1. Introduction
The bottleneck of drug pipeline has steadily increased time by time. To surmount the obstacles of some hydrophobic drugs and increment in therapeutic effectiveness, the advent of tailor-made nanocarriers involving in wisely engineering as well as precisely designing nanostructures has been developed for drug delivery. Functionalization of conventional polymers through physical adsorption or chemical incorporation are considered as the most prevalently effective method to improve the rapid clearance before accumulating in pathological sites as well as prolong the circulation time of pharmaceutical nanocarriers [[1], [2], [3]]. Among disparities of diversified biomaterial approaches, externally environmental stimulus such as temperature, pH, magnetic field, and so forth majorly has drawn greater awareness of novel ‘smart’ biopolymer fabrication [4,5]. Off all, discovery of the thermo-responsive biopolymers or amphiphilic molecules led to fabrication of several multifunctionally thermo-responsive grafted platforms which exhibited an effectively functional improvement of the original materials in biomedical applications.It would thoroughly review up-to-minute advances and some thermo-responsive polymers-based nanomaterials or bulky hydrogels in the ‘smart’ drug delivery systems (DDS) that covers from synthetic polymer to nature-driven biomaterials.
2. Thermosensitive polymers
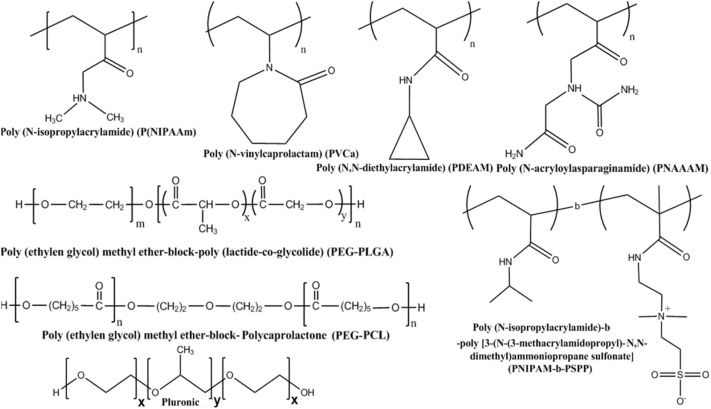
Temperature-stimulated polymers have considerably been synthesized and explored practical potential due to their intelligent performance.Some polymers could alternate the coil-to-globule state in aqueous solution while others presented their similar behaviors in organic solvents such as polystyrene in cyclohexane, polymethyl methacrylate in acetonitrile, etc. In regard to the literature objective, numerous polymers performing a phase transition in aqueous solution were herein discussed (seen in Fig. 1). Nevertheless, a polymer solution exhibited its exclusively different property with ambient temperature depending on chemical structure. Lower Critical Solution Temperature (LCST) and Upper Critical Solution Temperature (UCST) were the most vital criteria at which a solution transitioned from transparent to opaque. Moreover, thermosensitive polymers could be diversified among different LCST and UCST values. Belonged to LCST, Poly (N‑substituted (meth)acrylamide)s, typically, Poly (N‑isoacrylamide) (PNIPAM), which distinctively presented the amphiphilic nature upon heating, was the most heavily studied thermal-responsive candidate. At above the LCST (approximately 32 °C), PNIPAM composed of polar hydrophilic segments expelled the aqueous content to separate of hydrophobic chains out of aqueous solution and formed micellar particles with hydrophobically-edged water [6,7]. In keeping with the fact that Poly(N‑acryloyl‑N‑alkylpiperazine) based thermo-responsive polymers, temperature-regulated Lactam/pyrrolidone/pyrrolidine, Poly(dimethylaminoethyl methacrylate), Poly(alkyloxide) copolymers, Poly(vinyl ether)s and polyethers also functioned via this mechanism [8,9]. Besides, amphiphilic block polymer was produced as a soluble system below LCST and performed reversible phase transition properties after sol-gel conversion to improve its thermo-gelling behavior as well as maintain a sustainable release mechanism [10,11].
 Fig. 1. Examples of some synthetic ammphiphilic (co)polymers for biomedical applications.
Fig. 1. Examples of some synthetic ammphiphilic (co)polymers for biomedical applications.Opposite to the LCST polymer from the variety of quantity to its properties, UCST candidates took a mere contribution to thermo-responsive polymer and they turned into soluble when heating beyond UCST, contrarily to the insoluble state of LCST at the same condition [12]. Poly(acrylic acid‑co‑acrylamide) (P(AAA)) was a considerably typical polymer possessed an UCST value [13].UCST polymers generally existed in a small distribution of the temperature-regulated group, yet they were rarely observed under practically relevant condition [14].
Some of thermosensitive polymeric solutions indicated a distinct phase change which were utilized for delivering hydrophobic drugs and bioactive molecules in the human body (37 °C). Furthermore, the temperature-stimulated polymers have considerably been explored to conjugate to other biocompatible synthetic or natural polymers [15]. In the system, amphiphilic molecules were cleverly designed to maximize drug loading efficiency and control delivery in the nanoparticles or hydrogel platforms. The rationale lied behind their ‘smart” properties of the carriers in which phase transition occurred at the surrounding body temperature with improving hydrophobic drug loading efficiency in its hydrophobic domains or encapsulating others bioactive molecules (growth factors, genes, etc) via electrostatic interaction in the hydrophilic grafted polymer chains [16,17].In the study, we strikingly detailed some prevailing types of amphiphilic polymers and thermosensitive polymeric grafted platforms for functional improvement of conventional materials in biomedical applications.
3. Thermosensitive synthetic platforms
3.1. Liposomes
Liposome was composed by one or several phospholipid doubled-layers and an aqueous core which self-enclosed to spherical vesicles. Since being first described by Bangham in the mid-1960s, it has prevalently been integral to research and clinical applications, especially in drug delivery [[18], [19], [20]]. Due to its unique characteristics, not only were hydrophilic drugs entrapped in internal cavity, but also lipophilic and amphiphilic molecules could incorporate into membrane. Moreover, large internal cavity and biocompatible lamellar exterior beside surface charge of liposome efficiently delivered a variety of macromolecules (DNA, proteins, amphiphilic drugs and imaging agents) [[21], [22], [23], [24]].
Despite initial excitement of potency as drug delivery vehicles, short half-life with the insufficient disposition, inaccurate extrapolation of liposome to site of action, uncontrolled leakage along with fusion and reduced circulation time of liposome-based drugs considerably were key influencing the liposome's disappointment [25,26]. To circumvent the drawbacks associated with instability of self-enclosed structure, liposome has shifted from “first-generation conventional liposomes to “second-generation long-circulating liposomes” and third-regeneration multi-functionally temperature-responsive liposomes, obtained by modulating the lipid composition, size, charge, surface of the vesicle and its controlled release behavior (Fig. 2). Thanks to a wide range of physicochemical and biophysical properties, liposomes were easily modified to control their biological characteristics. A significant step in inducing an inefficient uptake of liposomes by mononuclear phagocyte system macrophages was the presence of PEG on the surface of the liposomal carrier (stealth liposomes), whose first successful clinical product in commercial was Doxil® [19,26,[28], [29], [30], [31]]. Follow the trend of promoting an effectively limiting drug release in the bloodstream, “heat-trigger-based-liposome” was designed with miscellaneous types: conventional temporal sensitive liposome (CTSL), lysolipid-containing low-temperature-responsive liposomes (LTSLs), thermal-dependent polymeric liposome. It newly emerged as a breakthrough of liposomal improvement. Remarkably, temperature sensitization of liposomes was recently achieved by cooperation of the lysolipid into the destabilized CTSLs to form stable pores at the melting phase transition temperature (Tm), increase temperature sensitivity to artificial plasma environment. Interestingly, conventional thermosensitive in which major additives of phospholipid membrane composition such as phosphatidylserine, phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol made profound impacts on ‘rigidity’ (T < Tm) or ‘fluidity’ of the bilayer (T > Tm) [[32], [33], [34], [35], [36]]. Of all, 1,2‑dipalmitoyl‑sn‑glycero‑3‑phosphocholine (DPPC) was the most prevailing component of TTSLs with Tm higher than blood heat [37]. The initial LTSLs, successfully formulated with DPPC: MPPC: DSPE-PEG-2000 in the molar ratio of 90:10:4, significantly promoted rapid drug release in comparison to other conventional liposomes [38]. Due to a large acyl-head group while single hydrocarbon tails, lysolipid favored the highly curved micelles resulting in stabilized accumulation at the boundary of bilayer upon increasing heat. Notably, Thermodox®, a clinical product was applied in dual with hyperthermia, a temperature upward phenomenon in patients with loco-regional breast carcinoma of the chest wall and combined with radiofrequency ablation in patients with primary or metastatic liver cancer [27].
 Fig. 2. The evolution of liposome was from conventional to second-generation long-circulating, then followed by third-regeneration multi-functionally temperature-responsive systems.
Fig. 2. The evolution of liposome was from conventional to second-generation long-circulating, then followed by third-regeneration multi-functionally temperature-responsive systems.Markedly, the temperature-triggered polymeric incorporation into liposome was another promising approach to functionalize non-thermosensitive formulations as well as improve some obstacles. This modification brought to light that membrane disruptiveness, desirably drug-controlled release upon temperature phase transition was considerably improved. Kono et al., initially introduced the N‑isopropylacrylamide and octadecyl acrylate to liposome by radical copolymerization under the dioxane using initiated azobisisobutyronitrile [39]. P(NIPAM-co-ODA)–modified-egg-yolk-phosphatidylcholine (EYPC)-liposome and lysolipid-dipalmitoylphosphatidylcholine (DPPC) - liposome pointed out a significantly increasing molecule release entrapped in their inner aqueous phases compared to original mild forms. Notably, the additional facilitation of sensitive polymer gave a significant synergy to the lysolipid liposome in temperature-controlled membrane disruption. F127-modified liposome modified di-oleoylphosphatidylcholine (DOPC)/cholesterol liposomes could deliver a small molecule fluorescent marker to cancer cells in vitro and in vivo [40]. T. Tagami et al. introduced DPPC/Poloxamer 188 Hybrid Liposome as an excellent administration of pulmonary treatment [41]. Admittedly, entrapped DOX could be rapidly liberated at 42 °C in vitro and presented a cytotoxic effects toward A549 cell lines while it minimized the rate at around 37 °C. Kono incorporated copoly(2‑(2‑ethoxy)ethoxyethyl vinyl ether‑block‑octadecylvinyl ether) to surface of liposome in order to maintain its stabilization at physiological temperatures due to large domination of cholesterol and high critical temperature of the block copolymer [42].
In other subsequent studies, thermo-reversible liposome has been extensively investigated with feasibly clinical potentials. For instances, grafting poly(Nisopropylacrylamide‑co‑acrylamide) (PNIPAM-AAM) and PEG onto liposomal surface ingeniously performed a high release and prolonged the stability of DOX along with averted protein adsorption in the serum [43],or cooperating poly(N‑(2‑hydroxypropyl)methacrylamide mono/dilactate) (pHPMA mono/dilactate) into liposomes could trigger the content release upon their cloud point temperature [44].
3.2. Polyester derivatives
Aside from abovementioned tailored-delivery systems regulated by temperature, body-friendly-degradable and biocompatible copolymers based poly(lactide) such as poly (ethylene glycol)‑co‑ poly (lactic acid‑co‑glycolic acid) (PEG-PLGA), poly (ethylene glycol)‑co‑polylactic acid (PEG-PLA), poly(l‑lactide‑co‑ɛ‑caprolactone) (PLCL), PNIPAam‑co‑poly(L:-lactic acid) (PNIPAam-PLLA), poly(lactide)‑tocopheryl polyethylene glycol succinate (PLA-TPGS) were diversified in the utilization [[45], [46], [47], [48]]. The amphiphilic copolymer solution transformed from its sol state (approximately 2–15 °C) to aqueous insoluble gel phase at blood heat and formed micellar infrastructure with hydrophobic inner cavity to entrap hydrophobic drugs while few molecules could be merely contributed in PEG domains (Fig. 3). Besides, drugs could be contrallably tracked out within hours or months and the combination to other polymers was versatile in further prolong the release profile. Hence, it boosted an intensive development of self-assembled nano-structures which were unswervingly administered to the specific sites and sustainably controlled with the release of incorporated or entrapped molecules.
 Fig. 3. The sol-gel phase change induced a profound impact on the conformational transformation of amphiphilic copolymer. A noticeable result from thermosensitive operation was micellar infrastructure formed with hydrophobic inner cavity to entrap hydrophobic drugs.
Fig. 3. The sol-gel phase change induced a profound impact on the conformational transformation of amphiphilic copolymer. A noticeable result from thermosensitive operation was micellar infrastructure formed with hydrophobic inner cavity to entrap hydrophobic drugs.Additionally, above thermosensitive polymeric derivatives were strongly captivated. Cho fabricated a Triogel (PLGA-b-PEG-b-PLGA) encapsulating triple of hydrophobic drugs coupled with gradually controlling the release profile in response to the physical gel erosion under blood-heat regulation [49]. Remarkably, Samyang's proprietary polymeric micelle technology in aqueous-insoluble-drug formulation has remedied the problems riddled of systemic and local delivery. Particularly, Genexol PM, a patented novel formulation of paclitaxel which progressed in the clinical trials (phase II of locally advanced head and neck squamous cell carcinoma, phase III of metastatic breast cancer and progressively carried out in phase IV) held high potential as a radio-sensitizer of chemo-radiation regiment [50,51]. Similarly noting that BIND-014 (investigated by BIND Therapeutics), a nanomedicine composed of Docetaxel encapsulated in an PSMA-targeted-amphiphilic copolymer grafted by poly (d,l‑lactide) (PLA) and PEG segments (via ACUPA) was promisingly applied in breast, head, neck cancer regiment, etc. (studied at stage I-III of clinical research). In another case, Docetacel-PM consisted of amphiphilic Polyvinylpyrolidone-grafted-Poly (d,l lactide acid) (PVP-b-PDLLA 38%) (DLLA 38 mol%) and CriPec formulated by Docetaxel-embedded- a physical cross-linking of a hydrophilic block coupled to a thermo-sensitive copolymer N‑2‑hydroxypropylmethacrylamidemonolactate (HPMAmLac1) and N‑2‑hydroxypropylmethacrylamidedilactate (HPMAmLac2) were flocked to a huge attention that was hailed as the leader in a wave of next-generation nanotechnology.
Among applicably widespread biomaterials, the nature-driven hydrophobic polyesters poly[(R)‑3‑hydroxybutyrate] (PHB) has been a prevailing research interest due to their biodegradability and biocompatibility [52]. Whereras, their potential in the biomedical field was considerably hampered with the rationale of hydrophobic behavior. To improve those obstacles, amphiphilic thermogels produced by a blend of PEG and hydrophilic functional groups have been on progress with various brilliant properties. Particularly, poly[(R)‑3‑hydroxybutyrate] based poly(ester-urethane)s was applied in blood contact implantation or injectable thermogel poly(PEG/PPG/PHB urethane)s could sustainably control drug release and act as a smart personalized medicine [[53], [54], [55]]. To follow this trend, a multifunctional thermosensitive polyester platforms combined with 2D transition metal dichalcogenides will be prospestive approaches in the bio-nanotechnology [56].
3.3. Dendrimer
Dendrimer has been emerging as one of the excellent candidates in the controlled-drug delivery system over other conventional nanoscale carriers [57,58]. Markedly, it was a well-controlled, nano-sized, radically symmetric polymer with unique three-dimensional highly-branched architecture [59,60]. Up to date, the class of dendrimer differentiated dependent upon the desired applications. Majority of popular outstanding dendrimers in drug delivery were categorized as poly(amidoamide) (PAMAM) [59,[61], [62], [63]], poly(propyleneimine) (PPI) [[64], [65], [66]], poly(l‑lysine) (PLL) [[67], [68], [69]]. Not only did the dendritic nanostructure potentially facilitate the high encapsulation together with maintaining suitable drug-released rate, but also facile modifications by subsequent corporation with smart polymers or hybrid formation considerably reduced side-effects of nanocarrier to improve their bio-compatibility. Yet, dendrimers with primary amine terminal groups had an influencing contribution to cell cytotoxicity comparing to which owned similar backbone but different surface components such as hydroxyl or carboxyl groups [70]. Exclusively, the labile end groups significantly promoted chemical corporations between dendrimer and polymeric molecules resulting in their solubility. In particularly, poly(ethylene glycol) (PEG)-PAMAM dendrimer provided an excellent increment of its affinity to aqueous molecules [[71], [72], [73], [74], [75]]. For long-circulating half-life and high accumulation in tumor tissue due to the (EPR) effect, it seemed reasonable to include a thermosensitive polymeric dendrimer to be external controlled drug release by physiological temperature. Take Poly‑(N‑substituted (meth)acrylamide)s–derivate as an example [76]. Poly (N‑isopropylacrylamide)(PIPAAm) multi-armed dendrimer or dendritic core-shell nanostructure via reversible addition-fragmentation chain transfer (RAFT) polymerization of PIPAAm chains was successfully synthesized in which catalytic activity and drug behavior controlled in response to change in polymer conformations could be applied to many “nanoscopic smart materials [77,78]. Le and coworkers employed its excellently temporal quality to improve the controlled water-insoluble-bioactive-compound delivery system with poly(N‑isopropylacrylamide) (PNIPAM)‑g‑polyamidoamine (PAMAM) G3.5 [79,80]. Toward a highlighting biocompatible nanocapsule, poly(N‑(2‑hydroxypropyl) methacrylamide) (pHPMA) group was introduced to the chain end of PAMAM dendrimers which could be applied as a nonimmunogenic polymer-drug conjugates [[81], [82], [83]]. Whereas, PNIPAM-modified dendrimers majorly bore resemblances of star-like rather than globular polymeric morphology, which resulted from inducing its molecular uniformity [84]. Consequently, isobutyramide (IBAM), n-butyramide (NBAM), cyclopropane carboxylic acid amide (CPCAM) temperature-sensitive segments were preferentially incorporated into dendrimer to retain the “globule” shape [[84], [85], [86]]. In another study relevant to PAMAM dendrimer, Nguyen et al. not only prepared a series highly lipophilic pluronics-conjugated PAMAM dendrimer but also gave a new conclusion that the hydrophobic drug encapsulation efficacy proportionally to the increase of lipophilic pluronicnanocarriers in comparison to other lesser lipophilic groups, which played a key role in delivering poorly water-insoluble drugs and applying to biomedicine [87]. Hydrophobic amino-acid residues, such as l‑phenylalanine and l‑leucine residues and carborane became favorable candidates of the thermo-dependent dendritic functionalization [[83], [84], [85], [86], [87], [88], [89], [90], [91]]. To date, elastin-mimetic dendrimers that a Val–Pro–Gly–Xaa–Gly peptide repetition conjugated to dendritic nanostructure exclusively exhibited a novel heat-controlled structure via the inverse transition temperature [92,93]. With some mentioned amphiphilic dendrimers-based nanocarriers, dendrimers could be decorated with targeting ligands [[94], [95], [96]] and fluorescent probes [97,98] which have been a forward-thinking approach to produce multifunctional dendrimers as demonstrated in Fig. 4.
 Fig. 4. Initial approaches of thermal-facilitated dendrimer in delivery system have gone beyond enhanced strategies in which targeting ligands and fluorescent probes were synergistically bound to dendrimer. When transporting in human body, the blood heat triggered amphiphilic properties along with site targets to produce multifunctional dendrimers.
Fig. 4. Initial approaches of thermal-facilitated dendrimer in delivery system have gone beyond enhanced strategies in which targeting ligands and fluorescent probes were synergistically bound to dendrimer. When transporting in human body, the blood heat triggered amphiphilic properties along with site targets to produce multifunctional dendrimers.3.4. Others thermal-responsive synthetic platforms
Over and above those discussed, copolymerized poly (ethylene glycol)‑b‑polypeptide micelles, particularly, an amphiphilic block copolymer NK105 was self-assembled by the hydrophilic polyethylene glycol (PEG) grafted with the hydrophobic 4‑phenyl‑1‑butanolate-functionalized polyaspartate have performed superior therapeutic efficacy on gastric and breast cancers than conventional PTX-Cremophor [99,100]. Similar is that micellar nanoparticlesNC-6004 Nanoplatin with cisplatin bonds were coordinated to polyamino acid to develop a novel brilliant drug without severe side-effects and to increase antitumor efficacy (at stage I-III of clinical trials in Asia) [101].In another case, squalenoylation technology induced self-assembly into nanosized aggregates of less water soluble hydrophobic compounds. The long-term stability, impressively high payload with initial burst release of squalenoyl-paclitaxel derivatives and linoleic acid-paclitaxel conjugate were significantly improved compared to that of naked drug [102,103].
4. Ampphiphilic derivatives of natural compounds
4.1. Polysaccharide-derived systems
Nature-driven polymeric materials have been evolved for decades in biomedical field encompassing drug-delivery and tissue-engineered scaffolds due to their biodegradability and biocompatibility [[104], [105], [106], [107], [108]]. Of all biomaterials, polysaccharides formed from repeating units joined together by glycoside bonds have been much investigated for biopolymers. Remarkably, polysaccharide bears a chemical structure versatility and biological performance resemblance to the extracellular matrix components, which were associated with immune responses. Thus, modification of natural to thermosensitive polymer significantly enhanced the mechanical strength and biocompatibility of the hydrogels or drug loading efficiency of the nanogels, as well as their interactions with the drugs [109,110].
4.1.1. Celluloses
Among the grafted polymers, cellulose, the most well-known abundant, renewable, and sustainable polysaccharide with condense unbranching β(1 → 4) linked d-glucose units via glycosidic bond [111,112]. Due to the body-friendly degradation polymer, high tensile strength, tunable porous shape, cellulose was an ideal material for tissue engineering applications [[113], [114], [115], [116]]. Yet, multiple hydrogen bonding interactions on the same or neighboring substituents profoundly influenced the highly crystalline-structured polymers, which reduced the hydration of cellulose [[117], [118]]. To exploit the high bio-compatibility as well as overcome minor drawback of poor dissolution, hydroxyl pendant groups were chemically modified into ether and cationic status. Notably, the aqueous-solution-of- cellulose-ethers crosslinked derivatives categorized as ethylcellulose (EC), methylcellulose (MC), hydroxypropyl methylcellulose (HPMC), hydroxyethyl cellulose (HEC) and carboxymethyl cellulose (CMC) [[119], [120]] gained much attentions in thermal-sensitization-driven approaches. Of all, MC has been increasingly studied their key role in bio-related applications with the rationale of its hydrophobic-hydrophilic non-equilibrium under temporal change [121]. Tate et al., introduced a bio-compatibly intracerebral scaffold to repair the central nervous system disorder [122]. Moreover, the PEG/and CS-SO3 entrapped MC-based injectable hydrogels significantly obviated the postoperative adhesion formation [123]. In another case, Kim and coworkers successfully fabricated an in-situ thermosensitive platform based on human adipose derived soluble ECM (sECM) and methylcellulose (MC) for therapeutic cell delivery and rapidly wound healing with minimal scars [124]. Besides, nature-driven or tailor-made polymers was grafted with cellulose to promote the thermo-sensitivity. Cellulose polymerized with N-isopropylacrylamide (NIPAAm) monomers by N, N‑methylene bisacrylamide (BisA) crosslinker or Poly(N‑isopropylacrylamide) (PNIPAAm) grafted methylcellulose (MC) widened the temperature range of swelling and deswelling of hydrogel [[125], [126]]. Similarly, hydroxypropyl cellulose‑graft‑poly(4‑vinyl pyridine) (HPC‑g‑P4VP) synthesized via ATRP, N‑isopropylacrylamide (NiPAAm) and acrylic acid (AA) polymerized from hydroxyethyl cellulose (HEC), hydroxypropyl cellulose‑graft‑poly(N,N‑dimethyl aminoethyl methacrylate) (HPC-g-PDMAEMA) copolymers were self-assembled under both temperature and pH stimulus [[127], [128], [129]].
Additionally, graft thermosensitive synthetic polymer was to impart the temperature-feedback cellulose without distortion of its desirable properties in drug delivery. Yan and coworker exclusively prepared a well-assembled cellulose-based poly(N,N‑dimethylaminoethyl methacrylate)‑graft‑poly(ε‑caprolactone) (EC-g-PDMAEMA-g-PCL) platform by ring-opening polymerization (ROP) and atom transfer radical polymerization(ATRP), which acted as highlighting drug nanocarriers for controlled drug release [130]. Wang et al., developed a Poloxamer 407/Carboxymethyl cellulose sodium (P407/CMCs) composite hydrogel which could parallel function moisture along with delivered drug in atopic eczema treatment [131]. Scheuble and his team engineered a thermoresponsive substrates based on methylated nanocrystalline cellulose (metNCC) and exclusively indicated its interfacial thermogelation influenced in brittle layers compared to commercial methylcellulose [132]. Moreover, temperature-stimulated bacterial cellulose whisker/Poly(NIPAM-co-BMA) was a particular instance of the soft tissue replacement application which controlled reversible thermosensitive phase behaviors with temperature changes [133].
4.1.2. Dextran/starch
Starch is composed of two carbohydrate polymers: amylose (a linear of α‑1,4‑linked glucose homopolymer, peculiarly existed in a α‑1,6‑linkage branch) and amylopectin (a highly branched amylose form).
Starch majorly conversed from powder to gel through gelatinization to be applicable in pharmaceutical potency. After being under gel transformation, amylopectin stabilizes effects, whereas amylose forms gels and has a strong tendency to form complexes with lipids and other components [134]. Dextran is a highly aqueous dissolved component and easily modified via reactive hydroxyl groups. Notwithstanding the disparity of extensive properties, both of starch and dextran have been modified to be outstanding biomaterials for nanoscale construction of cell culture engineering, drug delivery systems and further biomedical applications.
Starch functionalized by chemical reaction with hydroxylation, esterification, acylation, so forth has been in a massive investigation [[135], [136], [137], [138], [139], [140], [141]]. Besides, thermo-responsive starch derivate was among the main promising candidates. A thermal sensitive starch derivates was obtained by hydrophobic butyl groups incorporated into hydrophilic starch (2‑hydroxy‑3‑butoxypropyl starches, HBPS), or 3‑[2‑butoxy(ethoxy)m]‑2‑hydroxypropyl starch ethers (BEmS) was synthesized by hydrophilic oligo(ethylene glycol) spacers introduced into HBPS to shift its LCST ranging from 4.0 °C to 32.5 °C, and 17.5 °C to 55.0 °C,respectively [142,143].
Over and above that, dextran and its derivatives supplied prospective platforms for particular drug encapsulation and release which overcame the resistance of surface–bound dextran and nonspecific protein adsorption [[144], [145], [146]].
Dextran chemical hydrogels, modified by the covalent attachment of hydrocarbon groups (aliphatic or aromatic) via the formation of ether links; glycidyl methacrylate (GMD) or dimethacrylatepoly(ethylene glycol) (DMP) via radical polymerization under the presence of dextranase were considered promising drug and protein delivery systems to modulate the sustained rate of drug release [[147], [148], [149], [150], [151]].Notably, another essential field of dextran was massively investigated was that thermosensitive polymer grafted dextran to boost encapsulating efficacy and controlled release of hydrophobic drugs forward to LCST. It is worth pointing out the responsive dextran derivatives synthesized by multifold pathways such as poly(N‑isopropylacrylamide‑co‑N,N‑dimethylacrylamide [poly(NIPAAm‑co‑DMAAm)] by crosslinking reaction, self-assembled two-aqueous phase system poly(lactide‑co‑glycolide) (PLGA) based microparticleswith the utilization of Pluronic F127-containing ATPS could open promising design and concept of nano-scaled polymeric and liposome particles with strikingly brilliant physicochemical applied in drug delivery and other aspects of biomedicine [152,153].
4.1.3. Chitosan
Chitosan (CS), a linear natural polymer abundantly derived from marine, arthropod and insect skeleton, etc… has been under accelerating investigations for multi purposes of industry as well as biomedical branches [[154], [155], [156], [157], [158], [159]]. Chitosan exists as the extensive deacetylation of chitin (β‑(1–4)‑poly‑N‑acetyl‑d‑glucosamine) composed of units of d‑glucosamine and N‑acetyl‑glucosamine joined together via glycosidic bonds and highly soluble in appropriate acid solutions [160,161]. Remarkably, cationic property of CS considerably facilitate the gel formation or encapsulation of negatively-charged molecules as well as proteins through electrostatic interactions [162,163]. Likewise, CS could conjugate with hydrophobic segments which considerably enhanced the self-assembled amphiphilic particles [164]. The most vital properties of this biopolymer which have made them ideal candidates to fabricate polymeric tissue scaffolds, drug delivery, and wound dressing are: high porosity; biodegradability; predictable degradation rate; structural integrity; biocompatibility and muco-adhesion properties [[165], [166], [167], [168], [169]]. However eco-rich available CS is, it easily degraded inside human body majorly by the enzymatic activity of lysosome leading to uncontrolled-drug release issues. Moreover, physiochemical limitations of chitosan made it difficult to a scaffold for tissue regeneration. Hence, to overcome the bottleneck of CS and widen its applications, crosslinking functionalization or blending the polymer with other natural or synthetic substituents were the most approaches concerned. Multifold chemical modifications conducted such as oligomerization, alkylation, acylation, quaternization, hydroxyalkylation, carboxyalkylation, thiolation, sulfation, phosphorylation, and enzymatization. For instances, alkyl, carboxymethyl, hydroxyl or similar functional substitution could give a drastic rise to chitosan solubility without distortion of its cationic property [[169], [170], [171], [172]]. Chitosan with enzymatically-modified derivatives indicated a higher thermal stability and similar anti-pathogenic activities in comparison with its original form [173].
Besides, recent attention has been focused on the graft polymerization, especially, incorporation with smart polymers which undergo a reversible discontinuous volume phase change bywell-known external heat stimuli aqueous-solublepolymers such as Poly(N‑isopropylacrylamide)(PNIPAm), pluronic and poly(N‑vinylcaprolactam) (PVCL). PNIPAM after preliminarily being activated by free radical polymerization in the presence of chain-transfer agents, it was grafted onto chitosan chain with the coupling facilitation [174]. Bao and his colleagues admirably introduced a stimuli-responsive graft copolymer based on PNIPAm and natural platform CS, in which functionalized PNIPAM was grafted onto alkynyl-CS via click reaction variously induced response to consolutes in dual conditions (diluted and dense aqueous solutions) [175].
Additionally, closely to PNIPAM, Poly(vinyl caprolactam) (PNVCL) has been known to possess noteworthy properties for biomedical applications. PNVCL was incorporated into CS also based on grafting onto copolymerization, a novel approach prevalently applied with high efficiency. Prabaharan et al., introduced a novel copolymer synthesized by the grafted COOH-ended PNVCL segments onto CS with EDC and NHS condensing agents and settled a foundation to further research [176]. Follow the trend, thermosensitive CS derivatives were synthesized by grafting side chains of poly (N‑vinyl caprolactam) [PNVCL] to Chitosan backbone via amide reaction [177,178]. Nevertheless, not only did temperature-dependent PNIPAam/PNVCL-CS considerably improve bio-adsorption, bio-compatibility, pharmacokinetic characteristics but also lengthened the bio-circulation. Prabaharan indicated entrapped ketoprofen in PNVCL was slowly tracked down in plasma condition but high efficacy in tumor sides compared to original CS under the influence of temperature and pH [176]. Cao exhibited a novel thermosensitive PNIPAam–CS copolymer exhibiting in situ gel-forming properties and high tolerance and non-hazard in ocular hypertension, which could enhance capacity of Timolol maleate ophthalmic solution double more than which contained in conventional CS at the same concentration [179]. It was likely to 10‑hydroxycamptothecine (HCPT) [180], Paclitaxel [181]. Notably, CS-g-PNIPAAm was fabricated as an excellently mimicking microenvironment for stem cell regeneration [182]. Park and his group ingeniously synthesized a new thermosensitive injectable pluronic F127-grafted CS hydrogels which performed a great potential in tissue regeneration [183]. These authors also conjugated An RGD (Arg-Gly-Asp) peptide to the grafted copolymer and used as a cell-supporting scaffold for articular cartilage regeneration [184]. In another case, Gemcitabine, an oral administered anti-cancer drug, efficiently entrapped in the grafted copolymer at Hosseinzadeh's lab [185]. The system could be prolonged the drug and slower release rate than that of CS. Moreover, the drug-loaded nanoparticles (NPs) showed specific toxicity on HT-29 colon carcinoma cell line due to the muco-adhesive properties of carrier. Similarly, Tran and his colleagues developed a nanocurcumin-embedded hydrogel based thermosensitive polymer F-127-grafted CS in which the amphiphilic CS played a role as dispersant [186]. The nanocomposite material performed synergic impacts of nanocurcumin and the injectable chitosan hydrogel on enhancing burn/wound healing (Fig. 5). The same behavior of the thermosensitive nanocomposite gelatin-based hydrogel was recorded on wound healing [187].
 Fig. 5. Hydrophobic-bioactive Curcumin was throughout dispersed into self-assembled void of thermo-responsive F127-chitosan hydrogel via ultra-sonication. Besides, the temperature-sensitive nanocomposite gelatin-based hydrogel similarly induced the high effect to burn/wound healing compared to the above-mentioned chitosan.
Fig. 5. Hydrophobic-bioactive Curcumin was throughout dispersed into self-assembled void of thermo-responsive F127-chitosan hydrogel via ultra-sonication. Besides, the temperature-sensitive nanocomposite gelatin-based hydrogel similarly induced the high effect to burn/wound healing compared to the above-mentioned chitosan.Besides, functionalized CS derivatives has been approached to some such up-to-date applications such as more actively targeted temperature hindered polymers with folate-conjugated pluronic f127/chitosan core-shell nanoparticles, fibroblast nanocarriers in tissue engineering technology and the ocular compound delivery [[188], [189], [190], [191]].