydrogenase HytA-E in C. autoethanogenum is not possible during growth on CO, CO2, and H2 (Mock et al., 2015).
Electron bifurcation does not directly conserve energy in the form of ATP. However, the functionality is critical for maximal energy conservation in acetogens through the supply of Fdred for chemiosmotic ATP production through the Rnf complex and ATPase (Hess et al., 2016; Tremblay et al., 2012). Electron bifurcation is also employed for CO2 reduction to formate (E0ʹ [CO2/Formate] = −432 mV) (Thauer et al., 1977) either through coupled oxidation of Fd and NADPH using the FDH activity or direct reduction with H2using the bifurcating hydrogenase/FDH complex (Wang et al., 2013; Schuchmann and Müller, 2013). Furthermore, electron bifurcation might even be essential for energy homeostasis under certain conditions (see section “Energy homeostasis”) and is important for regulating the redox pools through the bifurcating Nfn transhydrogenase that acts as a redox interconversion valve (see section “Redox homeostasis”). The reader is referred to excellent reviews on electron bifurcation for more details (Herrmann et al., 2008; Buckel and Thauer, 2018; Peters et al., 2016; Müller et al., 2018).
3.3. Energy homeostasis
Challenging thermodynamics of acetogen metabolism makes maintenance of energy homeostasis critical for growth. Contrary to the significant fraction of ATP that both prokaryotes and eukaryotes generate from the catabolism of energy-rich sugar substrates through glycolysis using substrate-level phosphorylation (SLP), the WLP consumes one ATP per one acetyl-CoA formed from fixing CO or CO2 (Fig. 2). Acetogens produce ATP through electron-driven chemiosmosis using the Rnf or Ech activities and the ATPase (Fig. 2). Acetogens also acquire additional ATP using SLP from acetate production to achieve energy homeostasis, where the name acetogens is derived from (Fig. 2) (Liew et al., 2016). Acetogens like C. autoethanogenum and C. ljungdahli that possess the acetaldehyde:Fd oxidoreductase (AOR) activity, however, do not need to excrete acetate but can intracellularly convert it to ethanol and thereby couple ethanol and ATP production (Richter et al., 2016; Valgepea et al., 2017a; Mock et al., 2015; Bengelsdorf et al., 2016). This is significant as it diminishes the uncoupling effect of the proton motive force (PMF) by diffusion of extracellular acetic acid into the cytoplasm that is detrimental to chemiosmotic ATP production. Indeed, when higher steady-state biomass concentrations in autotrophic C. autoethanogenum chemostat cultures lead to higher extracellular acetic acid levels (i.e., higher diffusion of acetic acid into the cytoplasm), cells shift from acetate to ethanol excretion to maintain energy homeostasis (Fig. 4) (Valgepea et al., 2017a).
 Fig. 4. Regulatory mechanisms in anaerobic acetogens. The left side illustrates regulatory mechanisms of metabolism: top, by-product levels in low vs. high biomass concentration steady-state chemostats; middle, by-product levels in gas-limited vs. gas-excess cultures; bottom, inhibition of H2 uptake through Nfn driving force leading to metabolic oscillations. The right side illustrates regulation of transcription, translation, and metabolic fluxes: transcription is regulated through various promoter motifs and sigma (σ) factors and non-coding RNAs (ncRNA); translation is regulated through the 5′-untranslated region (5′UTR), its secondary structure, AU content, and ribosome binding site (RBS) sequence; metabolic fluxes are regulated post-translationally through thermodynamic regulation. TSS, transcription start site; RNAP, RNA polymerase. Figure was generated using Biorender.com.
Fig. 4. Regulatory mechanisms in anaerobic acetogens. The left side illustrates regulatory mechanisms of metabolism: top, by-product levels in low vs. high biomass concentration steady-state chemostats; middle, by-product levels in gas-limited vs. gas-excess cultures; bottom, inhibition of H2 uptake through Nfn driving force leading to metabolic oscillations. The right side illustrates regulation of transcription, translation, and metabolic fluxes: transcription is regulated through various promoter motifs and sigma (σ) factors and non-coding RNAs (ncRNA); translation is regulated through the 5′-untranslated region (5′UTR), its secondary structure, AU content, and ribosome binding site (RBS) sequence; metabolic fluxes are regulated post-translationally through thermodynamic regulation. TSS, transcription start site; RNAP, RNA polymerase. Figure was generated using Biorender.com.In addition to distinct ATP needs for anabolic reactions and polymerization of monomers (e.g., amino acids, nucleotides) into cellular macromolecules (e.g., proteins, DNA), growing cells spend energy to maintain metabolism. The sum of these energetic costs is termed maintenance energy, which includes the turnover of macromolecules, re-establishing ion gradients (e.g., PMF), and futile cycles (van Bodegom, 2007; Russell and Cook, 1995). It is difficult to experimentally quantify these ATP fluxes, so total maintenance energy can be estimated using genome-scale metabolic models (GEMs (Feist et al., 2007);). Maintenance energy costs in acetogens seem to range between ∼5 and ∼15 mmol of ATP/gram of cell dry weight (gCDW)/h during autotrophic growth on CO2+H2 or CO-containing gases on minimal medium (Valgepea et al., 2017a, 2018; Heffernan et al., 2020). Costs likely increase with increasing extracellular acetate and ethanol levels due to uncoupling of the PMF and the presence and increasing content of H2 due to the influx of protons without ATP synthesis. For example, the estimated ATP cost of PMF uncoupling from increased extracellular acetic acid levels closely matches the increase of maintenance energy with higher biomass concentrations (Valgepea et al., 2017a). Since maintenance costs are condition-dependent, it is more informative to compare the fraction of maintenance costs from total ATP production to evaluate the energy efficiency of metabolism. Notably, gas-fermenting C. autoethanogenumspends 26–44% of ATP for maintenance (Valgepea et al., 2017a, 2018; Heffernan et al., 2020), which is less than during heterotrophic growth (Valgepea et al., 2017b; Nagarajan et al., 2013) and less than seen in other bacteria (Lahtvee et al., 2014; Stouthamer, 1973; Teusink et al., 2006; Valgepea et al., 2011). This is further evidence for the high metabolic efficiency needed in acetogens operating at the thermodynamic edge of life.
Despite the energy-efficient metabolism, the acetogen product spectrum is challenged by energetics when fermenting carbon oxides (Molitor et al., 2017; Katsyv and Müller, 2020). Supplemental nutrients can provide valuable additional energy either directly through their catabolism (e.g., amino acids) or by serving as electron acceptors (e.g., nitrate). Indeed, supplementation of the amino acid arginine doubles the specific growth rate (μ) and nearly abolishes acetate excretion during autotrophic growth of C. autoethanogenum, effects that are predicted by a GEM (Valgepea et al., 2017b). The arginine effect comes from its catabolism through the arginine deiminase (ADI) pathway that yields ATP and allows to reorganize acetogen metabolism by activating the native pathway. The addition of arginine to CO2+H2 cultures and heterologous expression of the ADI pathway also improve growth and lower acetate production in A. woodii(Beck et al., 2020). Industrial application-wise, the additional price of arginine feeding (∼18,000$/ton) can be offset by the lack of need to add ammonia (∼11,000$/ton) as a nitrogen source as the ADI pathway yields 2 mol of ammonia per 1 mol of arginine. In addition to arginine, nitrate supplementation as a cheap electron acceptor is promising as it improves μ, biomass levels, and ATP production in C. ljungdahlii batch cultures, while no positive effects are seen for M. thermoacetica and A. woodii (Emerson et al., 2019). Nitrate addition to C. ljungdahlii bioreactor continuous cultures also improved growth and ethanol production but with a trade-off of stochastic inhibition effects leading to crash of metabolism (Klask et al., 2020). While (Emerson et al., 2019) propose that nitrate contributes to ATP production by facilitating a higher Rnf flux (i.e., higher ATPase flux) by acting as an electron sink for the complex, further studies are needed for the mechanistic understanding of nitrate metabolism in acetogens. The effects of other industrially less-relevant nutrient supplements are described in (Katsyv and Müller, 2020).
In addition to being essential for CO2 fixation in acetogens, electron bifurcation might also be essential for maintaining energy homeostasis. This is supported by GEM simulations showing that in silico growth of C. autoethanogenum on a CO-containing gas (syngas) is infeasible without the Fd-reducing and electron-bifurcating activity of the methylene-THF-reductase (MTHFR) for the reduction of methylene-THF through NADH oxidation (Valgepea et al., 2017a). Although MTHFR isolated from M. thermoacetica is electron-bifurcating towards reduction of benzyl viologen and not towards Fd isolated from Clostridium pasteurianum(Mock et al., 2014), these biochemical assays need to be repeated with ferredoxins isolated from the same organism due to potential enzyme specificities (Buckel and Thauer, 2018). We note that it was recently hypothesized that MTHFR might form a complex with Rnf in C. ljungdahlii for the reduction of methylene-THF through oxidation of Fd (Öppinger et al., 2021). As electron-driven chemiosmotic ATP conservation that relies on redox cofactors is essential for acetogens, maintenance of energy homeostasis is inherently coupled to redox homeostasis. The latter is discussed next.
3.4. Redox homeostasis
Redox homeostasis is central in acetogen metabolism as it is tightly coupled to cellular energetics (see the previous section) and carbon and electron flows (see next section). It is also a key factor determining carbon distribution into growth by-products (e.g., CO2, ethanol (Valgepea et al., 2018) and metabolic robustness in C. autoethanogenum (Mahamkali et al., 2020). In addition to the universal redox pairs of NADH/NAD+ and NADPH/NADP+, maintenance of redox balance in acetogens is also regulated through the Fdred/Fdox redox pair (Fig. 2). The latter pair might have the strongest impact on the phenotype as Fdox and Fdred pools are directly linked to carbon oxide fixation and chemiosmotic ATP generation (Fig. 2). Similar to the function of transhydrogenases in other organisms, acetogens can balance all three redox pairs with a single enzymatic activityusing the Nfn transhydrogenase as a redox valve to control levels of NADH and NADPH through coupling oxidation of Fd and NADH with reduction of NADP+(Fig. 2)(Wang et al., 2010). This is another important electron bifurcating activity providing valuable flexibility for acetogen metabolism. The role of Nfn might depend on the H2 content of the feed gas as an Nfn-deletion strain of C. autoethanogenum grows on a 35% CO, 10% CO2, 2% H2 gas mixture while growth is severely impaired on CO2+H2 and on CO with high H2 content (Marcellin et al., 2016). Also, the magnitude and even the direction of flux through Nfn seem to depend on the H2 content of the feed gas (see next section). Nfn's protein abundance in C. autoethanogenum is maintained high across autotrophic conditions, likely to promptly respond to redox perturbations (Valgepea et al., 2021).
Based on the stoichiometry and E0ʹs of reactant pairs of the Nfn transhydrogenase reaction (Fig. 2), the intracellular NADPH/NADP+ ratio is expected to be higher than the NADH/NAD+ ratio for maintaining sufficient driving force of the reaction (Buckel and Thauer, 2018). Indeed, measurements of intracellular concentrations of NADPH, NADP+, NADH, and NAD+ in C. autoethanogenum are generally consistent with the previous expectation as the NADPH/NADP+ ratio is ∼15-fold higher than the NADH/NAD+ ratio in syngas-limited chemostat cultures (Valgepea et al., 2017a; Mahamkali et al., 2020), ∼2-fold higher for a poly[(R)-3-hydroxybutyrate] (PHB)-producing recombinant strain (de Souza Pinto Lemgruber et al., 2019a), and >200-fold higher for both autotrophic and heterotrophic batch growth (Marcellin et al., 2016), while the NADH/NAD+ ratio is 2-fold higher in CO-limited chemostat cultures (Valgepea et al., 2018). Higher intracellular ratios of NADPH/NADP+ compared to NADH/NAD+are also seen in non-acetogen microorganisms such as Clostridium acetobutylicum (Amador-Noguez et al., 2011), Escherichia coli, and Saccharomyces cerevisiae (Bennett et al., 2009; Park et al., 2016). As expected (Amador-Noguez et al., 2011), the intracellular redox state of C. autoethanogenum becomes increasingly oxidized with a higher carbon flux to the reduced by-products ethanol and 2,3-BDO (Valgepea et al., 2017a).
The absolute values of NADPH/NADP+ and NADH/NAD+ ratios in gas-limited chemostat cultures of C. autoethanogenum are both below one (Valgepea et al., 2017a; Mahamkali et al., 2020; Valgepea et al., 2018; de Souza Pinto Lemgruber et al., 2019a) that could be explained in acetogens by a highly positive ratio (∼1000) for the third and key redox pair of Fdred/Fdox estimated using a thermodynamic flux analysis model (Mahamkali et al., 2020). Although this computationally predicted Fdred/Fdox ratio facilitated thermodynamically feasible metabolic flux distributions, experimental determination of the intracellular Fdred/Fdox ratio would be highly valuable for a comprehensive understanding of redox homeostasis in acetogens. Unfortunately, direct measurement of Fdox and Fdred pools seems extremely difficult as there are multiple types of ferredoxins in cells, they auto-oxidize and quantification using light absorption (i.e., absorption not unique compared to other iron sulfur proteins) or mass spectrometry (i.e., quantifying a protein and its charged metal ligands) is infeasible even if potential changes in cellular Fdox and Fdred pools from the depletion of CO or CO2 during culture sampling and quenching of metabolism are avoided.
The central importance of redox homeostasis in acetogen metabolism is well illustrated by the effect of changes in redox pair ratios on the ability of cells to self-organize metabolism to maintain metabolic robustness (Mahamkali et al., 2020). In this study, temporal oscillations in NADPH/NADP+ and NADH/NAD+ratios are reported in self-oscillating syngas-grown continuous cultures of C. autoethanogenum. The oscillations in redox lead to a redox imbalance and set limits to acetogen metabolism through the thermodynamic driving force of Nfn in terms of μ of cells and capability to co-utilize CO and H2 (Fig. 4). Uptake of H2by hydrogenases is resumed once inhibition from excess CO is relieved (i.e., CO becomes growth-limiting again). The oscillations were found to be the result of two events around the co-metabolism of H2 and CO. Hydrogenases are inhibited by excess CO (Thauer et al., 1974; Shima and Thauer, 2007; Adams, 1990). As such, co-metabolism occurs only when biomass is high enough to exhaust CO below the inhibitory limits. As hydrogenases are not inhibited, H2 uptake results in increased μ and ethanol production until the AOR driving force is exhausted, which ultimately results in the crash. Notably, such oscillations could not be triggered in CO-grown continuous cultures of C. autoethanogenum. To better understand the effect of the redox balance on limits of acetogen metabolism, it would be relevant to investigate further whether metabolic oscillations are dependent on the H2 content of the feed gas and if similar oscillations can be seen for other acetogens.
3.5. Carbon distribution and electron balancing
Understanding metabolic regulation behind carbon distribution is vital for both comprehensive descriptions of metabolism and commercialization of any industrial bioprocess. Carbon distribution is particularly linked to electron balancing in anaerobic acetogen gas fermentation utilizing carbon oxides solely (e.g., no nitrate supplementation) as cell biomass and growth by-products (e.g., acetate, ethanol) are the only electron acceptors. While only H2 can supply electrons during growth on CO2+H2, CO acts as a carbon and electron source. However, even H2 is directly linked to carbon fixation as cells can use H2 for direct reduction of CO2 to formate within the WLP in addition to generating reducing equivalents from its oxidation (Fig. 2). Electron balancing in acetogens is realized through transferring electrons between donors and acceptors using the reduced redox cofactors Fdred, NADPH, and NADH as electron carriers. It is important to note that CO, CO2, and H2 freely diffuse across cell membranes. Acetogens growing on these gases, therefore, lack the possibility to balance the supply and demand of carbon and electrons through controlling substrate influx using transporters that exist for heterotrophic growth of organisms. Thus, the architecture of acetogen metabolism must ensure exceptionally flexible regulation to rapidly respond to changes in gas influx (i.e., availability) and maintain cellular homeostasis. A good example of this is the immediate counterbalancing of lowered supply of Fdred from loss of H2 uptake with increased supply from CO oxidation and CO2 dissipation during C. autoethanogenum growth on syngas (Valgepea et al., 2017a; Mahamkali et al., 2020). In another case, C. ljungdahlii efficiently redirects reducing equivalents from biomass synthesis towards ethanol production when growth becomes nutrient-limited with excess syngas supply (Fig. 4) (Richter et al., 2013, 2016; Martin et al., 2016).
Some acetogens such as E. limosum can additionally ferment carbon oxides to butyric acid (Chang et al., 2001), and C. carboxidivorans to hexanoic acid, hexanol, and butanol (Phillips, 2015). The three closely related acetogens C. autoethanogenum, C. ljungdahlii, and C. ragsdalei, can natively produce acetic acid, lactic acid, 2,3-BDO, and a significant amount of ethanol from carbon oxides. It seems that acetogens such as A. woodii and M. thermoacetica that lack the AOR functionality of coupling ethanol and ATP production can produce ethanol either minimally or not at all (Bengelsdorf et al., 2016; Shin et al., 2016). This is consistent with observations in C. autoethanogenum and C. ljungdahliithat in silico ethanol is solely produced using the AOR activity through acetate (compared to the direct “conventional route” from acetyl-CoA) (Valgepea et al., 2017a, 2018; Mahamkali et al., 2020; Heffernan et al., 2020), both transcript and protein expression of the primary AOR–AOR1–greatly exceed that of genes of the “conventional route” (Richter et al., 2016; Valgepea et al., 2017a; Nagarajan et al., 2013; Marcellin et al., 2016; Valgepea et al., 2021; Al-bassam et al., 2018), and deletion of AOR activities nearly abolishes ethanol production (Liew et al., 2016). Simplified theoretical estimations of ATP gains or costs from the autotrophic production of some native and non-native compounds in acetogens can be found in (Fast and Papoutsakis, 2012; Katsyv and Müller, 2020).
Numerous studies have investigated the effects of environmental conditions, medium and gas composition, cultivation mode, among others, on autotrophic carbon distribution in acetogens. Here, we will only cover steady-state data (Table 2) obtained using continuous cultures (e.g., chemostat) as steady-state conditions (e.g., constant pH, biomass, by-products, μ) enable the unequivocal definition of the physiological state of the cells and thus unbiased quantification of condition-dependent metabolism, including carbon distribution (Adamberg et al., 2015; Hoskisson and Hobbs, 2005). Similar to the effect of pH on the product profile in acetone-butanol-ethanol (ABE) fermentation with C. acetobutylicum (Jones and Woods, 1986), lower pH increases ethanol (“solventogenesis”) and decreases acetate (“acetogenesis”) production in C. autoethanogenum (Safo et al., 2021) and C. ljungdahlii (Klask et al., 2020). Interestingly, the same effect of higher ethanol and lower acetate with the additional increase of carbon flux into 2,3-BDO is seen with higher gas-liquid mass transfer rates (i.e., biomass concentration) in C. autoethanogenum (Valgepea et al., 2017a, 2018; Schatschneider et al., 2018). The effect of gas composition on carbon distribution is substantial as supply and increasing the content of H2 in CO gas mixtures strongly diminishes carbon loss into CO2 and proportionally enhances the ethanol-to-acetate ratio in C. autoethanogenum (Valgepea et al., 2018; Diender et al., 2019). Notably, supplementing CO2+H2 cultures of C. autoethanogenum with just 2% CO significantly improves the CO2 fermentation performance (Heffernan et al., 2020). Both greater mass transfer and increased H2 supply in A. woodiichemostats channel carbon from biomass into acetate (Novak et al., 2021). Also, the nature of growth-limitation in continuous cultures affects carbon distribution considerably as C. ljungdahlii cultures with excess gas supply (i.e., “nutrient-limited”) show a much higher ethanol/acetate ratio compared to cultures limited by gas-liquid mass transfer (Richter et al., 2013, 2016; Martin et al., 2016).
Table 2. Effect of steady-state fermentation characteristics on autotrophic carbon distribution in acetogens.
| Characteristic | Effect | Organisms | References |
|---|---|---|---|
| pH↑ | ethanol↑ and acetate↓ |
C. autoethanogenum C. ljungdahlii |
Klask et al. (2020); Safo et al. (2021) |
| Gas-liquid mass transfer/biomass concentration↑ | ethanol↑, 2,3-BDO↑, and acetate↓ | C. autoethanogenum | Valgepea et al., 2017a, Valgepea et al., 2018; Schatschneider et al. (2018) |
| acetate↑ and biomass↓ | A. woodii | Novak et al. (2021) | |
|
Feed gas composition H2 supply and ↑ H2content in CO gas mixtures |
CO2↓ and ethanol/acetate↑ | C. autoethanogenum | Valgepea et al. (2018); Diender et al. (2019) |
|
Feed gas composition 2% supply of CO in CO2+H2 gas mixtures |
ethanol/acetate↑, μ↑, biomass concentration↑ | C. autoethanogenum | Heffernan et al. (2020) |
|
Feed gas composition ↑ H2 content in CO + CO2 gas mixtures |
acetate↑ and biomass↓ | A. woodii | Novak et al. (2021) |
| Growth-limitation | Gas excess (nutrient-limited) vs. gas-limited cultures = ethanol/acetate↑ | C. ljungdahlii | Richter et al., 2013, Richter et al., 2016; Martin et al. (2016) |
While CO serves both as a carbon and electron source for acetogens, fixing CO2needs an additional supply of electrons. H2 is well-suited for this as acetogens can metabolize it at high rates, and renewable and cheap H2 is becoming increasingly available for industrial applications. Attractive alternative possibilities for electron supply are the electrotrophic nature of some acetogens to receive electrons from electrodes (Nevin et al., 2011) and artificial photosynthesis systems to attain electrons directly from light (Sakimoto et al., 2016; Zhang et al., 2018; Jin et al., 2021). See section “Industrial gas fermentation” below for more details.
3.6. Metabolic flux distribution
Genome-scale metabolic models (GEM) for acetogens have been useful to understand acetogen metabolism. Since the first GEM for C. ljungdahlii was published (Nagarajan et al., 2013), it was rapidly followed by GEMs for M. thermoacetica (Islam et al., 2015), C. autoethanogenum (Marcellin et al., 2016), and the C. ljungdahlii model expanded to an metabolism and macromolecular synthesis (ME) model (Liu et al., 2019). The models have been valuable tools for predicting intracellular fluxes and phenotypic behavior, explaining byproduct distribution according to cellular homeostasis, and creating synthetic cocultures. For example, the C. autoethanogenum model was elegantly used to identify novel sources of energy by feeding arginine to supply ATP (Valgepea et al., 2017b). The GEM model was also used to show how CO2 is reduced to formate in the WLP using H2 energy through the formate-H2 lyase activity of the HytA-E/FDH complex for gas mixtures containing CO and CO2 (Valgepea et al., 2017a, 2018; Heffernan et al., 2020). The models showed that using this strategy, cells are able to conserve Fdred, ensuring maximum energy efficiency extracted from H2. Importantly the models also capture the pivotal role of the electron-bifurcating Nfn complex in energy conservation, independently of the H2 concentration, showing the importance of the Nfn complex, which acts as a metabolic valve for redox species for the cellular control of metabolism. The latter functionality further highlights the efficiency of acetogen metabolism as it saves valuable Fdred for driving ATP synthesis. Recently, RNA-seq analysis, 13C metabolite-tracing, and a GEM were used to demonstrate the presence of the glycine synthase-reductase pathway (GSRP) and reductive glycine pathway (RGP) to fix C1 compounds in C. drakei (Song et al., 2020).
Recently, two acetogen GEM models were used to increase syngas conversion to medium-chain fatty acids by co-culturing C. autoethanogenum with Clostridiumkluyveri (Benito-Vaquerizo et al., 2020). The models were first used to understand the ratios of ethanol and acetate needed by the cells and to predict additional substrates (succinate) to increase the production of larger chain products. This study is significant given the energy limitations of acetogens and the limitations to produce longer chain carbon products in pure cultures. The multi-species GEM reconstruction was able to distinguish between products and feeds as both, C. autoethanogenum and C. kluyveri, share many products that can be metabolized and produced simultaneously. Community flux balance analysis (FBA) is helpful to predict flux distribution and exchange fluxes between species through detailed validation by chemostat experiments. Together the community GEM model predicted accurately the production of long-chain metabolites from syngas, including butyrate and hexanoate, which may be a valuable tool for producing desirable longer carbon chain products in light of the above mentioned thermodynamic and energy limitations of acetogen metabolism, which are ultimately constrained by a tightly controlled regulatory mechanism as discussed below.
3.7. Regulatory mechanisms of metabolism
As discussed earlier, acetogens possess a primitive metabolism in which regulation is simple yet complex and defies many paradigms. The flexibility of their metabolism is a defining trait of acetogens that has ensured subsistence over millennia. This is due to a relatively small set of enzymes to ensure cellular homeostasis through a carefully balanced redox stoichiometry. This is achieved in contrast to aerobic metabolism, where O2 provides an unlimited oxidant to supply energy as electrons are transferred. In acetogens, electron acceptors are very limited and have low redox potentials, often leading to thermodynamic barriers. In addition to electron bifurcating enzymes, acetogens adjust their product spectrum to balance redox via Nfn. By changing the ratio of reduced and oxidized products at low and high biomass, C. autoethanogenum is able to regulate metabolism and ensure metabolic robustness (Fig. 4) (Valgepea et al., 2017a; Mahamkali et al., 2020). This delicate balance results in ample product spectrum under different gases and gas-excess conditions. For example, depending on the biomass concentration in chemostat cultures, acetogens produce different quantities of acetate and ethanol. These byproduct changes occur without transcriptional changes, as shown by RNA sequencing, despite global carbon redistribution (Valgepea et al., 2017a). Additionally, C. ljungdahliican efficiently balance redox when growth becomes nutrient-limited with excess syngas supply by redirecting reducing equivalents from biomass towards ethanol (Fig. 4) (Richter et al., 2013, 2016; Martin et al., 2016).
More and more evidence suggest that metabolism in acetogens is controlled by the concentration of intracellular metabolites rather than at the mRNA or protein level (Fig. 4) (Richter et al., 2016; Valgepea et al., 2017a, 2021; Mahamkali et al., 2020). This is despite findings which show that the unique metabolism of acetogens uses novel promoter motifs and unique sigma factorsto control levels of crucial genes involved in autotrophy (see next section) (de Souza Pinto Lemgruber et al., 2019b). In fact, acetogens are able to commit to the production of acetate or ethanol depending on the redox metabolic requirements regulated by AOR and the demand for Fd.
3.8. Regulation of transcription, translation, and metabolic fluxes
In addition to identifying regulatory mechanisms for maintenance of energy and redox homeostasis, quantification of regulation of transcription, translation, and metabolic fluxes are also important for both fundamental understandings of acetogen metabolism and advanced metabolic engineering of cell factories (Fig. 4). Differential RNA sequencing (dRNA-seq) (Sharma et al., 2010) is a valuable technology for mapping transcriptional architecture. dRNA-seq shows that in four acetogens transcription start sites (TSSs) are dominantly primary (within ∼250 nt upstream of an annotated gene) (Al-bassam et al., 2018; Song et al., 2017; Shin et al., 2018; de Souza Pinto Lemgruber et al., 2019b), most genes contain just one primary TSS (de Souza Pinto Lemgruber et al., 2019b), and significant condition-dependency (e.g., autotrophic vs. heterotrophic growth) of TSSs including for key genes in central metabolism (Shin et al., 2018; de Souza Pinto Lemgruber et al., 2019b). Single-nucleotide resolution reveals a preference for purine (A/G) and pyrimidine (C/T) in acetogens for transcription initiation at +1 and −1 position of primary TSSs, respectively (Song et al., 2017; Shin et al., 2018; de Souza Pinto Lemgruber et al., 2019b), which is commonly seen in bacteria (Sharma et al., 2010). Also, the Shine-Dalgarno sequence (AGGAGG) for ribosome binding is well-conserved in acetogens (Al-bassam et al., 2018; Song et al., 2017; Shin et al., 2018; de Souza Pinto Lemgruber et al., 2019b).
Importantly, dRNA-seq facilitates a more accurate genome-wide prediction of promoter motifs. In addition to the identification of the well-known Pribnow box and −35 TTGACA and −10 TATAAT (TATA box in eukaryotes and archaea) promoter motifs in acetogens (Al-bassam et al., 2018; Song et al., 2017; Shin et al., 2018; de Souza Pinto Lemgruber et al., 2019b; Shin et al., 2021), dRNA-seq led to the discovery of a new promoter motif (termed Pcauto) in C. autoethanogenum that is associated with essential genes for autotrophic growth (de Souza Pinto Lemgruber et al., 2019b). Both the housekeeping sigma factor σAand a TetR-family transcriptional regulator can activate transcription from Pcautowhile the latter also binds to the RNA polymerase, suggesting it might act as a sigma factor in C. autoethanogenum. Further work into TetR is needed as no change in C. autoethanogenum phenotype is seen with ∼30-fold down-regulation of TetR (Fackler et al., 2021b). Transcriptional regulation seems to vary between acetogens as transcription of essential autotrophic genes (e.g., WLP, hydrogenases) in E. limosum is initiated from the −35 TTGACA and −10 TATAAT motif likely by σA (Song et al., 2017). dRNA-seq data also suggest that non-coding RNAs (ncRNA) might play a role in transcriptional regulation in acetogens (Song et al., 2017; Shin et al., 2018).
Optimal regulation of translation is especially relevant for energy-limited acetogens as the synthesis of proteins has a significant ATP cost. Ribosome profiling (Ribo-seq) together with RNA-seq sheds light on translational regulation through quantification of genome-wide translational efficiencies(TEs; i.e., the ratio between transcripts being translated to all transcripts of a gene) (Ingolia et al., 2009; Ingolia, 2014). Relevance of translational regulation in acetogens is suggested by the negligible fraction of leaderless transcripts that lack upstream signals to regulate TEs (i.e., transcripts with a 5′-untranslated region <10 nt) (Al-bassam et al., 2018; Song et al., 2017; Shin et al., 2018; de Souza Pinto Lemgruber et al., 2019b). Indeed, translational regulation of gene expression in acetogens seems widespread as transcriptional (RNA-seq data) and translational (Ribo-seq data) levels of genes are moderately correlated (Al-bassam et al., 2018; Shin et al., 2021; Song et al., 2018). Notably, TEs of genes in key pathways for carbon and energy metabolism in C. ljungdahlii and A. woodiiare differentially enhanced in response to change in growth substrates (Al-bassam et al., 2018; Shin et al., 2021). A negative correlation between transcript expression and TE in E. limosum and A. woodii further supports translational buffering in acetogens (Shin et al., 2021; Song et al., 2018). Features of the coding region and secondary structure of the 5′-untranslated regions of transcripts have the dominant effect on TEs (Al-bassam et al., 2018; Shin et al., 2021; Song et al., 2018).
While Ribo-seq data are good proxies for protein expression (Mori et al., 2021), proteomics is needed to quantify absolute protein concentrations. Absolute proteome quantification in autotrophic C. autoethanogenum cultures reveals prioritization of acetogen proteome allocation for the WLP, energy metabolism (e.g., acetate and ethanol synthesis, hydrogenases, Rnf, ATPase), and translation (Valgepea et al., 2021). Notably, significant proteome resources are also invested into proteins with unknown functions. Data further indicate which isoenzymescould catalyze important metabolic fluxes in central metabolism. These observations point to the need for large-scale experimental determination of genotype-phenotype relationships in acetogens.
Understanding the regulation of metabolic fluxes is equally essential to understanding the regulation of transcription and translation as fluxes realize catabolism of substrates, biomass synthesis through anabolism, and energy and redox homeostasis. Flux regulation is also a critical factor for engineering the production performance (titer, rate, yield) of a bioprocess. Flux changes between different cultures of C. autoethanogenum or C. ljungdahlii are not proportional to changes in transcript or protein expression, i.e., metabolic fluxes in acetogens do not seem to be controlled through transcription or translation (Richter et al., 2016; Valgepea et al., 2017a; Valgepea et al., 2018; Al-bassam et al., 2018). The same holds true for self-oscillating chemostat cultures of C. autoethanogenum, where thermodynamic metabolic flux analysis shows post-translational regulation of fluxes through reaction thermodynamics (Mahamkali et al., 2020). Importantly, integration of absolute proteomics and flux data in autotrophic C. autoethanogenum cultures reveals that adjustments in metabolic fluxes were dominantly accompanied by changing apparent in vivocatalytic rates of enzymes (kapp) rather than their concentrations (Valgepea et al., 2021). The systems-level analysis further showed that enzymes catalyze high-rate fluxes with both high concentrations and high in vivo catalytic rates. Post-translational regulation of metabolic fluxes is expected for energy-limited acetogens as it is energetically the least costly mechanism.
3.9. Genotype-phenotype relationships
Identifying the links between genotype and phenotype for any organism is fundamental for a comprehensive understanding of their metabolism and advancing rational metabolic engineering of cell factories. This is especially relevant for less-studied and non-conventional organisms, such as acetogens, since the prediction of gene functionalities based on homology is less reliable. Furthermore, experimental determination of genotype-phenotype relationships is essential for identifying condition-specific functionalities of both isoenzymes and proteins with unknown functions. We refer the reader to recent reviews summarizing the understanding and studies of genotype-phenotype relationships in acetogens (Bourgade et al., 2021; Jin et al., 2020; Zhang et al., 2020). While the development of genetic modification tools for acetogens has progressed rapidly (see section “Strain engineering”), the throughput of generation of genetically modified strains (e.g., gene knock-outs, -downs, -ins) and quantification of their phenotypes (e.g., fermentation, systems-level analysis) needs to be significantly increased in order to catch up with the understanding of metabolism in the most-studied microorganisms (e.g., E. coli, Bacillus subtilis, S. cerevisiae). At least one effort towards the latter goal in acetogens is underway by the authors of this review together with collaborators aiming to map genotype-phenotype links for every gene in C. autoethanogenum(ARC Center of, 2021).