1. Introduction
Each person consumes a certain amount of products, but during this process much waste is produced and discarded. The disposal of excess products and waste has been at the forefront of combating climate change. In modern societies, the average amount of waste generated by each consumer is staggering and continually increasing. It is estimated that the typical inhabitant of Western Europe produces more than 450 kg of garbage per year [1]. It must be taken into account that the whole world is committed to economic development and the continuous improvement of living conditions, thus the problem of the depletion of the limited resources of our planet and the management of waste becomes crucial. The global consumption and depletion of materials was highlighted in the Millennium Development Goals formulated in the year 2000 by the United Nations. A set of goals were formed with a heavy importance on the 7th Goal: To Ensure Environmental Sustainability [2]. Additionally, the 2008 Waste Framework Directive includes a 50% recycling target for waste from households, to be fulfilled by 2020 [3]. In 2014, in the United States, approximately 258 million tonnes of municipal solid waste (MSW) was generated. Over 89 million tonnes of the municipal waste was recycled and composted (34.6 wt%), over 33 million tonnes were combusted with energy recovery and 136 million tonnes were placed in landfills [4]. Generally, MSW covers waste from households. However, bulky waste, waste from commerce and trade, office buildings, institutions and small businesses, yard and garden waste, street sweepings, the contents of litter containers and market cleansing waste are included, too. In an expanding and developing economy, MSW is usually defined as the waste produced in a municipality, and it may be classified as either hazardous or non-hazardous. The MSW impact on the environment and quality of life is mainly related to air, water, and soil contamination. Land use, odours, and prejudice against certain types of waste treatment should also be taken into account [5].
1.1. Composition of MSW
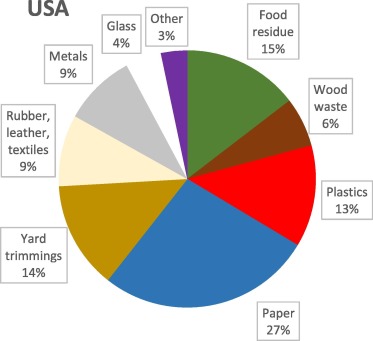
Municipal solid waste collected mainly from households consists of plastics, paper, metals, textiles, organic waste, leather, rubber, metals, glass, ceramics, soil materials and miscellaneous other materials. Fig. 1, Fig. 2, Fig. 3 show the average composition of MSW in the USA [6], China [7] and Europe [8]. Typical household waste contains a wide range of materials that vary significantly in composition depending on the type of community and its consumers’ incomes and lifestyles, and its degree of industrialisation, institutionalism and commercialism. In general, the highest waste generation is correlated with the highest income. Moreover, even the season of year or the number of persons living in a household influence the amount and composition of waste. For example, more food waste and less paper is generated during summer. Additionally, the larger the community, the more garbage is produced per capita [9].
 Fig. 1. Composition of MSW in the USA [6].
Fig. 1. Composition of MSW in the USA [6]. Fig. 2. Composition of MSW in China [7].
Fig. 2. Composition of MSW in China [7]. Fig. 3. Composition of MSW in Europe [8].
Fig. 3. Composition of MSW in Europe [8].1.2. Waste pyrolysis
Pyrolysis is the thermochemical decomposition of organic material at high temperature and in the absence of oxygen or in an atmosphere of inert gases. Compared to combustion, pyrolysis has a lower process temperature and lower emissions of air pollutants such as polybrominated diphenylethers (PBDEs) [10], [11]. Additionally, the scale of pyrolysis plants is more flexible than incineration plants [12]. Nowadays, pyrolysis is getting attention for its flexibility to generate a combination of solid, liquid and gaseous products in different proportions just by the variation of operating parameters such as temperature or heating rate. It also provides an opportunity of transforming materials of low-energy density into bio-fuels of high-energy density, at the same time recovering high value chemicals [13], [14]. One of the great advantages of this process is that many types of raw material can be used, including industrial and domestic residues. Different types of pyrolysis have been developed: fast, catalytic fast, intermediate, slow, vacuum. In practice, the processes of thermal treatment of waste can operate with a small amount of air present. Eventually this may lead to a partial gasification. In those cases, pyrolysis occurs in the inner zone of the bed. These processes are sometimes described as “quasi-pyrolysis” [15]. Other methods of thermal conversion also used in waste management are carbonisation, gasification and torrefaction. Expected yields of products from different types of biomass thermal conversion are shown in Fig. 4. Furthermore, different type of organic matter can be used to charge the reactors such as wood, organic waste (soft and hard biomass) residues from agriculture, forestry, pulping industry. Some examples are forest residues [16], [17], [18], [19], food [20], [21] and agricultural waste [22], [23]. The fractions of MSW subjected to pyrolysis mainly consist of paper, cloth, plastics, food waste and yard waste. Moreover, different types of reactors have been developed, such as fixed bed, fluidized bed, tubular and pyroformed reactor or haloclean rotatory kiln. It is then easy to understand the high variability of conditions and consequently of residues obtainable. Prerequisite for the successful application of pyrolysis is the appropriate choice of input materials and the setting of optimal process conditions. For these reasons, the suitability or unsuitability of selected types of waste and their mixtures for the pyrolysis process has been verified many times by laboratory experiments with subsequent assessment of the quantity and quality of the individual products of pyrolysis [24].
 Fig. 4. Approximate spectra of products from different modes of thermal conversion of biomass [25].
Fig. 4. Approximate spectra of products from different modes of thermal conversion of biomass [25].2. Types of pyrolysis reactors used to utilize different domestic waste
The reactor type being used for the pyrolysis of waste has to be given great importance because of the large amount of heat to be transferred across the reactor wall to ensure material degradation [26]. Reactors described in literature used in the pyrolysis of different wastes include fixed bed reactors, batch or semi-batch reactors, rotary kilns, fluidized bed reactors, microwave assisted reactors and some innovative solutions like plasma or solar reactors. For many years, scientists have explored the mechanisms of this process in laboratories around the world, so slow, fast and intermediate pyrolysis are already well known. Several industrial waste plants successfully use or have used those processes like Waste Pyrolysis Plant “Burgau” located in Germany, which was working for 30 years (until 2015) [27]. However, there is still a big challenge to make pyrolysis economically viable, thus the next studies should focus on the implementation of the latest developments in pilots and on an industrial scale. In this section it will be highlighted which of these solutions could be implemented as industrial solutions, instead of a use only on a laboratory scale.
Usually the pyrolysis process is conducted under atmospheric pressure. In contrast, vacuum pyrolysis is carried out under very low pressures, which can be about 5 kPa. Vacuum pyrolysis has some advantages compared to pyrolysis under atmospheric pressure because of the short residence time of organic vapour in the reactor and the low decomposition temperature, which reduces the occurrence and intensity of secondary reactions [28]. This type of reactor has been used in the pyrolysis of different types of household waste, such as wood [29], plastics [30], [31], printed circuit boards [32] and tyres [33]. However, vacuum pyrolysis is difficult to achieve in practice, thus there is no information about using this process in the pyrolysis of MSW on an industrial scale.
2.1. Fixed bed reactors and batch reactors
Fixed bed reactors are the simplest solution available in laboratory-scale studies, because they are easy to design. In this type of reactor, the feedstock is placed in the reactor (often stainless steel), which is heated externally. The most popular solution is an electric furnace. Before the experiment the reactor is flushed by an inert gas (e.g. N2, Ar) and the gas flow is maintained during the whole process in order to provide an anaerobic atmosphere. The gases and vapours obtained are discharged from the reactor during the pyrolysis, but char is usually removed after the process. The fixed bed reactor is characterized by a low heating rate. Furthermore, the feedstock does not move during the process, thus it is difficult to imagine a uniform heating of a large portion of MSW on an industrial scale. This type of reactor could be used on a larger scale provided that the technology used (e.g. heat pipes) enables better heat transfer [34]. Anyway, the fixed bed reactor is a good instrument for providing experimental information on the parameters of pyrolysis and its products.
In general, batch reactors are a closed system with no input or output of reactants or products while the reaction is being carried out resulting in high conversion. On the other hand, semi-batch reactors allow the addition of reactants and the removal of products, while the process is occurring. However, products are not uniform from batch to batch and increasing the scale is problematic [35]. The other disadvantages of this type of reactor are a long solid residence time and the difficulty of removing char.
2.2. Fluidized bed reactors
Typically, fluidised-bed reactors are used to study the behaviour of fast pyrolysis and to investigate the secondary cracking of oil at longer residence times. Fluidised-bed reactors are characterized by a high heating rate and a good blending of the feedstock. Therefore, such reactors are widely used in laboratory studies in order to describe the influence of temperature and residence time on pyrolysis behaviour and products [12]. This type of reactor seems to be a good solution for waste polymer pyrolysis. For example, polymer pyrolysis in a fluidised-bed reactor can provide remarkable advantages over the processes in other reactors in which heat is not transferred as efficiently for the cracking of polymers because polymers have a very low thermal conductivityand high viscosity [12].
On the other hand, there are important difficulties in using fluidized-bed reactors to utilize MSW. First, the raw material provided to the reactor must be tiny, so it could float in the fluid. Second, there is a big problem with separating the char from the bed material. Thus, this type of reactor is seldom used in large-scale projects. On the other hand, Ding et al. [36] proved, that it is possible to obtain high quality pyrolytic oil from MSW in a fluidized-bed reactor. However, the system was complicated and it would be problematic to scale-up. This system is shown in Fig. 5. The difficulties in sample preparation further complicate the system, and contribute towards the issues regarding viability and industrial scale up. After collection, sorting and drying, the components of MSW were crushed to small pieces. Biomass was pulverised to powder and bulked (with the maximum size being no more than 1 cm). Plastics and papers were crushed (chopped) to pieces of a length less than 5 mm. Then the components were mixed together again. The mixed raw material was dried in the oven at 80 °C for more than 48 h before each experiment, to ensure the elimination of moisture.
 Fig. 5. Pyrolysis of MSW system based on fluidized bed reactor [36].
Fig. 5. Pyrolysis of MSW system based on fluidized bed reactor [36].2.3. Spouted bed reactor
This reactor is suitable for handling particles of irregular texture, fine particles, sticky solids and those with a wide size distribution. Furthermore, the system has a great versatility with regard to gas flow, allowing operation with short gas residence times. Additionally, the excellent movement of the solids in this reactor, which leads to high heat transfer rates between phases, makes this reactor suitable for flash pyrolysis. Moreover, the conical spouted bed reactor is appropriate for continuous operation, which is especially relevant for the implementation of biomass pyrolysis at larger scale [37]. Spouted beds have also been applied successfully in studies of the pyrolysis of a number of polymers including polystyrene [38], polyethylene [39], polypropylene and polyethylene terephthalate [40]. The spouted bed reactor presents interesting conditions for the pyrolysis of waste plastics due to low bed segregation and lower attrition in comparison with the bubbling fluidized bed [39]. In this type of reactor waste plastics melt as they are being fed into the reactor and provide a uniform coating around the sand particles due to their cyclic movement. It also offers high heat transfer between phases and smaller defluidization problems with sticky solids from plastics. The solid flow pattern and the action of the spout decreases the formation of agglomerates [41]. The scheme of the conical spouted bed reactor used in pyrolysis of plastics is shown in Fig. 6. However, there is no information about the use of this reactor with mixed MSW, because they need very small pieces of feedstock to provide the advantages mentioned above.
 Fig. 6. Scheme and design parameters of conical spouted bed reactor used in pyrolysis of waste plastics [42].
Fig. 6. Scheme and design parameters of conical spouted bed reactor used in pyrolysis of waste plastics [42].2.4. Rotary kiln reactor
Rotary kiln reactors have been used in the slow pyrolysis of MSW in plants located in Germany and Japan among others. They usually carry out the process in temperatures around 500 °C with a residence time of about 1 h. This is the only type of reactor, which has been successfully implemented as a practical industrial solution at various scales so far [12], [43], [44]. However, even they may need some pre-treatment of MSW before pyrolysis. Waste should be sorted in order to remove unwanted materials and then shredded [12]. On the other hand, the preparation of waste as feedstock for the pyrolysis process is quite simple. Conventional recycling plants have to use a wide list of expensive and complicated devices, which can separate different type of polymers.
It is important, that solid wastes of various shapes, sizes and heating values can be fed into a rotary kiln either in batches or continuously; this feature allows an extensive use of this type of reactor. Rotary kilns offer better heat transfer to the feedstock than fixed beds and at the same time they are less complicated in operation than fluidized beds. The residence time of the feedstock in the reactor is a very important parameter in the pyrolysis process because it determines the energy received by the charge at a given heating rate. In rotary kilns, residence time is usually a function of the mean volumetric flow and the rotational speed of the kiln and this was studied by Fantozzi et al. [45]. Furthermore, a rotary kiln pyrolyser has many advantages over other types of reactors. For example, the slow rotation of an inclined kiln enables a good mixing of wastes, thus it is possible to obtain more uniform pyrolytic products. Also, the flexible adjustment of residence time can make the pyrolysis reaction easy to perform at optimum conditions [46]. Fig. 7. shows the lab-scale pyrolysis system based on a rotary-kiln reactor.
 Fig. 7. Pyrolysis system based on rotary kiln reactor: (1) thermometer; (2) bearing; (3) gear transmission; (4) electrical furnace; (5) rotary kiln; (6) temperature controller; (7) seal; (8) tube type condenser; (9) filter; (10) total flow meter; (11) computer; (12) gas sampling device; (13) tar reservoir; (14) feed and discharge opening; and (15) adjustable speed electrical machinery [47].
Fig. 7. Pyrolysis system based on rotary kiln reactor: (1) thermometer; (2) bearing; (3) gear transmission; (4) electrical furnace; (5) rotary kiln; (6) temperature controller; (7) seal; (8) tube type condenser; (9) filter; (10) total flow meter; (11) computer; (12) gas sampling device; (13) tar reservoir; (14) feed and discharge opening; and (15) adjustable speed electrical machinery [47].2.5. Microwave assisted reactors
Microwaves lie between infrared and radio frequencies in the electromagnetic spectrum. The wave lengths of microwaves are between 1 mm and 1 m with corresponding frequencies between 300 GHz and 300 MHz, respectively. The two most widely used microwave frequencies are 915 MHz and 2.45 GHz. Microwave energy is derived from electrical energy and most of the domestic microwave ovens use the frequency of 2.45 GHz [48]. Microwave pyrolysis used in waste to energy processes was studied by Lam and Chase [49]. They accurately characterized this process, but they concluded that the growth of industrial microwave heating applications is hampered by an apparent lack of the understanding of microwave systems and the technical information for designing commercial equipment for this type of pyrolysis.
The combination of microwaves and pyrolysis has attracted much attention due to the nature and many advantages of microwave heating. The most important advantages provided by microwaves are uniform and rapid internal heating of large biomass particles, immediate response for rapid start-up and shut down, high energy efficiency, no need for agitation and controllability [50]. This solution was proposed to provide pyrolysis of paper [51], biomass [48] and plastics [26]. The comparison of conventional and microwave-based heating was summarized by Yin [52] and it is shown in Table 1. However, as observed in other studies, the system faces similar issues to the fluidized-bed reactor - the feedstock particles must be very small and also the organic vapours should be removed from the reactor very quickly in order to prevent secondary cracking reactions. Additionally, high operating costs connected with high electrical power consumption must be taken into account.
Table 1. Comparison of microwave and thermal heating [52].
| Microwave dielectric heating | Conventional thermal heating |
|---|---|
| Conversion of energy | Transfer of energy |
| In-core volumetric and uniform heating: the whole material heated simultaneously, energetic coupling at molecular level | Superficial heating: via convection/conduction |
| Rapid and efficient | Slow, inefficient, limited by material thermal conductivity |
| Selective: rapid intense heating for polar substances and ineffective for non polar substances | Non-selective |
| Dependent on material’s properties | Less dependent on material’s properties |
| Hot spots: an effect due to inhomogeneities of microwave field or dielectric properties within a material | No ‘hot spots’ |
| Precise and controlled heating: the energy input starts and stops immediately when the power is turned on or off | Less controllable |
2.6. Plasma reactors
Plasma is an ionized gas considered by many to be the fourth state of matter, next to solid, liquid and gas. It can be considered as a gaseous mixture of negatively charged electrons and positively charged ions, which is created by heating a gas intensively or by subjecting a gas to a strong electromagnetic field. We can distinguish two main groups of plasmas, i.e. the high temperature or fusion plasmas and the low temperature plasmas or gas discharges. Thermal plasma generation can be achieved using a direct current or an alternating current electrical discharge or using radio frequency induction or a microwave discharge. Even a 2.45 GHz magnetron available from a commercial microwave oven can be used to produce plasma.
When carbonaceous particles derived from waste are injected into a plasma, they are heated very rapidly by the plasma, then the volatile matter is released and cracked giving rise to hydrogen and light hydrocarbons such as methane and acetylene [53]. Plasma pyrolysis is becoming of increasing interest due to its manageability, it enables fast heating, and can work effectively at relatively low power consumption [51]. Huang and Tang [53] reviewed thermal plasma pyrolysis technologies in the treatment of organic waste. Thermal plasma pyrolysis of organic waste produces only two streams: a combustible gas and a solid residue, both of which are useful and easy-to-handle products. Gas yields vary between 50 and 98 wt%. This combustible gas is composed of H2, CO, C2H2, CH4, and C2H4 and has a heating value in the range of 4–9 MJ/Nm3. Thus it can be used directly as a fuel in various energy applications such as direct firing in boilers, gas turbines or gas engines. Guddeti et al. [54] reported that the solid residue from polypropylene pyrolysis contained almost 100% carbon. They observed some novel carbon structures, indicating the potential of several high value applications of this solid carbon such as production of high surface area catalysts, carbon adsorbent or electronic applications such as super capacitors.
2.7. Solar reactors
A very interesting solution for heating the pyrolysis reactor was proposed by Zeng et al. [55]. They investigated the pyrolysis of beech wood in a laboratory-scale solar reactor. The pyrolysis experiments were carried out in a transparent Pyrex balloon reactor under an argon flow. The wood pellet was placed in a graphite crucible insulated with black foam and located at the focus of a 1.5 kW vertical-axis solar furnace. The schematic of the experimental setup is shown in Fig. 8. This construction allows the system to reach temperatures between 600 °C and 2000 °C without any additional heating sources. The aim of this study was to check the effect of temperature and heating rate on char composition and structure. The highest char yield was about 14%, which was obtained at 600 °C with a heating rate of 50 °C/s and the lowest char yield was about 6.5% when the temperature and heating rate were 2000 °C and 450 °C/s.Fig. 9
 Fig. 8. Schematic of the solar pyrolysis experimental setup [55].
Fig. 8. Schematic of the solar pyrolysis experimental setup [55]. Fig. 9. Chemical composition of wood, wt% [61].
Fig. 9. Chemical composition of wood, wt% [61].Although this is a laboratory-scale study, it deserves more attention. The possibility of using renewable energy resources to provide energy to endothermic reactions makes pyrolysis more environmentally friendly. In addition, the energy efficiency increases. This should be the direction of the latest design solutions.
3. Types of waste treated by pyrolysis and products obtained
3.1. Wood and garden waste
Each kind of biomass potentially can be used as an energy source replacing conventional fossil fuels. Municipal Solid Waste may contain significant amounts of garden waste, which are generated during maintenance of private gardens and public parks. It consists of an organic fraction (e.g. grass clippings, hedge cuttings, material from pruning, leaves, and wood) and an inorganic fraction (e.g. soil and stones) [56]. Used furniture, waste construction wood, chopsticks and toothpicks also appear in the category of household rubbish. The biomass residues can be processed in order to recover their organic content in a useful form [57]. It is noteworthy, that biomass is neutral in terms of CO2impacts, it emits as much CO2 when burned as it had previously absorbed from the atmosphere, the net effect is zero [58]. In general, wood biomass is composed of three main components (hemicellulose, cellulose and lignin), with some extractives [59], and it can be divided into two basic groups: soft and hard wood. A simple chemical composition of them is shown in Fig. 8. and During the pyrolysis process the hemicellulose breaks down first at temperatures of about 200–250 °C, then cellulose decomposes in the temperature range of 240–350 °C and finally lignin is pyrolysed at temperatures of about 280–500 °C. In general, the pyrolysis of wood requires a temperature of at least 300–375 °C [59], [60].
In 2015, Dalla Vecchia Torri et al. [62] published the characterisation of bio-oils derived from hard and soft wood using fast and intermediate pyrolysis. In particular their study focussed on Eucalyptus sp. (hardwood formed by syringil-guaiacyl lignin) and Norwegian spruce Picea abies (softwood formed by guaiacyllignin) and the use of the most appropriate pyrolysis system for fast pyrolysis (a bubbling fluidized bed reactor), also using an alternative and more robust system and pyrolysis process (fixed bed reactor and intermediate pyrolysis). The residues were then characterized using time of flight gas chromatography and gas chromatography with quadrupole mass spectrometry. The influence of final temperature (400, 550 and 700 °C) and mass (5, 7 and 9 g) were investigated, while nitrogen flow (1 mL/min) and heating rate (100 °C/min) were kept constant in a procedure similar to the one used by Faccini et al. [17]. Raw bio-oil obtained from intermediate pyrolysis with Eucalyptus sp. chips had a yield of 49 ± 1.3%, while Picea abies residues provided 50 ± 5.7% oil. Bio-oils from intermediate pyrolysis were mainly composed of phenol, followed by ketones in both biomasses, while bio-oils from the fast pyrolysis of Eucalyptus residues showed a slight change in composition, with ketones as the major class, followed by phenols. Furthermore, a great number of phenolic compounds in intermediate pyrolysis bio-oils was noticed, especially methoxy derivatives of lignin breakdown, and these are related to slower heating rates, which allow the increase of secondary reactions and, consequently, the production of oxygenated compounds of lower molecular weight. In 2016, Widiyannita et al. [63] also investigated the pyrolysis of hard wood (ulin wood) at 300 °C, 400 °C, 500 °C, 600 °C and 700 °C. As expected, the temperature significantly influenced the characteristics of pyrolysis products. The optimum temperature to produce a liquid product was 400 °C, and the highest amount of char was obtained at a temperature of 300 °C. The highest temperature pyrolysis produced char with the lowest pore size. The gas yield was the lowest pyrolysis product and it consisted mainly of CO, CO2 and CH4.
Kim et al. [64] checked the influence of reaction conditions on bio-oil production from the pyrolysis of construction waste wood. They used waste wood collected at a landfill site. It was a mixture of plywood, particle board, scantlings and natural wood. Material was pulverized and dried before pyrolysis. Decomposition of waste wood began at 200 °C and mass reduction took place until the temperature was about 400 °C. For both reactors used (batch reactor and fluidized bed reactor) the gas yield increased with increasing temperature (from 400 °C to 550 °C), whereas the char yield decreased with increasing temperature. The maximum oil yield was obtained at 500 °C: 54.2% and 59.9% for the batch and fluidized bed, respectively. Higher temperatures are preferable for generating gas. The gas fraction was composed mainly of CO, CO2and light hydrocarbons (C1-C4). A huge amount of carbon oxides (approximately 90%) is produced by decarbonylation of carbonyl groups and decarboxylation of carboxylic acid groups. The CO2 fraction decreased with increasing temperature but the fractions of CO and C1-C4 species increased with increasing temperature. The fluidized bed reactor produced larger quantities of CO and hydrocarbons. The moisture content in bio-oil was 20–30%, which is typical for oils obtained from biomass. The moisture reduces the viscosity thus improving the fluidity and atomisation characteristics, but on the other hand decreases the heating value and can cause phase separation in the bio-oil obtained. In the applications of a fluidized bed reactor the moisture content was lower than in the case of a batch reactor. The oils consisted of the following six categories: acids, oxygenates, aromatics, phenolics, N-compounds and hydrocarbons. Oxygenates and acids reduce the heating value and stability of the oil. In the batch reactor they consisted of almost 60% of the oil, slightly less in a fluidized bed reactor, and the amount of them decreased with increasing temperature. Phenolics – which improve the oil quality - represented approximately 30% of the oils in a batch reactor and around 40% in a fluidized bed reactor and the yield increased with temperature. The authors concluded, that a fluidized bed reactor is favourable for the production of bio-oil from wood waste. Furthermore, those reactors can be particularly useful for the production of bio-oils from the waste of the wood industry, which produces huge amounts of sawdust.
Construction wood is comparatively rare in MSW, but furniture can be found more often in waste from households. Compared with raw wood, furniture wood contains oils, adhesives, paints and varnishes used in fabrication. One of the most popular additives is formaldehyde-based resins. Thus the composition of products - especially gases - from the pyrolysis of waste furniture should be measured, to ensure there are no threats to the environment and to people. In 2010 Heo et al. [65] investigated the fast pyrolysis of waste furniture sawdust. Before the experiment the sawdust was dried at 110 °C for 24 h. The pyrolysis was carried out in a fluidized bed reactor at temperatures ranging from 400 to 550 °C. The gas composition at 450 °C was 28.0% CO, 62.3% CO2, and 9.7% light hydrocarbons (C1–C4). When the temperature was increased, the char yield decreased from about 35.8% at 400 °C to 21.3% at 550 °C, while more pyrolysis vapours were released. The oil yield was a maximum of 58.1% at 450 °C and then it decreased at higher temperatures. It was noticed, that the water content in the liquid phase was high (40–60 wt%) and it might be caused by the various organic additives in waste furniture. The major components of oils were acids, oxygenates, phenolics and PAHs (polycyclic aromatic hydrocarbons). As shown in Fig. 10., the amounts of acids rapidly increased with increasing temperatures up to 500 °C. On the other hand, the oil became rapidly less oxygenated at temperatures above 500 °C because of the secondary decomposition of the pyrolysis vapours. The amounts of phenolics were high at 400 and 550 °C as there was a gradual decrease in the amount of most of the phenolic compounds, while certain compounds (phenol, 2 and 3-methyl-phenol, and 1,2-benzedediol) rapidly increased with increasing temperature.
 Fig. 10. Changes in composition of oil with increasing temperature in a fluidized bed reactor, in the fast pyrolysis of waste furniture sawdust [65].
Fig. 10. Changes in composition of oil with increasing temperature in a fluidized bed reactor, in the fast pyrolysis of waste furniture sawdust [65].Moreno and Font [66] studied the kinetics of waste furniture pyrolysis and the evolved gases for tests carried out at 500 °C and 850 °C in a tubular reactor. In the volatile gases evolved, the differences between furniture wood waste and solid wood are in the occurrence and concentration of nitrogen compounds. In the pyrolysis of furniture wood waste NH3 (ammonia) was found, in the pyrolysis of raw solid wood the amount of NH3 was insignificant. Other nitrogen compounds such as acetonitrile, 2-propenenitrile, pyrrole and pyridine, 2-methyl appear in the furniture wood waste, too. The nitrogen content can be due to the nitrogenated organic resins, such as formaldehyde-based resins. The concentration of PAHs was also measured. Significant amounts of naphthalene, acenaphthylene, acenaphthene, fluorine, phenanthrene, anthracene, fluoranthene, pyrene and benzo[a]anthracene were found at 850 °C. From this study, there is a need to control the emission of toxic gases from pyrolysis, especially when real waste is treated. This case shows the difference between the pyrolysis of raw solid wood (practically not found in garbage) and real wood waste. The yields of gas, oil and char from the pyrolysis of wood biomass are summarized in Table 2.
Table 2. Products from the pyrolysis of wood biomass.
| Type of wood | Pyrolysis condition | Pyrolysis products | References | |||||
|---|---|---|---|---|---|---|---|---|
| Reactor | Tempera-ture, °C | Heating rate, °C/min | Gas | Oil/tar | Char | |||
| Yield, wt% | Yield, wt% | Details | Yield, wt% | |||||
| Poplar (hard wood) | Fixed bed | 400 | 50 | ∼26.2 | ∼41.8 | Anhydrosugars, furans, aldehydes, ketones, acids, phenols, and hydrocarbons; pH: 3.1–3.9; heating value: 11.85–14.39 MJ/kg | ∼32 | [74] |
| 500 | ∼30 | ∼43 | ∼27 | |||||
| 600 | ∼31.7 | ∼41.6 | ∼26.7 | |||||
| Pine (soft wood) | Fixed bed | 300 | 10 | ∼10 | ∼25 | At low temperatures: wide variety of light molecular carbohydrates and their derivatives, such as saccharide, furan, carboxylic acid, ester, ketone and aldehyde (no D-glucose); at higher temperatures: many guaiacols and phenols (without PAHs) | ∼65 | [75] |
| 400 | ∼18 | ∼53 | ∼29 | |||||
| 500 | ∼16 | ∼60 | ∼24 | |||||
| 600 | ∼19 | ∼60 | ∼21 | |||||
| 700 | ∼20 | ∼59 | ∼21 | |||||
| Soft wood bark | Vacuum | 500 | 27.4 | 45.0 a | Separated into bottom and upper layer; composition: carboxylic acids, polyaromatic compounds such as naphthalenes, phenanthrenes and anthracenes, aliphatic hydrocarbons (C21–C27), sterols, methyl esters, phenols, benzenediols, furans, cyclopentens, alcohols and sugars; pH: 2.34–3.03; heating value: 24.3–42.4 MJ/kg | 27.6 | [61] | |
| Hard wood bark | 12 | 19.9 | 55.9 a | 26.2 | ||||
| Eucalyptus (hard wood) | Semi-batch | 400 | 20 | ∼20.5 | ∼45 | Phenol derivatives, heterocyclic derivatives, aromatic carboxylic acid derivatives; pH:1.8–2.9; heating value: 16.093 MJ/kg; flash point: 68 °C | ∼34.5 | [76] |
| 500 | ∼23.5 | ∼47.5 | ∼29 | |||||
| 600 | 30.35 | 43.7 | 25.95 | |||||
| Spruce (soft wood) | Horizontal tube reactor | up to 752 °C | 28.9b | 39.7b | Two liquid phases: an aqueous phase containing a wide variety of organo-oxygen compounds of low molecular weight and an oil phase containing insoluble organics of high molecular weight; the highest yield in 527 °C; sulphur content: 0.01 wt% | 32.4b | [60] | |
| Bamboo c | Fluidized bed | 400 | 100 | ∼19 | ∼27 | Very hydrophilic and thermo-active; with temperature increasing, percentage of moisture increased, while heating value of oil decreased; heating value: 21.41–28.15 MJ/kg | ∼26 | [77] |
| 500 | ∼20 | ∼31 | ∼20 | |||||
| 600 | ∼24 | ∼27 | ∼18 | |||||
| 700 | ∼33 | ∼18 | ∼17 | |||||
- a
-
oils + aqueous phase.
- b
-
average composition.
- c
-
moisture was measured separately.
There is a lack of information about the pyrolysis of mixed garden waste, because this is mainly converted into compost, which seems be a reasonable solution to the problem of disposal. However, this process needs both a long time period and controlled conditions to result in a good quality fertilizer. If the available area is limited and the amounts of garden and food waste generated are significant, there is a need to propose a faster method to treat this type of garbage. In this case pyrolysis could be considered. Hedman et al. [67] warn against the uncontrolled burning of garden and domestic waste, because the emissions of dibenzodioxins, dibenzofurans and polychlorinated biphenyls are alarming. Some studies investigated the pyrolysis of leaves [68], [69], branches [70], [71], bark [61] and grass [72], [73], but usually only certain species. Despite this it can be assumed that garden waste will behave during pyrolysis alike other types of biomass.
3.2. Food waste
In some areas food residue accounts for more than a half of the total waste generation. It is estimated that as much as 50% of the food produced is wasted before and after reaching the consumer, amounting to over 1.3 billion tonnes per year of food produced globally for human consumption [78]. Food waste contains lipids, carbohydrates, amino acids, phosphates, vitamins and other substances containing carbon, thus it can be a valuable source of fuels [79]. The food waste can be divided into several groups as follows: organic crop residues, catering waste and derivatives (including used cooking oils), animal by-products and mixed domestic food waste [78]. The pyrolysis of selected food wastes, such as fruit peels [80], [81], [82], potatoes peels [83], nuts shells [84], [85], [86] or bones and meat [87], [88] has been investigated and reported in literature. However, many of works focused on bio-chars. Girotto et al. [89]described the problem of food waste utilization to produce useful products such as bio-oils. Pyrolysis was mentioned as a method with potential in treatment of food waste, but the effectiveness of the process is strongly dependent of waste composition. Pyrolysis of mixture of food waste have been considered in limited applications so far, because of the high composition variability of this waste.
Liu at el. [90] investigated the treatment of food waste by pyrolysis with microwave heating. Food waste was collected from a residential area in China. Fruits, plastic and shells were removed from the raw food waste, thus the remaining three main components were white rice, vegetable leaves, and meat/ bones, with proportions of 32.69%, 44.23% and 23.08%, respectively. The measurement of the temperature profiles of food waste under different microwave powers was one of the main aims of this work. The composition of the products obtained has not been considered. When the microwave power was increased from 300 to 600 W, the yield of solid residue decreased sequentially, the gas yield increased continuously, and the bio-oil yield first increased, and then decreased. The optimal level of power for pyrolysis was 400 W. At the same time Zhang at al. [91] studied the fast pyrolysis of food waste. The conduct of the study was very similar to the investigation mentioned above. For the fast pyrolysis of food waste at 600 °C, there were various oxygenates in the pyrolysis vapour product (e.g., acetic acid; furfural; 2-cyclopenten-1-one, 2-hydroxy-; 2-cyclopenten-1-one, 2-hydroxy-3-methyl-; cyclopropyl carbinol; 1,4:3,6-dianhydro-πd-glucopyranose; benzofuran, 2,3- dihydro-), and there were almost no hydrocarbons and aromatics. Moreover, the oxygen content in the pyrolysis vapour product was very high - 32.26%.
Apart from the main components of pyrolysis products it is worth considering the presence of other potentially unsafe compounds. In fact, the transformation of materials during pyrolysis can produce many pollutants, such as sulphurous compounds, heavy metals, nitrogen compounds, etc. The concentration of these components is also heavily dependent on the composition of the raw material in the process. Debono and Villot [92] tried to find the reaction pathway of nitrogen compounds during the pyrolysis of various organic wastes. They examined food waste and sewage sludge from cruise ships and also common softwood from gymnosperm trees (each alone and as a mixture). A homogenous portion of 5 g of waste was placed in the reactor, which was heated at 20 °C/min to 500 °C, while purged by argon as a carrier gas. When heated, wastes were transformed into char, tars and gas. The nitrogen distribution in condensable products (char and tars) was high so that the nitrogen in the wastes is presumably stable. Researchers observed the presence of 18 nitrogen compounds in the pyrolysis gas·NH3, HCN (hydrogen cyanide) and three types of compounds were identified: nitriles, heterocyclic compounds and amides. In the tars were identified 72 nitrogen compounds, which can be divided into six families: nitriles, heterocyclic compounds with one nitrogen atom, heterocyclic compounds with two nitrogen atoms, amides, amines and oximes. In organic wastes, like waste food, the main sources of nitrogen are the proteins. Hence they can be considered as the main sources of nitrogen products. Therefore, the pathway proposed in this study was based on the degradation of proteins.
In 2016 studies on the pyrolysis of food waste were carried out in Ostrava, Czech Republic. Grycová et al. [79] conducted studies on the pyrolysis of samples of waste cereal and peanut crisps at a final temperature of 800 °C. They obtained 62% and 46% of oils with a heating value 12 MJ/kg and 25 MJ/kg from peanut crisps and cereal, respectively. However, they recommended its further use for energy recovery after the separation of water, because of the noticeable water content. The gas yield was about 15–25 wt% and the gaseous components were analysed. The variation of the gas compositions as a function of temperature was clear: there was an increase in temperature accelerated hydrogen evolution. On the other hand, the concentrations of measured hydrocarbons and carbon monoxide decreased with the increasing temperature as described by Kalinci et al. [93]. The sorption capacity of chars was investigated, too. The surface area of tested pyrolysis chars was very small (below 10 m2/g) [79]. Thus, in order to use them further, their surface area could be increased by activation and/or some chemical treatments. What is commendable, this analysis looked at all of the pyrolysis products, though to a limited extent.
3.3. Paper
Households, offices and commercial establishments are the three main sectors for paper consumption. White paper is used for academic purposes, newsprint paper is used in newspapers and corrugated cardboard is widely used in packaging. In addition, various types of paper are used for different purposes, for instance, glossy paper is used for magazines. Furthermore, paper use is widely classified into three categories, namely, industrial use (for filtering, packaging, electrical use, and wrapping), cultural use (for printing, writing, newspaper, and currency), and food packaging (for candy wrappers, food wrappers, tea bags, coffee cups and filters) [94]. This enormous consumption of paper makes it the major component of the combustible fraction of solid waste, accounting for about one third of typical municipal solid wastes. It is an appropriate combustible material and has low contents of nitrogen and sulphur. It may have sufficient feedstock for waste-to-energy utilization [51]. In 2015, 71.5% of all paper consumed in Europe was recycled, corresponding to 1.2 million tonnes more than the 70% target in 2010. In Europe, paper fibres are reused 3.5 times on average, and the world average is 2.4. Theoretically paper can be reused six to seven times, but usually it is impossible in practice [95]. Table 3. below summarises the product allocation from the pyrolysis of different type of waste paper.
Table 3. Yields from pyrolysis of different paper waste products.
| Type of pyrolysis | Reactor |
Tempe-rature, °C |
Heating rate, °C/min | Type of waste | Product yields, wt% | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Gas | Oil | Char | ||||||
| Vacuum pyrolysis, pressure: 5 mmHg | Tubular furnace | 300 | 10 | Waste paper | 12.66 | 42.18 | 45.16 | [96] |
| 330 | 13.56 | 42.97 | 43.57 | |||||
| 360 | 13.88 | 44.04 | 41.08 | |||||
| 390 | 15.32 | 45.57 | 39.11 | |||||
| 420 | 16.17 | 46.86 | 36.97 | |||||
| 450 | 17.74 | 47.03 | 35.23 | |||||
| Slow, reaction time: 1 h | Semi-batch | 400 | 10 | ∼23 | ∼41 | 36 | [103] | |
| 500 | ∼23 | ∼45 | ∼32 | |||||
| 600 | ∼24 | 48 | ∼28 | |||||
| 700 | ∼31 | ∼42 | ∼27 | |||||
| 800 | ∼39 | ∼36 | ∼25 | |||||
| 900 | ∼44 | 33 | 23 | |||||
| Slow, reaction time: 15 min | Fixed bed | 800 | 10 | Paper biomass | ∼53 | ∼15 | ∼32 | [104] |
| Slow | Semi-batch | 325 | 20 | Paper cups | 26.67 | 30.33 | 43 | [14] |
| 350 | 21.27 | 39.23 | 39.5 | |||||
| 375 | 16.87 | 47.13 | 36 | |||||
| 400 | 13.87 | 52.53 | 33.6 | |||||
| 425 | 26.87 | 42.33 | 30.8 | |||||
| Preheated lab-scale facility | Batch reactor | 400 | – | Printing paper | ∼32 | ∼25 | ∼43 | [98] |
| 500 | ∼38 | ∼21 | ∼41 | |||||
| 600 | ∼41 | ∼19 | ∼40 | |||||
| 400 | Cardboard | ∼31 | ∼34 | ∼35 | ||||
| 500 | ∼40 | ∼31 | ∼29 | |||||
| 600 | ∼50 | ∼22 | ∼28 | |||||
| Slow; reaction time 2 h | Packed-bed | 400 | 10 | Cardboard | ∼31 | ∼39 | ∼30 | [105] |
| 500 | ∼32.5 | ∼39.5 | ∼28 | |||||
| 600 | ∼32 | ∼42 | ∼26 | |||||
| Fast pyrolysis | Rotating Microwave Reactor | Below 200 | – | Office paper | 15 | 42 | 43 | [50] |
| Intermediate; reaction time: 150 s | Flow screw | 650 | Tetrapak® cartons | 61 | 16 | 24 | [102] | |
| 850 | 76 | 5 | 19 | |||||
| Slow; reaction time: 1 h | Semi-batch | 400 | 5 | Tetrapak® cartons | 23.9 | 39.4a | 36.7b | [106] |
| 500 | 23.3 | 51.2a | 25.5b | |||||
| 600 | 23.9 | 50.7a | 25.4b | |||||
- a
-
char + aluminium.
- b
-
tar + wax,
3.3.1. Waste paper
Li et al. [96] investigated waste paper pyrolysis in tubular furnace pyrolysis equipment. The process was conducted at different pyrolysis temperatures and heating rates to check the product distribution, yields of pyrolysis products, and oil composition. They did not consider the composition of other pyrolysis products. Waste paper used in that experiment was collected from the waste material market. Before pyrolysis the waste paper was dried at 90 °C for 12 h to reduce the moisture content. The maximum bio-oil yield of 49.13% was achieved at a temperature of around 420 °C with a heating rate of 30 °C/min. The yield of gas increased with increasing pyrolysis temperature. The highest yield of gas of 18.23% and the lowest yield of char of 33.43% were obtained with a heating rate of 30 °C/min at a pyrolysis temperature of 450 °C. At a higher temperature, the secondary decompositions of the char and secondary cracking of vapours take place and enrich the contents of the gas product. The results of spectroscopic and chromatographic analysis showed that oil consists of many different chemical classes and there were four main different compounds in it: anhydrosugars, carboxyl compounds, carbonyl compounds and aromatic compounds. The oil consisted of two phases, one aqueous containing water, but also including appreciable quantities of acetic acid and other oxygenated compounds, and one non-aqueous containing tar, and levoglucosan. In contrast, the main components of oil obtained from the pyrolysis of paper pulp at 800 °C in descending order were naphthalene, styrene, benzene 1-ethynyl-4-methyl- and phenol [97].
Zhou et al. [98] studied the behaviour of printing paper and cardboard during pyrolysis at 400 °C, 500 °C and 600 °C. The aim of this study was to simulate real fixed bed gasifier conditions. For both type of waste, syngas yields increased, whereas char and tar yields decreased with increasing temperature from 400 °C to 600 °C. The temperature for maximum tar yield from printing paper was around 400 °C. Cardboard pyrolysis produced a higher tar yield and a lower char yield than printing paper. The gas produced consisted of CO2, CO, H2, CH4 and other light hydrocarbons (C2-C4) and their concentration was dependent on the process temperature. The major oil components were phenolics, benzenes, naphthalenes, benzofurans and cyclopentens. Aliphatic compounds occupied a quite small fraction of the oil. This extensive study could be very useful in predicting the behaviour of paper biomass during pyrolysis. However, it would have been good to have added the heating value of the pyrolysis products and some additional properties, which would have made it easier to evaluate their potential applications.
3.3.2. Waste paper cups
Paper cups are designed for single use and then must be disposed or recycled. Paper cups are not usually recycled; in general the disposal either leads to landfill or they are burnt in a mixture of general waste. Recycling paper cups could be potentially difficult because of their composition as a combination of paper and paraffin. A basic cup is typically made of 95 wt% of paper (cellulose wood pulp) and 5 wt% of polyethylene for coating, to improve its water resistivity and resistance to heat [14]. Paper cups may consume more non-renewable resources than cups made of polystyrene foam. This is because the wood for the paper cups has to be transported by road or rail to the manufacturing plant. The petrochemicals needed to make polystyrene cups is taken to the plant through a pipeline. Moreover, only about half the wood chips are turned into pulp paper cups. Bark and some wood waste is burned to supply energy for the process, which finally requires about 12 times as much steam, 36 times as much electricity and twice as much cooling water as the process used to make a polystyrene cup [99]. This is alarming information.
Several researchers investigated various aspects of the pyrolysis of waste paper cups [14], [99], [100]. For example, Jankovic [99] investigated the pyrolysis of paper cups at different heating rates up to the final temperature of 700 °C using thermo-analytical techniques aimed at obtaining a detailed mechanistic scheme of the process under non-isothermal conditions. He identified the active pyrolysis zone in a temperature range of 250–400 °C with three decomposition stages and one sub-stage. The activation energy was considered as a constant value 135.8 kJ/mol. Singh et al. [100] also made thermogravimetric analysis of waste paper cup pyrolysis. They found, that the weight loss started at 300–400 °C and finished around 500 °C. Air and nitrogen were compared as the flow medium, and the pyrolysis temperature was higher in the case of the nitrogen atmosphere. At the same time, Biswal et al. [14] studied the pyrolysis of paper cup waste in a semi batch reactor at a temperature range from 325 °C to 425 °C. The reaction time was reduced with increase in temperature (from 24 to 8 min). The maximum liquid yield was 52% at a temperature of 400 °C. The amount of gas decreased with rising temperature up to 400 °C and then increased. The highest yield of gas (26.87 %) was gained at 425 °C. The groups of compounds present in the pyrolytic oil were aldehydes, ketones, carboxylic acids, esters, alkenes, and alkanes. It was found that the pyrolytic oil contained many compounds having carbon chain length in the range of C6–C18. The physical properties of pyrolytic oil obtained were similar to other pyrolytic oils and poor quality fuels. The gross calorific value of the pyrolytic oil was 23 MJ/kg and its water content was 9.1%. It is interesting that the pour point was minus 12 °C, which may lead to freezing problems in colder regions with sub-zero climates.
3.3.3. Tetrapak® cartons
The multi-layer polycoated paperboards, often called ‘tetrapaks’ or ‘tetra briks’, are widely used as aseptic packages for beverages like milk, juice or wine. This packaging system allows products once considered perishable to be distributed and stored without refrigeration for periods of up to six months or even more. The components in tetra pack are generally kraft paper (about 70%), low-density polyethylene (approximately 25%) and aluminium foil (remainder – about 5%) [101]. The conscientious consumer, who wants to segregate waste, will have a serious problem with classifying those containers, so most of them will enter mixed waste and finally be sent to landfill. There is an urgent need to find a proper treatment method for them. Pyrolysis seems to meet the requirements, because it is a suitable treatment method both for paper and plastics. Additionally, the aluminium can be removed relatively easily from solid residue.
Haydary [102] studied the pyrolysis of aseptic packages aimed at maximum gas production and minimum tar fraction. The total amount of gas produced and also the content of H2 and CO in gas increased with increasing temperature, while the content of hydrocarbons in gas and the total amount of liquid yield decreased. The maximum gas yield was 76% at 850 °C. The liquid fraction consisted of organic oils and water. The water and organic phases were not separated and studied in detail. Solid residue yields varied between 20 % and 25%. The proportion of carbon in the solid product decreased from 68% at 650 °C to 52% at 850 °C with the temperature increasing. However, the content of ash in the solid product increased from 21% to 26%. Aluminium residue was easily separated from the solid product. At temperatures below 750 °C, the Al was obtained without any visible structural or chemical changes, but colour and structure of the Al foil changed at higher temperatures. The remaining part of the solid product was formed of low sulphur and nitrogen solid fuel with an ash content of around 22% and heating value of 15 MJ/kg. This study shows that pyrolysis allows the effective treatment of even the most difficult waste of complex composition without damage to the environment. However, the accurate analysis of oils should be carried out in order to characterize the process entirely.
3.4. Rubber
Rubber compounds may appear in domestic waste streams, although this component is small and typically does not exceed a few percent. The biggest source of natural and synthetic rubber in waste is scrap tyres. In 2013, the used tyres in European Union countries were estimated at 3.6 million tonnes [107]. In the U.S. about 4 million tonnes was generated in 2015 [108]. Apart from tyres, other sources of rubber can be cable insulation, shoe soles and gloves, etc.
3.4.1. Tyres
Since the pyrolysis of waste tyres has been widely considered previously [109], [110], [111], [112], [113], it will be just briefly reviewed. In general, when whole used tyres are processed, four output streams are produced: gas, liquid (oil), solid (char) and steel. The composition of each fraction strongly depends on the pyrolysis conditions used and on the tyre composition. Temperatures of about 500 °C are considered to be optimal for the pyrolysis of tyres. The pyrolysis solid residue is mesoporous material with an average heating value of 30 MJ/kg, composed of reinforcing carbon black used in tyre production and other inorganic compounds formed during the pyrolytic process [33]. The char yield varies between about 35 and 55 wt%. The liquid-phase of pyrolysis products is usually named pyrolysis oil. Its main compounds are xylenes, trimethylbenzenes, dimethylstyrenes, dimethylindenes and limonenes and some heteroatom-containing compounds [114]. Oil yields vary between 38 and 56 wt% and the heating value is about 40–43 MJ/kg [115]. Gas obtained from the pyrolysis of waste tyres can range from a few per cent to more than ten percent of the products. It has a high heating value, up to about 84 MJ/Nm3 or 42 MJ/kg [110]. It can be said that gas-phase products from waste tyre pyrolysis generally are a mixture of paraffins, olefins (other hydrocarbons also appear), carbon oxides, hydrogen and small amounts of sulphur and nitrogen compounds.
The pyrolysis of waste tyres usually aims to maximize the yield of the liquid-phase product, because of the valuable chemicals obtained from it. Another way of improving the economics of the process is the acquisition of activated carbon from char. Moreover, the high calorific value of the pyrolysis gas meets the energy requirements of the process and also allows the production of surplus electricity.
3.4.2. Hand gloves
Kaminsky et al. [116] studied the pyrolysis of natural rubber from hand gloves, which are commonly used in households. They obtained 18.2 wt% of gas, 80.6 wt% of oil and tar and 1.2 wt% of carbon black. The gas fraction consisted mainly of methane, CO2, ethane and propene. Hydrogen, CO, H2S and other light hydrocarbons also appeared. A representative sample of the raw pyrolysis oil was distilled and the distillate was separated in two phases. The polar phase consisted mainly of water, but the second phase consisted of a large variety of aliphatic and aromatic compounds such as isoprene, toluene and xylene. Additionally, the carbon black production at 600 °C was very low.
3.5. Textiles
Textile waste is considered as one of the fastest growing sectors in terms of household waste, because sales of new textiles and clothing continually increase and each new cloth finally will join the waste stream. The clothing and textile wastes are composed of synthetic materials such as acrylic, nylon and polyester fibres and natural materials such as wool, flax, leather, silk and cotton. Some studies concerning the pyrolysis of textiles are summarized below. However, there is lack of comprehensive analysis of this topic in the literature. Balcik-Canbolat et al. [117] investigated the pyrolysis of mixed waste textile fibres in a batch reactor. However, they analysed the composition of gas and char only to a limited extent and they completely neglected the liquid phase. Additional studies have been focused on char; examples of products obtained from pyrolysis of textiles are shown in Table 4.
Table 4. Product yields from waste textiles: slow pyrolysis.
| Reactor type | Tempe-rature, °C | Heating rate, °C/min | Gas yield, wt% | Oil yield, wt% | Char yield, wt% | References |
|---|---|---|---|---|---|---|
| Batch bed | 500 | 5 | ∼2 | ∼40 | ∼58 | [118] |
| 700 | ∼8 | ∼41 | ∼51 | |||
| 900 | ∼10 | ∼42 | ∼48 | |||
| Packed bed | 300 | 10 | ∼8 | ∼17 | ∼75 | [13] |
| 400 | ∼14 | ∼35 | ∼31 | |||
| 500 | ∼12 | ∼60 | ∼28 | |||
| 600 | ∼22 | ∼49 | ∼29 | |||
| 700 | ∼37.5 | ∼42.5 | ∼20 | |||
| 800 | ∼40 | ∼40 | ∼20 | |||
| Fixed bed | 450 | 5 | 60.35 | 14.00 | 25.65 | [119] |
| 500 | 66.32 | 15.41 | 18.27 | |||
| 550 | 53.35 | 29.74 | 16.91 | |||
| 600 | 54.26 | 29.49 | 16.25 | |||
| Packed bed | 400 | 10 | ∼32.5 | ∼42.5 | ∼25 | [105] |
| 500 | ∼32 | ∼45.5 | ∼22.5 | |||
| 600 | ∼43 | ∼34 | ∼23 |
Reed and Williams [120] examined five samples of natural fibres: hemp, flax, jute, coir and abaca, for their potential to produce activated carbon from pyrolytic char by physical activation. All of them consist mainly of cellulose (about 60%) and hemicellulose (about 12 wt% to 20 wt%) and smaller amounts of lignin, except coir, which contains more lignin (41–45 wt%) and less cellulose (36–43 wt%). The five biomass waste types were pyrolysed in a fixed bed reactor at a heating rate of 2 °C/min to the final temperature of 450 °C under nitrogen flow. The highest product yield was the liquid, which was composed of a hydrocarbon liquid with high water content. The char yield varied between 24.6 wt% from jute and 34.4 wt% from coir. The gas yield did not exceed 30 wt%, and its composition was dominated by CO, CO2, H2, CH4 and C2H6 with minor concentrations of other hydrocarbon gases up to C4. This composition is typical for pyrogas obtained from biomass.
Cotton is composed mainly of natural polymeric – cellulose. Thus, this material should behave like other biomass materials during pyrolysis. Chowdhury and Sarkar [13] developed the processing of Indian textile waste composed mainly of cotton. They used a fixed bed reactor heated to the temperature range 300 °C to 900 °C. The oil yield increased with increasing temperature and reached 60 wt% at 500 °C and then decreased. The highest heating value of the oil was 20 MJ/kg. Similar results were obtained by Yang et al. [105] during their investigations on the pyrolysis of textiles. In contrast the char yield decreased continuously from 75 wt% at 300 °C to 17 wt% at 900 °C. The maximum gas yield was about 40 wt% at 800 °C. The heating value of the char increased gradually from 20 to 32 MJ/kg as the temperature increased from 300 °C to 500 °C. The char was analysed using SEM. Micrographs characterized the shape and size of the char particles and their porous surface structure [13].
Acrylic textile fabric is one of the most commonly used polymers in the textile field. With acrylic fibres, the constituent polymer chains must contain at least 85% of cyanoethane (acrylonitrile) groups. The remaining 15% consists of other groups that assist fibre processing and allow the addition of several useful properties to the fibres [121]. Nahil and Williams [118] pyrolysed acrylic textile waste in a static bed reactor at temperatures between 500 °C and 900 °C. The main objective of their study was the production of activated carbon products. The char mass decreased as the pyrolysis temperature was increased from 500 °C to 900 °C, while oil and gas yields increased. However, the proportion of fixed carbon increased and the moisture content decreased with increasing temperature, thus the chars obtained at 800 and 900 °C were used in activation processes. The properties of chars obtained from acrylic textiles are shown in Table 5. The surface areas of the pyrolysis chars were less than 2 m2/g. Activation of the pyrolysis chars with steam produced a marked increase in the surface area and porosity. The maximum surface area was obtained from char generated at 800 °C and then steam activated at 900 °C and this was 619 m2/g.
Table 5. Properties of chars and activated carbons from acrylic textile waste [118]
| Pyrolysis tempe-rature, °C | Char yield, wt.% of pyrolysis products | Fixed carbon, wt% of char | Moisture, wt.% of char | Activation tempe-rature, °C | C content, wt% of activated carbon | BET surface area, m2/g | Pore volume, m3/g |
|---|---|---|---|---|---|---|---|
| 900 | ∼48 | 79.5 | 1.8 | 800 | 84.8 | 52 | 0.018 |
| 850 | 84.2 | 204 | 0.088 | ||||
| 900 | 87.4 | 373 | 0.180 | ||||
| 800 | ∼50 | 75.9 | 3.2 | 800 | 77.8 | 148 | 0.059 |
| 850 | 79.7 | 352 | 0.151 | ||||
| 900 | 87.4 | 619 | 0.302 | ||||
| 700 | ∼51 | 72.3 | 4.6 | ||||
| 600 | ∼54 | 69.2 | 4.8 | ||||
| 500 | ∼58 | 62.6 | 4.5 |
3.6. Plastics
The world production of plastic materials reached 269 million tonnes in 2015. China is the largest producer of plastic materials, followed by Europe and NAFTA. The total European demand for plastic in 2015 was 49 million tonnes for PP, PE, PVC, PUR, PET and PS in descending order [122]. However, in municipal waste the largest fractions are: PE, PP, PS, PET and PVC [123]. Plastics are much less likely to biodegrade than other organic materials in MSW. They form a heterogeneous mixture of various components with unstable internal structure and changeable external characteristics. Moreover, the contents of plastic waste vary with the region and the season [124]. As most plastics are not biodegradable, their deposition in landfills is not a desirable solution from an environmental standpoint. There is also a lot of controversy about the incineration of these wastes, due to the release of toxic and greenhouse gases [57]. For example, only very high incineration temperatures can prevent the release of dioxins and furans from plastics, but this requires huge quantities of energy. Another disadvantage of traditional incineration is that it completely destroys all organic matter, which could be valuable for different purposes. The effective treatment of plastic waste is a challenge for the protection of the environment and natural resources. The pyrolysis of plastics has been reviewed previously by Sharuddin et al. [35]. They concluded that pyrolysis has great potential to convert plastic waste to valuable, energy-bearing liquid oil, gas and char. Therefore, it is one of the best solutions for plastic waste conversion and it is also economical in terms of operation. The flexibility that it provides in terms of desired products can be achieved by changing operating parameters accordingly. However, the authors did not focus on real plastic waste mixtures, thus the following sections will expand this topic.
3.6.1. High density polyethylene (HDPE) and low density polyethylene (LDPE)
High and low density polyethylene are the largest component of waste plastic. HDPE can be characterized as a long linear polymer chain with a high degree of crystallinity and low branching which leads to high strength properties. In contrast, LDPE has more branching that results in weaker intermolecular forces, thus lower tensile strength and hardness. However, LDPE has better ductility and it is easier to mould [35]. HDPE is resistant to many different solvents and has a wide variety of applications: bottle caps, food storage containers, plastic bags, backpacking frames, banners, folding chairs and tables, fuel tanks for vehicles, piping, storage sheds, 3-D printer filaments and many more. LDPE is widely used for manufacturing various containers, dispensing bottles, wash bottles, packaging foam etc. Plastic bags are the most popular use of LDPE.
Ahmad et al. [125] studied the quality of oil obtained from HDPE pyrolysis over a temperature range of 250 – 400 °C. They found, that at 250 °C no cracking was observed. At 350 °C the conversion of HDPE into oils was the highest and oil yield reached 80.88 wt%. However, at 400 °C the gas yield had grown to 45.29 wt%, causing a decrease in oil production. The liquid fraction obtained from HDPE was enriched in naphtha range hydrocarbons with a preponderance of both gasoline and diesel range hydrocarbons. The distribution of paraffinic, olefinic, and naphthenic hydrocarbons in oil was 59.70, 31.90, and 8.40 wt%, respectively. It is worth noting, that the Diesel index calculated for HDPE was 31.05 (Diesel – 40), which means, that this liquid fuel had excellent combustion properties. Kumar and Singh [126] used a semi-batch reactor and temperatures between 400 and 550 °C in order to process HDPE. And finally, Mastral et al. [127] conducted the pyrolysis of the same plastic above 650 °C. They used a fluidized bed, thus the solid residue was not measured, because this would present great difficulties. It is possible to see the behaviour of HDPE during pyrolysis in wide range of temperatures. Fig. 11. shows the results obtained by researchers mentioned above. It can be concluded that temperatures between 350 °C and 550 °C are the most appropriate for the pyrolysis of HDPE if the aim is to obtain liquid. Lower temperatures result in higher char yields. On the other hand, very high temperatures also reduce liquid yields.